Small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells improve postoperative cognitive dysfunction in mice with diabetes
Hai-Li Lang, Yan-Zhi Zhao, Ren-Jie Xiao, Jing Sun, Yong Chen, Guo-Wen Hu , Guo-Hai Xu,
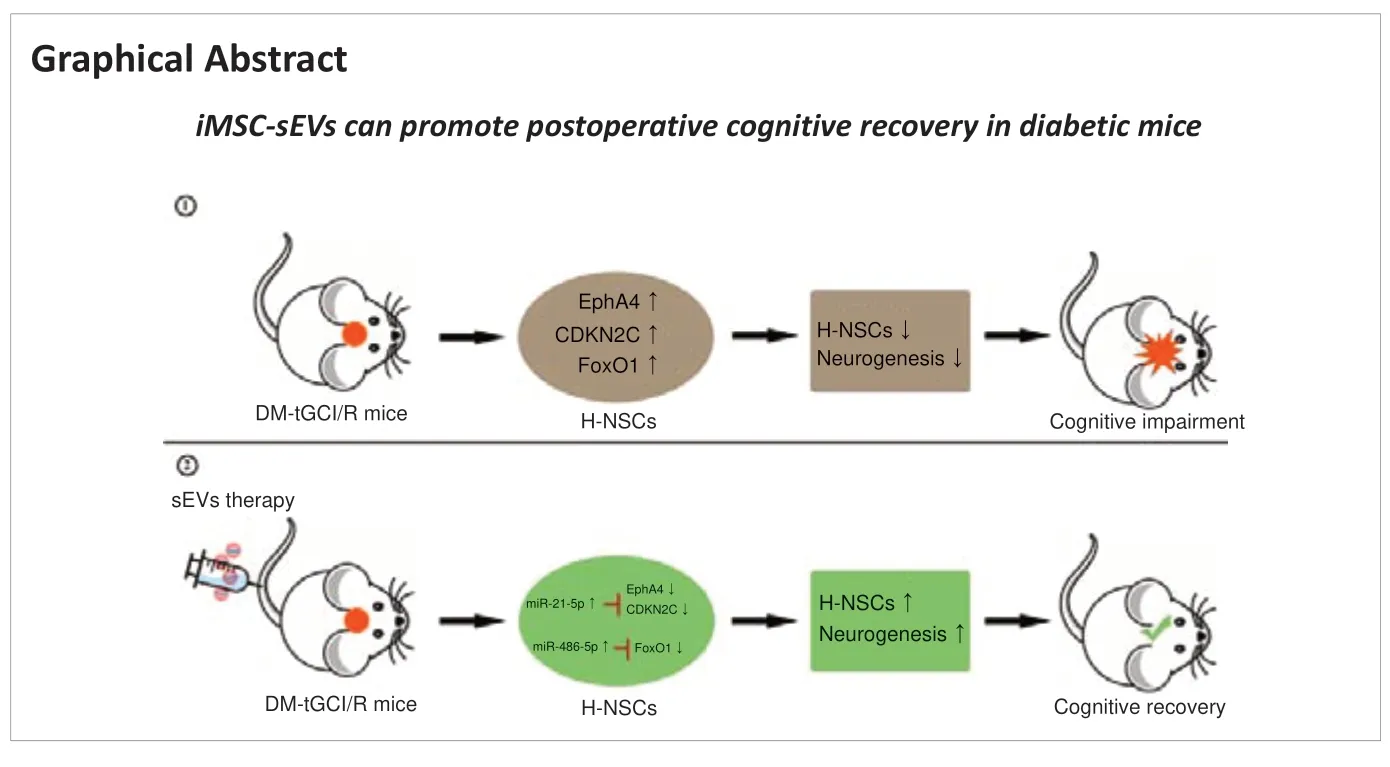
Abstract Postoperative cognitive dysfunction (POCD) is a common surgical complication. Diabetes mellitus (DM) increases risk of developing POCD after surgery. DM patients with POCD seriously threaten the quality of patients’ life, however, the intrinsic mechanism is unclear, and the effective treatment is deficiency. Previous studies have demonstrated neuronal loss and reduced neurogenesis in the hippocampus in mouse models of POCD. In this study, we constructed a mouse model of DM by intraperitoneal injection of streptozotocin, and then induced postoperative cognitive dysfunction by transient bilateral common carotid artery occlusion. We found that mouse models of DM-POCD exhibited the most serious cognitive impairment, as well as the most hippocampal neural stem cells (H-NSCs) loss and neurogenesis decline. Subsequently, we hypothesized that small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells (iMSC-sEVs) might promote neurogenesis and restore cognitive function in patients with DM-POCD. iMSC-sEVs were administered via the tail vein beginning on day 2 after surgery, and then once every 3 days for 1 month thereafter. Our results showed that iMSC-sEVs treatment significantly recovered compromised proliferation and neuronal-differentiation capacity in H-NSCs, and reversed cognitive impairment in mouse models of DM-POCD. Furthermore, miRNA sequencing and qPCR showed miR-21-5p and miR-486-5p were the highest expression in iMSC-sEVs. We found iMSC-sEVs mainly transferred miR-21-5p and miR-486-5p to promote H-NSCs proliferation and neurogenesis. As miR-21-5p was demonstrated to directly targete Epha4 and CDKN2C, while miR-486-5p can inhibit FoxO1 in NSCs. We then demonstrated iMSC-sEVs can transfer miR-21-5p and miR-486-5p to inhibit EphA4, CDKN2C, and FoxO1 expression in H-NSCs. Collectively, these results indicate significant H-NSC loss and neurogenesis reduction lead to DM-POCD, the application of iMSC-sEVs may represent a novel cell-free therapeutic tool for diabetic patients with postoperative cognitive dysfunction.
Key Words: diabetes mellitus; hippocampus; induced pluripotent stem cell; mesenchymal stem cell; miRNA; neural stem cell; neurogenesis; postoperative cognitive dysfunction; signaling pathway; small extracellular vesicle
Introduction 609 Methods 610 Results 611 Discussion 615
Introduction
Postoperative cognitive dysfunction (POCD) is one of the most common complications in surgical patients, presenting as impaired cognitive function that may persist for months or even years after surgery (Steinmetz and Rasmussen, 2016). Transient or repeated global cerebral ischemia during surgery can induce cerebral ischemia/reperfusion injury, causing neuronal damage and neuroinflammation, which is regarded as the key pathogenesis of POCD (van Harten et al., 2012; Hovens et al., 2016). POCD is also an important comorbidity and complication of diabetes mellitus (DM) (Biessels and Whitmer, 2020), and multiple studies have demonstrated that patients with DM are at increased risk of developing cognitive dysfunction after surgery (Thourani et al., 1999; Kadoi et al., 2005). The increasing prevalence of DM (Saeedi et al., 2019) is associated with a rise in the number of diabetic surgical patients and an according increase in the incidence of DM-POCD, further leading to increased morbidity and mortality, prolonged hospital stays, impaired long-term cognitive function, and decreased quality of life (Daiello et al., 2019). It is therefore necessary to explore new effective strategies to prevent and treat DM-POCD.
Maintaining cognitive function requires structural and functional integrity of the hippocampus. Hippocampal neural stem cells (H-NSCs) and their neurogenesis play crucial roles in maintaining and restoring hippocampal structure and hippocampus-dependent brain functions, by increasing the production of functional granule neurons that integrate into existing hippocampal circuits (van Praag et al., 2002; Toda and Gage, 2018). Similar to other cognitive dysfunction diseases (Boese et al., 2020; Hu et al., 2020, 2021), we previously demonstrated that hippocampal neurons were lost and hippocampal neurogenesis was decreased in mice with POCD, and showed that restoring H-NSCs and promoting their neurogenesis aided cognitive recovery (Sun et al., 2021). However, the physiological changes in H-NSCs and whether improving their neurogenesis promotes cognitive recovery in DMPOCD remain unclear.
Small extracellular vesicles (sEVs), including classical exosomes, are natural nano-sized particles secreted by cells, which participate in intercellular communication and influence recipient cell behavior via the delivery of functional biomolecules (Gualerzi et al., 2021; Negahdaripour et al., 2021; Zhang et al., 2022). Stem cell-derived sEVs are an attractive therapeutic strategy in regenerative medicine due to their promising pro-regenerative effects, lack of aneuploidy risk, and low possibility of immune rejection (Constantin et al., 2020; Rahmani et al., 2020). Induced pluripotent stem cell-derived mesenchymal stem cells (iMSCs) are a promising cell source for autologous cell therapies in regenerative medicine because of their easy acquisition, powerful proliferation, and therapeutic function (Jakob et al., 2020). sEVs secreted by iMSCs (iMSCsEVs) promoted angiogenesis (Hu et al., 2015), skin cell proliferation (Kim et al., 2018), and bone regeneration (Zhu et al., 2017). We therefore hypothesized that iMSC-sEVs might exhibit powerful neurogenesis-promoting functions that could aid cognitive recovery in DM-POCD.
The present study aimed to examine changes in cognitive function and hippocampal neurogenesis in a mouse model of DM-POCD, and to investigate the biological function and potential mechanism of iMSC-sEVs in regulating H-NSC proliferation and neuronal differentiation, with the goal of developing an effective treatment strategy for DM-POCD.
Methods
Generation of iMSCs from human induced pluripotent stem cells (iPSCs)
In accordance with our previous study (Hu et al., 2015), the human iPSC line (IPS-S cell line, RRID: CVCL_C876) was provided by the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Liao et al., 2008), and cultured and expanded on human ESC-Qualified BD Matrigel (BD Biosciences, Sparks, MD, USA)-coated plates in mTESR1 (StemCell Technologies, Vancouver, BC, Canada). iPSCs were identified by immunofluorescent staining of Nanog, OCT4, SSEA4, TRA-1-81, and alkaline phosphatase (Beyotime Biotechnology, Shanghai, China, Cat# C3206) (Hu et al., 2020). When the iPSCs reached 80‒90% confluence, mTESR1 was replaced with Dulbecco’s modified Eagle medium-low glucose (Corning, Tewksbury, MA, USA) containing 10% (vol/vol) fetal bovine serum (Life Technologies, Grand Island, NY, USA) and 2 mM L-glutamine. After 14 days of culture, the cells were serially trypsinized (0.25% trypsin/1 mM ethylene diamine tetraacetie acid; Life Technologies) and reseeded three times. Cells at passage 4 generally had a morphology resembling MSCs and were used for identification and further experiments.
iMSC identification
As described previously (Hu et al., 2015), we used the easyCytesystem (Guava Millipore, Billerica, MA, USA) to analyze iMSC surface antigens. CD29-PE, CD90-PE, CD105-PE, and HLA-DR-PE conjugated monoclonal antibodies, and isotype-matched mouse monoclonal antibodies (all BD Biosciences) were used at the manufacturer’s recommended concentrations. iMSC multipotency was tested by osteogenic and adipogenic differentiation. For adipogenic differentiation, iMSCs were induced using alpha minimum essential medium (Corning) supplemented with 10% fetal bovine serum, 100 µM indomethacin (Cayman Chemical, Ann Arbor, MI, USA), 1 µM dexamethasone (Sigma, St. Louis, MO, USA), 10 µg/mL insulin (Sigma), and 0.5 mM isobutylmethylxanthine (Life Technologies). Cells were stained with Oil Red O (Beyotime Biotechnology) after 14 days of induction. For osteogenic differentiation, iMSCs were induced by Dulbecco’s modified Eagle mediumhigh glucose (Corning) supplemented with 10% fetal bovine serum, 50 µg/mL ascorbic acid-2-phosphate (Merck, Darmstadt, Germany), 10 mM b-glycerophosphate (Sigma), and 100 nM dexamethasone (Sigma). Cells were stained with Alizarin red (Beyotime Biotechnology) after 21 days of induction. Images were acquired using a phase-contrast microscope (Leica, Wetzlar, Germany).
iMSC-sEV isolation
iMSC-sEVs were isolated from iMSC-conditioned medium (iMSC-CM) by differential ultracentrifugation, as described previously (Hu et al., 2020). Briefly, iMSC-CM was centrifuged at 300 ×g
for 10 minutes, 2000 ×g
for 20 minutes, and 10,000 ×g
for 30 minutes, respectively. The supernatant was then filtered through a 0.22 µm sterilized filter (Millipore, Bedford, MA, USA) to remove large EVs, followed by ultracentrifugation twice at 100,000 ×g
for 114 minutes using an SW 32 Ti Rotor Swinging Bucket rotor (K factor of 256.8, 28,536 r/min; Beckman Coulter, Fullerton, CA, USA) to pellet the iMSC-sEVs. Finally, the pellet was used for identification experiments or resuspended in phosphate-buffered saline (PBS) and stored at ‒80°C. All centrifugation steps were performed at 4°C.iMSC-sEV identification and particle parameter measurement
The morphology of the iMSC-sEVs was observed by transmission electron microscopy (Hitachi H-7650, Tokyo, Japan). Briefly, 10 µL iMSC-sEV-enriched solution was placed on a formvar-carbon coated grid (300 meshes) and left to dry, fixed in 1% glutaraldehyde, stained with saturated aqueous uranyl oxalate, and imaged (Hu et al., 2020). The size distribution and particle concentration of the iMSC-sEVs were analyzed using an N30 NanoFlow Analyzer (NanoFCM Inc., Xiamen, China). The concentration of EVs was calculated according to the ratio of side-scatter intensity to particle concentration in standard polystyrene nanoparticles, and the size distribution was calculated according to the standard curve (Hu et al., 2020). The expression of sEV markers (CD63, TSG101, GM130 and β-actin) was determined by western blotting (Hu et al., 2020). To calculate the iMSC-sEV parameters, the number of iMSCs and the volume of iMSC-CM were quantified, the iMSC-CM was used to isolate iMSC-sEVs by differential ultracentrifugation, the size distribution and particle concentration of the pellets were measured using a nanoflow cytometer (NanoFCM Inc., Xiamen, China), and the protein concentration of the iMSCsEVs was quantified using a BCA Protein Assay Kit (Beyotime Biotechnology; Cat#P0012).
Animal experiments
Male C57BL/6J mice (n
= 128, age 4‒6 weeks, weight 15‒20 g) were obtained from Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, China; license No. SCXK (Hu) 2012-0002) and housed in a specific pathogen-free animal laboratory at the Experimental Animal Center of the Medical College of Nanchang University (Institutional approval number for laboratory animal studies: SYXK (Gan) 2015-0001). The animal studies were approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University [No. (2018)099] in 2018. All experiments were designed and reported according to the Animal Research: Reporting ofIn Vivo
Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). In this study, mice were anesthetized by inhalation of 5% isoflurane (RWD Life Technology, Shenzhen, China) and anesthesia was maintained by 1.5% isoflurane.The mice were divided into the following groups: (1) Normal-sham, Normaltransient global cerebral ischemia/reperfusion (tGCI/R), DM-sham, and DMtGCI/R groups; (2) DM-sham + PBS, DM-sham + sEVs, DM-tGCI/R + PBS, and DM-tGCI/R + sEVs groups; and (3) DM-tGCI/R + PBS, DM-tGCI/R + sEVs-NC, and DM-tGCI/R + sEVs-IN groups.
We induced DM according to the animal model protocols of the Diabetic Complications Consortium (Hu et al., 2019). DM was induced in 2-month-old mice by intraperitoneal injection of streptozotocin (10 mg dissolved in 1 mL 0.1 M citrate buffer; Sigma) at 50 mg/kg for 5 consecutive days. Normal control animals received the same volume (0.1 mL) of citrate buffer. Two months after the last streptozotocin injection, blood glucose levels were assayed in tail vein blood, and mice with random blood glucose concentrations > 16.7 mM and glycosylated hemoglobin > 6.5% were considered to have DM (Hu et al., 2019) and were used for subsequent experiments.
According to our previous study (Sun et al., 2021), we induced tGCI/R injury in mice by transient bilateral common carotid artery occlusion surgery to imitate POCD. Briefly, mice were anesthetized and the right and left common carotid arteries were obstructed for 10 minutes, followed by reperfusion for 10 minutes, repeated three times. The threading was then removed and the incision was sutured. Mice in the sham group were subjected to the same surgical procedure without ligation of the arteries. The PBS- and iMSC-sEVtreated groups received 100 µL PBS or iMSC-sEVs (1 × 10particles dissolved in 100 µL PBS), respectively, by tail vein injection, beginning on the second day after surgery and once every 3 days for 1 month thereafter. The mice were injected with 50 mg/kg 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen, Carlsbad, CA, USA; Cat# E10187) 3 and 1 days before sacrifice, to label proliferating cells.
Morris water maze
The Morris water maze test was employed to assess the spatial learning and memory abilities of the mice, as described previously (Sun et al., 2021). Briefly, the latency to escape onto the platform and the distance from the starting quadrant to the platform were recorded as indicators of spatial learning. In each trial, mice were given a maximum of 60 seconds to find the submerged platform. Spatial memory was assessed by spatial probe trial on day 5 of the trial. The time the mice spent in the target quadrant or the times they crossed the platform position within 60 seconds were recorded. All data were collected and analyzed by two participants who were blinded to the group conditions, using the SuperMaze animal behavior record and analysis system (Xinran Mdt InfoTech Ltd., Shanghai, China).
Immunofluorescence staining
Mice were anesthetized with 5% isoflurane and perfused immediately. The brain was harvested and immersed in paraformaldehyde at 4°C for 12 hours, followed by dehydration in 20%, 30%, and 35% (w/v) sucrose solutions at 4°C, respectively. Brain sections and cultured cells (H-NSCs, iPSCs) were prepared and subjected to immunofluorescence staining as described previously (Hu et al., 2020; Sun et al., 2021). The primary antibodies (4°C, overnight) were as follows: rabbit anti-SRY-box transcription factor 2 (Sox2; 1:100; Abcam, Cambridge, UK; Cat# ab92494; RRID: AB_10585428), mouse anti-glial fibrillary acidic protein (GFAP; 1:100; Abcam; Cat# ab10062; RRID: AB_296804), rabbit anti-β-III tubulin (1:100; Abcam; Cat# ab18207; RRID: AB_444319), rabbit anti-doublecortin (DCX; 1:100; Abcam; Cat# ab18723; RRID: AB_732011), rabbit anti-Nanog (1:100; Abcam; Cat# ab109250; RRID:AB_10863442), rabbit anti-organic cation/carnitine transporter 4 (OCT4; 1:100; Abcam; Cat# ab19857; RRID: AB_445175), mouse anti-stage-specific embryonic antigen-4 (SSEA4; 1:100; Abcam; Cat# ab16287, RRID: AB_778073), and mouse anti-T cell receptor alpha locus-1-81 (TRA-1-81; 1:100; Abcam; Cat# ab16289; RRID: AB_2165986). The secondary antibodies (25°C, 1 hour, 1:400; Invitrogen) were as follows: Alexa Fluor® 594 goat anti-rabbit (Cat# A11012; RRID: AB_141359) or anti-mouse (Cat# A21125; RRID:AB_141593) IgG (H+L), Alexa Fluor® 488 goat anti-rabbit (Cat# A32731; RRID: AB_2633280), or anti-mouse (Cat# A32723; RRID: AB_2633275) IgG (H + L). EdUcells were stained using a Click-iT Edu Alexa Fluor 594 Imaging Kit (Cat# C10339; Life Technologies). Briefly, the Click-iT kit azide and buffer additive were thawed in a light-protected box and diluted with dHO to make a working 1× solution. After three rinses in PBS, the reaction cocktail was made following the manufacturer’s protocol. Brain sections or cultured cells were incubated in the EdU cocktail for 30 minutes. Nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI; Beyotime Biotechnology, Shanghai, China) and at least 10 images were acquired using an Olympus IX73 fluorescence microscope (Olympus Corporation, Tokyo, Japan). The number of positively stained cells was calculated using ImageJ software V1.8.0 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012). Image acquisition and positive cell counts were performed by two participants who were blinded to the group conditions.
H-NSC isolation, proliferation, and neuronal differentiation
Mice were anesthetized and sacrificed by cervical dislocation. H-NSCs were isolated from the hippocampus of mice in the different groups, as described previously (Sun et al., 2021), and cultured in Dulbecco’s modified Eagle/F12 medium supplemented with 2% B27 (Gibco, Waltham, MA, USA; Cat# 12587010), 20 ng/mL epidermal growth factor (ProSpec Bio, Rehovot, Israel), 20 ng/mL basic fibroblast growth factor (ProSpec Bio), 5 µg/mL heparin (Sigma), and 1% penicillin/streptomycin (Gibco).
H-NSC proliferation and differentiation assays were performed as described previously (Sun et al., 2021). For the proliferation assay, 20,000 cells were cultured in complete NSC medium for 4 days and then treated with 10 µM EdU (Life Technologies; Cat# A10044) for 4 hours. H-NSCs were dissociated, plated, fixed, and subjected to immunofluorescence staining. For the neuronal-differentiation assay, 50,000 cells were plated on poly-L-lysine hydrobromide (Sigma; Cat# P8920)-coated 48-well plates and cultured with neural basal medium (Gibco) containing 2% B27 and 1% fetal bovine serum for 5 days. The cells were then fixed and subjected to immunofluorescence staining.
In vitro
iMSC-sEV uptake assay
Fluorescent carbocyanine dye (DiO; Life Technologies) was used to label iMSC-sEVs, as described previously (Hu et al., 2020). After incubation with DiO for 30 minutes at 37°C, the labeled iMSC-sEVs were washed with PBS and pelleted by three rounds of differential ultracentrifugation. H-NSCs were incubated with DiO-labeled iMSC-sEVs (1 × 10particles/mL) for 12 hours, rinsed twice with PBS, fixed, and stained with DAPI. Images were acquired using an Olympus IX73 fluorescence microscope.
iMSC-sEV microRNA expression profiling and delivery of miRNA inhibitors to iMSC-sEVs
iMSC-sEV miRNAs were isolated using an miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentration and purity of the RNA samples were detected using a NanoDropND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Microarray analysis was performed on an Illumina NextSeq 500 (Illumina, San Diego, CA, USA) and analyzed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
We searched the PubMed database for microRNAs (miRNAs), involved in promoting either NSC proliferation or neuronal differentiation using the search terms: ([NSCor neural stemor neural progenitoror NPC]) AND ([miRNAor microRNA]]) AND ([neurogenesisor proliferation]). Searches were limited to English language articles.
miRNA inhibitors of miR-21-5p and miR-486-5p and a negative control were obtained from GenePharma (Shanghai, China). All the nucleotides in the inhibitors contained 2′-O-Me modifications at every base and a 5′-Cy3-containing amino linker. The sequences of the inhibitors are listed inTable 1
. An Exo-Fect™ siRNA/miRNA Transfection Kit (System Biosciences, Mountain View, CA, USA) was used to deliver the miRNA inhibitors into the iMSC-sEVs in accordance with the manufacturer’s instructions.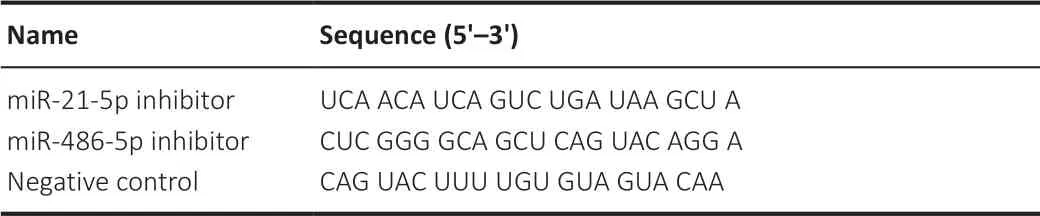
Table 1 |Sequences of miRNA inhibitors
Real-time polymerase chain reaction (qPCR) analysis
The expression levels of miRNAs in iMSC-sEVs and H-NSCs were determined by qPCR. iMSC-sEV miRNAs were isolated using an miRNeasy Mini Kit, as described previously (Hu et al., 2020), and reverse transcribed using a miScript II RT Kit (Qiagen). PCR was carried out using an ABI Prism 7900HT Real Time System (Applied Biosystems, Carlsbad, CA, USA) with a miScript SYBR Green PCR Kit (Qiagen) and miScript Primer Assay (Qiagen). The miScript Primer Assays for the target miRNAs are listed inTable 2
. Data were analyzed using the cycle threshold (Ct) value. Each experiment was performed in triplicate. Total RNA was extracted from H-NSCs using TRIzol (Life Technologies), as described previously (Hu et al., 2015), and the RNA concentration was measured with a Nanodrop 2000 reader (Thermo Scientific). The primer sequences for Eph receptor A4 (Epha4
), cyclin-dependent kinase inhibitor 2C (CDKN2C
), and forkhead box O1 (FoxO1
) are summarized inTable 3
. Data were analyzed using SDS 2.4 software, and expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH
) using the ΔΔCt method. Each qPCR was performed in triplicate for yield validation.
Table 2 |Sequences of microRNA primers
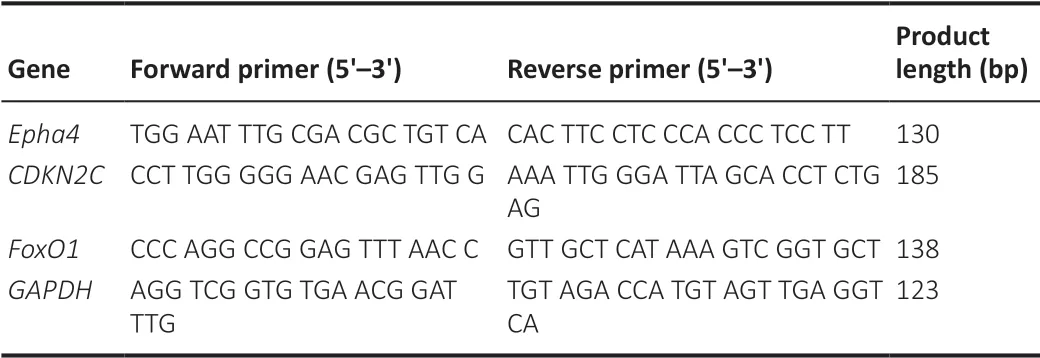
Table 3 |Primers used for qPCR
Western blot assay
The levels of proteins in iMSC-sEVs, H-NSCs, and hippocampus tissue were determined by western blot. iMSC-sEV protein, H-NSC whole protein, and hippocampus protein were harvested using radio-immunoprecipitation assay lysis buffer supplemented with protease inhibitor (Beyotime Biotechnology, Cat# P1006), as described previously (Hu et al., 2020). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Darmstadt, Germany). The primary antibodies were as follows: rabbit anti-CD63 (1:1000; Abcam; Cat# ab134045; RRID: AB_2800495), mouse anti-tumor susceptibility gene 101 (TSG-101; 1:1000; Abcam, Cat# ab83; RRID: AB_306450), mouse anti-Golgi matrix protein (GM130; 1:500; Abcam; Cat# ab169276; RRID: AB_2894838), mouse anti-synaptophysin (SYP; 1:1000; Abcam; Cat# ab8049; RRID: AB_2198854), mouse anti-growth associated protein 43 (Gap43; 1:1000; Abcam; Cat# ab277627), rabbit anti-β-actin (1:1000; Abcam; Cat# ab179467; RRID: AB_2737344), rabbit anti-Epha4 (1:1000; Abcam; Cat# ab264047), rabbit anti-CDKN2C (1:1000; Abcam; Cat# ab192239), and rabbit anti-FoxO1 (1:1000; Abcam; Cat# ab52857; RRID: AB_869817). The membranes were incubated with horseradish peroxidaselinked rabbit anti-mouse IgG (1:3000; Cell Signaling Technology; Cat# 58802; RRID: AB_2799549) or horseradish peroxidase-linked goat anti-rabbit (1:3000; Cell Signaling Technology; Cat# 7074; RRID: AB_2099233) secondary antibody. The proteins were detected by enhanced chemiluminescence (Thermo Scientific) and imaged using an Image Quant LAS 4000 mini biomolecular imager (GE Healthcare, Little Chalfont, UK).
Statistical analysis
Evaluators were blinded to treatment assignments. The sample size was based on our previous study (Sun et al., 2021). Data were analyzed using Prism 8 software (GraphPad Software, San Diego, CA, USA) by analysts who were blinded to the purpose of the study. All data are presented as mean ± standard error (SEM). Differences among the groups were analyzed by oneway analysis of variance followed by Bonferronipost hoc
test in the absence of equivalent variance.P
< 0.05 was deemed to be statistically significant.Results
Diabetes accelerates H-NSC inactivation and cognitive dysfunction in mice suffering from tGCI/R injury
Thein vivo
studies are summarized inFigure 1A
. We initially investigated the effects of DM on cognitive dysfunction in mice suffering from tGCI/R injury using the Morris water maze test to assess their spatial learning and memory abilities. The escape latency and swimming distance on day 5 of the training trials were both longer in normal mice with tGCI/R injury (Nor + tGCI/R group), DM mice with sham injury (DM + sham group), and DM mice with tGCI/R injury (DM + tGCI/R group) than in normal mice with sham injury (Nor + sham group). In addition, the escape latency and swimming distance were longer in DM + tGCI/R mice compared with Nor + tGCI/R and DM + sham group mice (escape latency:P
= 0.0006 for DM + tGCI/Rvs
. Nor + tGCI/R; swimming distance:P
= 0.0003 for DM + tGCI/Rvs.
Nor + tGCI/R;Figure 1B
andC
). In the probe trials, mice in the Nor + tGCI/R, DM + sham, and DM + tGCI/R groups spent less time in the target quadrant and crossed the platform position fewer times than mice in the Nor + sham group, with mice in the DM + tGCI/R group showing the poorest performances (spent time:P
= 0.0001 for DM + tGCI/Rvs
. Nor + tGCI/R; crossed times:P
= 0.0047 for DM + tGCI/Rvs
. Nor + tGCI/R;Figure 1D
andE
).We also detected expression levels of the synapse-related proteins SYP and Gap43 in the hippocampus to further estimate cognitive function. Expression levels of SYP and Gap43 were lower in Nor + tGCI/R, DM + sham, and DM + tGCI/R mice compared with Nor + sham mice, with the lowest levels in the DM + tGCI/R group (SYP:P
= 0.0001 for DM + tGCI/Rvs
. Nor + tGCI/R; Gap43:P
= 0.0001 for DM + tGCI/Rvs
. Nor + tGCI/R;Figure 1F
). These data indicated that both tGCI/R injury and DM impaired cognitive function, suggesting that DM patients suffering from tGCI/R injury may experience more serious cognitive impairment than patients without DM.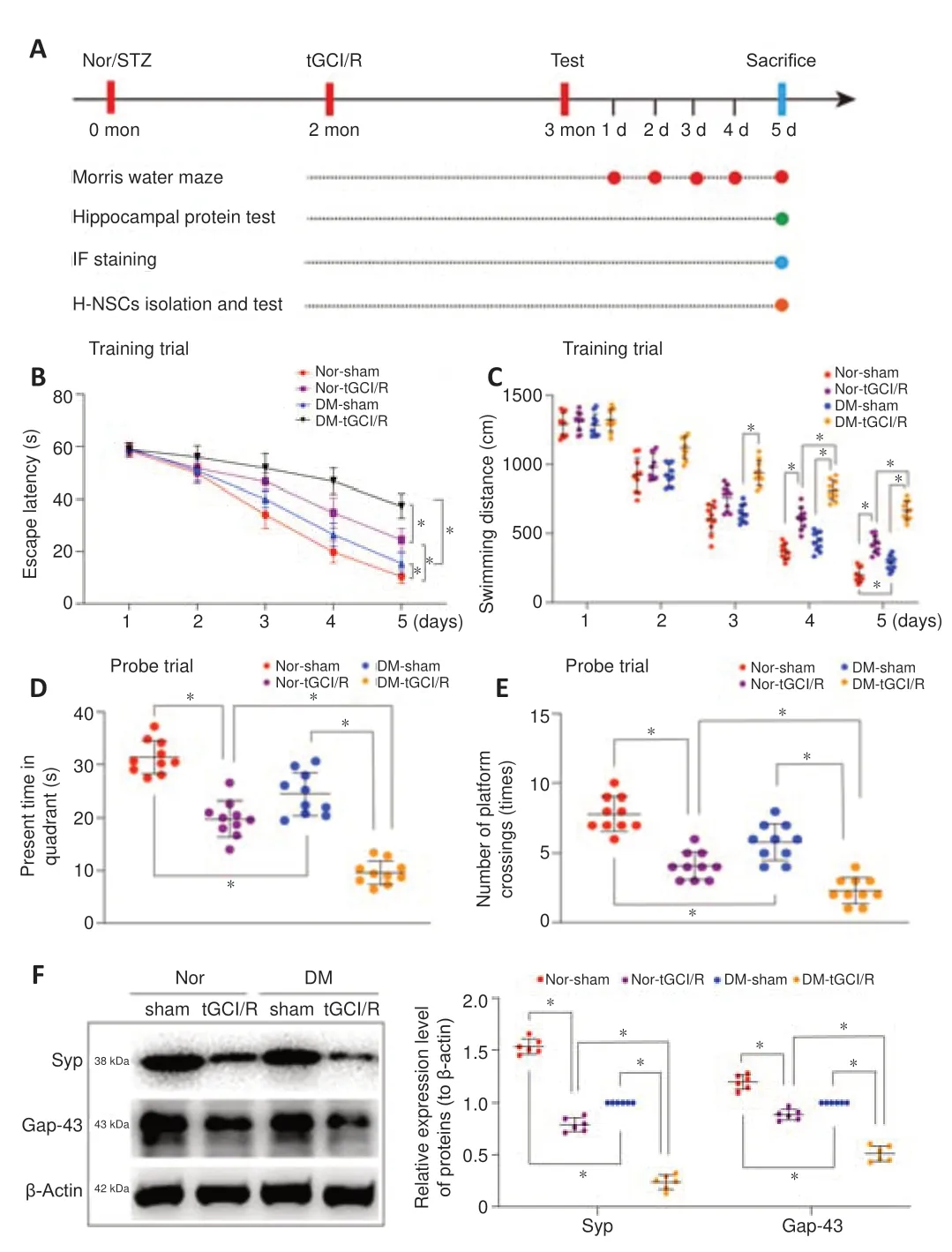
Figure 1 |Cognitive impairment in DM-tGCI/R mice. (A) In vivo experiments. (B‒E) Spatial learning and memory abilities in mice were tested by Morris water maze (n = 10/group). Mice in the DM + tGCI/R group showed the worst spatial learning and memory abilities among the four groups. (F) Western blot analysis of hippocampal SYP and Gap43 in mice (n = 6/group). Mice in the DM + tGCI/R group showed the lowest expression levels of hippocampal SYP and Gap43 among the four groups. All data are presented as mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DM: Diabetes mellitus; Gap43: growth associated protein 43; SYP: Synaptophysin; tGCI/R: transient global cerebral ischemia/reperfusion.
H-NSCs play a crucial role in maintaining and restoring cognition (Toda and Gage, 2018). Their decline thus directly reduces neuroplasticity and cognitive function, while their proliferation and neurogenesis help restore hippocampal structure and function (Hu et al., 2020). We therefore calculated the numbers of H-NSCs (Sox2/GFAP) and newly generated immature neurons (DCX) in the subgranular zone (SGZ) of mice in the four groups, as described previously (Hu et al., 2021). The numbers of Sox2/GFAPcells and DCXcells were higher in Nor + sham mice compared with Nor + tGCI/R, DM + tGCI/R, and Nor + sham mice, and the numbers of Sox2/GFAPcells and DCXcells were lower in DM + tGCI/R mice compared with Nor + sham and Nor + tGCI/R mice (Sox2/GFAP:P
= 0.0001 for DM + tGCI/Rvs.
Nor + tGCI/R; DCX:P
= 0.0001 for DM + tGCI/Rvs
. Nor + tGCI/R;Figure 2A–D
). We also isolated H-NSCs from mice in the four groups and compared their proliferation and neuronal differentiation abilitiesin vitro
. The percentage of proliferating H-NSCs (EdU/DAPIcells) was higher in the Nor + sham group compared with the other three groups and lower in the DM + tGCI/R group compared with the Nor + tGCI/R and DM + sham groups (P
= 0.0001 for DM + tGCI/Rvs.
Nor + tGCI/R;Figure 2E
andF
). After induction, the percentage of differentiated neurons (β-III tubulincells) was significantly higher in the Nor + sham group compared with the Nor + tGCI/R, DM + sham, and DM + tGCI/R groups, and was lowest in the DM + tGCI/R group (P
= 0.0001 for DM + tGCI/Rvs
. Nor + tGCI/R;Figure 2G
andH
). These results indicated that the proliferation and neuronal differentiation capacities of H-NSCs were decreased in mice after exposure to DM or tGCI/R injury. H-NSC activation was further decreased in DM mice suffering from tGCI/R injury, leading to adverse effects on neurogenesis and cognitive function.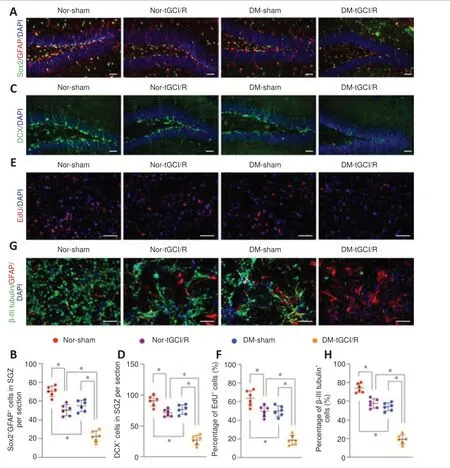
Figure 2 |H-NSC proliferation and neuronal differentiation deficits in DM-tGCI/R mice. (A) Immunofluorescence (IF) staining of Sox2+ (Alexa Fluor 488, green) and GFAP+ (Alexa Fluor 594, red), and DAPI nuclear staining (blue) in the hippocampus and (B) estimated numbers of Sox2+/GFAP+ cells. The number was lowest in the DM + tGCI/R group. (C) IF staining of hippocampal proliferative immature neurons (DCX+; Alexa Fluor 488, green) and DAPI nuclear staining (blue) and (D) estimated numbers of DCX+ cells. The number was lowest in the DM + tGCI/R group. (E) IF staining of EdU (Alexa Fluor 594, red) incorporation and DAPI nuclear staining (blue) in isolated H-NSCs and (F) quantification of EdU+/DAPI+ cells in DAPI+ cells. The percentage of proliferating H-NSCs (EdU+/DAPI+ cells) was lowest in the DM + tGCI/R group. (G) IF staining of neuronal differentiation (β-III tubulin+, Alexa Fluor 488, green; GFAP+, Alexa Fluor 594, red; DAPI, blue) in isolated H-NSCs and (H) quantification of β-III tubulin+ (Alexa Fluor 488, green)/DAPI+ cells among DAPI+ cells. The percentage of differentiated neurons (β-III tubulin+ cells) was lowest in the DM + tGCI/R group. Scale bars: 50 µm in A, B; 100 µm in C, D. All data are presented as mean ± SEM (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DCX: Doublecortin; DM: diabetes mellitus; EdU: 5-ethynyl-2′-deoxyuridine; GFAP: glial fibrillary acidic protein; H-NSCs: hippocampal neural stem cells; IF: immunofluorescence; tGCI/R: transient global cerebral ischemia/reperfusion.
iMSC generation and iMSC-sEV identification
iPSCs were grown clonally (Figure 3A
) and shown to be positive for alkaline phosphatase (Figure 3B
) and pluripotency-related markers, including OCT4, Nanog, TRA-1-81, and SSEA4 (Figure 3C
). After induction in MSC-induction medium, iPSCs lost their typical morphology and formed a monolayer of larger spindle-shaped cells at the border of the colonies (Figure 3Da
). After trypsinization and passaging three times, the cells developed a uniform fibroblast-like morphology (Figure 3D-b
). iMSCs possessed powerful adipogenic (Figure 3E
) and osteogenic differentiation capabilities (Figure 3F
) and were positive for MSC-positive markers including CD29, CD90, and CD105, and negative for MSC-negative markers, such as HLA-DR (Figure 3G
). These results indicated that the iPSCs were successfully differentiated into MSCs.iMSC-sEVs isolated from iMSC-CM by differential ultracentrifugation exhibited a size distribution of approximately 100 nm, with a characteristic cup-shaped morphology under transmission electron microscopy (Figure 3H
). Western blot analysis showed that iMSC-sEVs also expressed the exosomal markers CD63 and TSG101, but not express the Golgi matrix protein GM130 and β-actin (Figure 3I
), indicating no contamination of the isolated iMSC-sEVs by cellular components (Hu et al., 2020). Flow cytometry analysis identified particles with a mean diameter of 73.9 ± 26.3 nm and a concentration of 8.1 × 10± 0.6 × 10particles/mL (Figure 3J
). We further calculated the particle concentration and protein concentration by evaluating the yield of iMSC-sEVs. The mean particle concentration was 5.62 × 10± 1.17 × 10particles/mL iMSC-CM and 547.4 ± 64.6 particles/cell (Figure 3K
). The mean protein concentration was 731.9 ± 86.2 ng/mL iMSC-CM and 9.6 × 10± 1.8 × 10ng/particle.iMSC-sEVs enhances H-NSC activity to restore cognitive function in DM-tGCI/R mice
Thein vivo
studies are summarized inFigure 4A
. iMSC-sEVs were injected intravenously into mice in the different groups to investigate their therapeutic effects. We first investigated the effects of iMSC-sEVs in promoting cognitive recovery in DM mice suffering from tGCI/R injury. The escape latency and swimming distance on day 5 of the training trials were shorter in DM-sham + sEVs and DM-tGCI/R + sEVs mice compared with DM-sham + PBS or DMtGCI/R + PBS mice, respectively (escape latency:P
= 0.0004 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS; swimming distance:P
= 0.0002 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS;Figure 4B
andC
). In probe trials, DM-sham + sEVs and DM-tGCI/R + sEVs mice spent longer in the target quadrant and crossed the platform more times than DM-sham + PBS and DM-tGCI/R + PBS mice (spent time:P
= 0.0016 for DM-tGCI/R + sEVsvs.
DM-tGCI/R + PBS; crossed times:P
= 0.0014 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS;Figure 4D
andE
). Moreover, expression levels of hippocampal SYP and Gap43 were higher in DM-sham + sEVs and DM-tGCI/R + sEVs mice compared with DM-sham + PBS and DM-tGCI/R + PBS mice (SYP:P
= 0.0001 for DM-tGCI/R + sEVsvs.
DM-tGCI/R + PBS; Gap43:P
= 0.0001 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS;Figure 4F
). These results indicated that iMSC-sEVs possessed a powerful ability to reverse cognitive deficits in DM mice suffering from tGCI/R injury.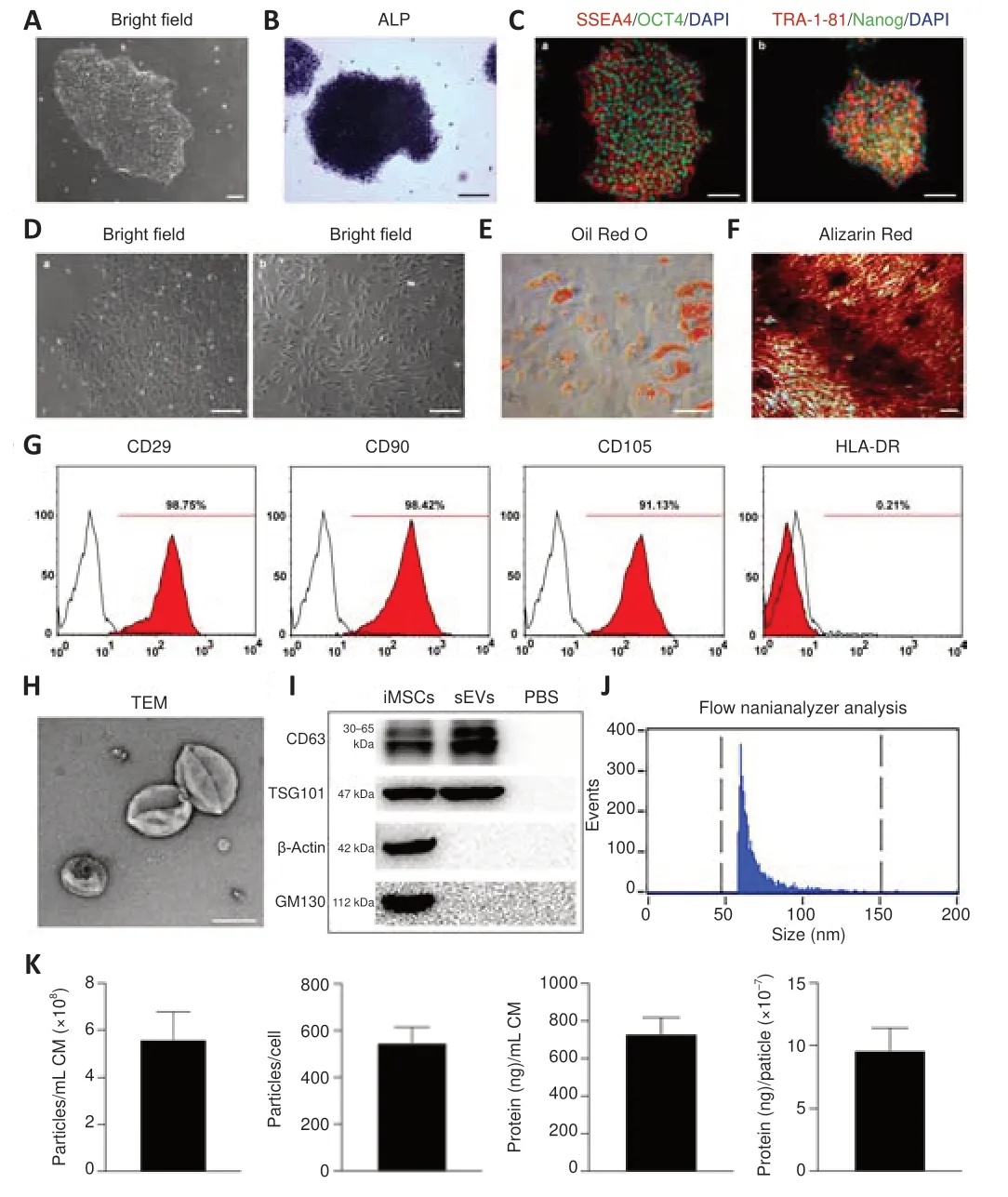
Figure 3 |Generation of iMSCs and characterization of iMSC-sEVs. (A) Morphology of clonally grown iPSC colonies in bright field. (B) Alkaline phosphatase staining of iPSCs. (C) IF staining of OCT4 (Alexa Fluor 488, green), Nanog (Alexa Fluor 488, green), TRA-1-81 (Alexa Fluor 594, red), and SSEA4 (Alexa Fluor 594, red) in iPSCs. iPSCs were positive for pluripotency-related markers, including OCT4, Nanog, TRA-1-81, and SSEA4. (D) Intermediate phase of differentiation of iPSCs into iMSCs (a) and complete differentiation into fibroblast-like cells (b). After induction in MSC-induction medium, iPSCs began to lose their typical morphology and formed a monolayer of larger spindleshaped cells at the border of the colonies. (E) Oil Red O staining of small lipid droplets in multi-differentiated iMSCs on day 21. (F) Alizarin red staining of osteogenic mineralization in multi-differentiated iMSCs on day 14. (G) Flow cytometric analysis of mesenchymal positive markers including CD29, CD90, and CD105, and negative markers including HLA-DR. Black histograms represent isotype controls and red solid peak represents the marker indicated. (H) Morphology of iMSC-sEVs observed by transmission electron microscopy. Scale bars: 100 µm in A, C, F; 150 µm in B; 500 µm in D; 50 µm in E; 100 nm in H. (I) Western blot showing presence of sEV markers including CD63 and TSG101, and negative for GM130 and β-actin. (J) Particle size distribution and concentration of iMSCsEVs measured by nanoflow cytometry. (K) Yield of iMSC-sEVs was evaluated in terms of particle concentration and protein concentration: the mean particle concentration was 5.62 ± 1.17 × 108 particles/mL iMSC-CM and 547.4 ± 64.6 particles/per cell; the mean protein concentration was 731.9 ± 86.2 ng/mL iMSC-CM and 9.6 ± 1.8 × 10‒7 ng/particle. All data presented as mean ± SEM (n = 6). ALP: Alkaline phosphatase; CM: conditioned medium; iMSCs: induced pluripotent stem cell-derived mesenchymal stem cells; iPSCs: induced pluripotent stem cell; sEVs: small extracellular vesicles; TEM: transmission electron microscope.
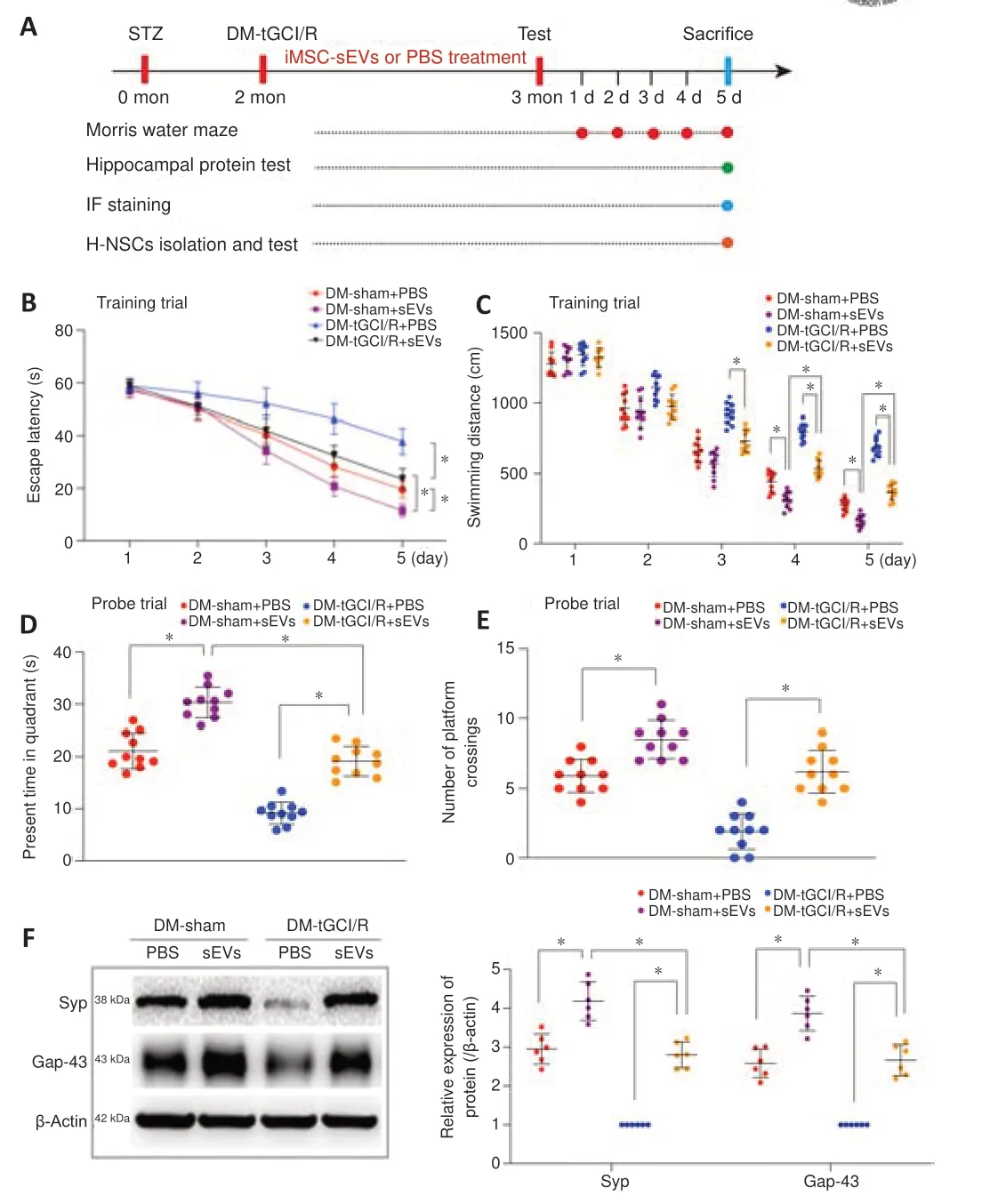
Figure 4 | iMSC-sEVs reverse cognitive impairment in DM-tGCI/R mice. (A) In vivo experiments. (B‒E) Spatial learning and memory abilities in mice were tested by Morris water maze (n = 10/group). iMSC-sEVs reversed spatial learning and memory deficits in DM mice suffering from tGCI/R injury. (F) Western blot analysis of hippocampal SYP and Gap43 in mice (n = 6/group). iMSC-sEVs increased expression levels of hippocampal SYP and Gap43 in DM mice suffering from tGCI/R injury. All data presented as mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DM: Diabetes mellitus; Gap43: growth associated protein 43; (iMSC-)sEVs: induced pluripotent stem cell-derived mesenchymal stem cells-derived small extracellular vesicles; PBS: phosphate buffer saline; SYP: synaptophysin; tGCI/R: transient global cerebral ischemia/reperfusion.
We also detected the numbers of H-NSCs (Sox2/GFAP) and newly generated immature neurons (DCX) and their proliferation statuses (EdU) in the SGZ. The application of iMSC-sEVs (DM-sham + sEVs and DM-tGCI/R + sEVs groups) significantly increased the number of Sox2/GFAPand Sox2/ EdUcells in the SGZ compared with vehicle-treated mice in the DM-sham (DMsham + PBS group) and DM-tGCI/R groups (DM-tGCI/R + PBS group), (Sox2/GFAP:P
= 0.0002 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS; Sox2/ EdU:P
= 0.0015 for DM-tGCI/R + sEVsvs.
DM-tGCI/R + PBS;Figure 5A–D
). Although the numbers of Sox2/GFAPand Sox2/EdUcells in the SGZ were lower in DM-tGCI/R + sEVs compared with DM-sham + sEVs mice, the numbers were similar to those in DM-sham + PBS mice. In addition, the numbers of total DCXand DCX/EdUcells were higher in DM-sham + sEVs and DM-tGCI/R + sEVs mice compared with DM-sham + PBS and DM-tGCI/R + PBS mice (DCX:P
= 0.0004 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS; DCX/EdU:P
= 0.0002 for DM-tGCI/R + sEVsvs.
DM-tGCI/R + PBS;Figure 5E–G
). Proliferation and neuronal-differentiation assays showed that the percentages of proliferating H-NSCs (EdU/DAPIcells) and differentiated neurons (β-III tubulincells) were significantly higher in the DM-sham + sEVs and DM-tGCI/R + sEVs groups compared with the DM-sham + PBS and DM-tGCI/R + PBS groups (EdU/DAPI:P
= 0.0001 for DM-tGCI/R + sEVsvs.
DM-tGCI/R + PBS; β-III tubulin:P
= 0.0001 for DM-tGCI/R + sEVsvs
. DM-tGCI/R + PBS;Figure 5H–K
). These results indicated that iMSC-sEVs possessed a powerful ability to promote H-NSC proliferation and neurogenesis in DM mice suffering from tGCI/R injury, resulting in cognitive recovery.iMSC-sEVs promote H-NSC proliferation and neuronal differentiation by transferring miR-21-5P and miR-486-5P
We further investigated the mechanism by which iMSC-sEVs promoted H-NSC proliferation and neuronal differentiation. Internalization assay showed that, after incubation for 12 hours, DiO-labeled iMSC-sEVs were internalized by H-NSCs isolated from DM-tGCI/R mice (Figure 6A
). We then detected the proliferation and neuronal differentiation-promoting functions of H-NSCs isolated from DM-tGCI/R micein vitro
. The percentages of proliferating H-NSCs and differentiated neurons were significantly higher in the iMSC-sEVs group compared with the PBS and non-treatment (Non) groups (EdU/DAPI:P
= 0.0001 for sEVsvs
. PBS,P
= 0.0006 for sEVsvs.
Non; β-III tubulin:P
= 0.0001 for sEVsvs
. PBS,P
= 0.0001 for sEVsvs
. Non;Figure 6B
andC
). These results further demonstrated that iMSC-sEVs promoted hippocampal neurogenesis in mice suffering from tGCI/R injury. miRNAs are key molecules in sEVs helping to modulate recipient cell function (Hu et al., 2020; Tan et al., 2020). We therefore used next-generation sequencing to identify the expression levels of miRNAs in iMSC-sEVs (Additional Table 1
). We further searched published studies to identify candidate miRNAs that promoted either NSC proliferation or neuronal differentiation or were highly enriched in iMSC-sEVs. We filtered out 234 iMSC-sEV miRNAs by miRNA sequencing and identified 69 miRNAs involved in promoting neurogenesis by a literature search (Figure 7A
andAdditional Table
2
). Among these, 49 miRNAs were coexpressed and their counts per million values are shown inFigure 7B
. The 10 miRNAs with the highest expression levels were miR-451a, miR-21-5p, miR-486-5p, miR-25-3p, miR-26a-5p, miR-92a-3p, miR-320a-3p, let-7a-5p, let-7b-5p, and miR-134-5p. The levels of these miRNAs in iMSC-sEVs were confirmed by qPCR, and the expression levels of miR-21-5p and miR-486-5p were significantly higher than those of the other miRNAs (Figure 7C
). The expression levels of miR-21-5p and miR-486-5p in H-NSCs isolated from DM-tGCI/R mice increased 11.3-fold and 7.4-fold, respectively, after incubation with iMSC-sEVs for 24 hours (Figure 7D
). Because miR-21-5p and miR-486-5p were most highly expressed in iMSCsEVs and could be transferred to DM-tGCI/R H-NSCs, we hypothesized that iMSC-sEVs promoted H-NSC proliferation and neurogenesis in DM-tGCI/R mice by transferring miR-21-5p and miR-486-5p.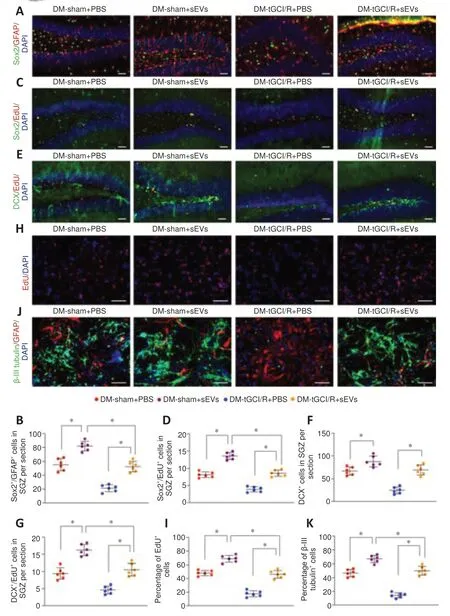
Figure 5 |iMSC-sEVs promote H-NSC proliferation and neurogenesis in the SGZ in DM-tGCI/R mice. (A) IF staining of Sox2+ (Alexa Fluor 488, green) and GFAP+ (Alexa Fluor 594, red) in the hippocampus and (B) estimated numbers of Sox2+/GFAP+ cells. iMSC-sEVs improved the number of H-NSCs in DM mice suffering from tGCI/R injury. (C) IF staining of proliferative H-NSCs (EdU+, Alexa Fluor 594, red; Sox2+, Alexa Fluor 488, green) in the hippocampus and (D) estimated numbers of EdU+/Sox2+ cells Alexa. iMSC-sEVs promoted H-NSC proliferation in DM mice suffering from tGCI/R injury. (E) IF staining of hippocampal proliferative immature neurons (EdU+, Alexa Fluor 594, red; DCX+, Alexa Fluor 488, green) and (F, G) estimated numbers. iMSC-sEVs promoted hippocampal neurogenesis in DM mice suffering from tGCI/R injury. (H) IF staining of EdU incorporation in isolated H-NSCs and (I) quantification of EdU+/DAPI+ cells among DAPI+ cells. iMSC-sEVs promoted H-NSC proliferation in H-NSCs isolated from DM mice suffering from tGCI/R injury. (J) IF staining of neuronal differentiation in isolated H-NSCs and (K) quantification of β-III tubulin+/DAPI+ cells among DAPI+ cells. iMSC-sEVs promoted H-NSC neuronal differentiation in H-NSCs isolated from DM mice suffering from tGCI/R injury. Scale bars: 50 µm in A‒C; 100 µm in D, E. All data presented as mean ± SEM (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DCX: Doublecortin; DM: diabetes mellitus; EdU: 5-ethynyl-2′-deoxyuridine; GFAP: glial fibrillary acidic protein; H-NSCs: hippocampal neural stem cells; IF: immunofluorescence; (iMSC-)sEVs: induced pluripotent stem cellderived mesenchymal stem cells-derived small extracellular vesicles; SGZ: subgranular zone; tGCI/R: transient global cerebral ischemia/reperfusion.
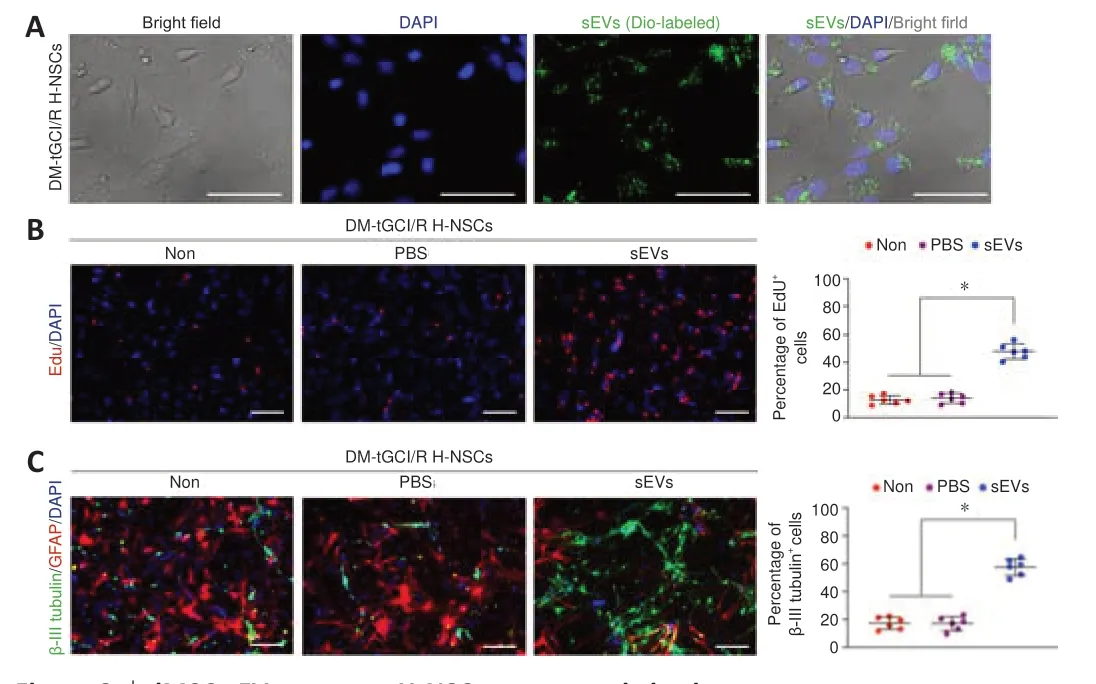
Figure 6 |iMSC-sEVs promote H-NSC neurogenesis in vitro.(A) IF staining of DiO-labeled iMSC-sEVs (Alexa Fluor 488, green) internalized by DM-tGCI/R H-NSCs. (B) IF staining of EdU incorporation in DM-tGCI/R H-NSCs and quantification of EdU+ (Alexa Fluor 594, red) /DAPI+ cells among DAPI+ cells (n = 6/group). iMSC-sEVs promoted proliferation of H-NSCs isolated from DM mice suffering from tGCI/R injury. (C) IF staining of neuronal differentiation in DM-tGCI/R H-NSCs and quantification of β-III tubulin+ (Alexa Fluor 488, green)/DAPI+ cells among DAPI+ cells (n = 6/group). iMSC-sEVs promoted neuronal differentiation of H-NSCs isolated from DM mice suffering from tGCI/R injury. Scale bars: 50 µm in A; 100 µm in B, C. All data presented as mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DM: diabetes mellitus; Edu: 5-ethynyl-2′-deoxyuridine; H-NSCs: hippocampal neural stem cells; IF: immunofluorescence; (iMSC-)sEVs: induced pluripotent stem cell-derived mesenchymal stem cells-derived small extracellular vesicles; tGCI/R: transient global cerebral ischemia/reperfusion.
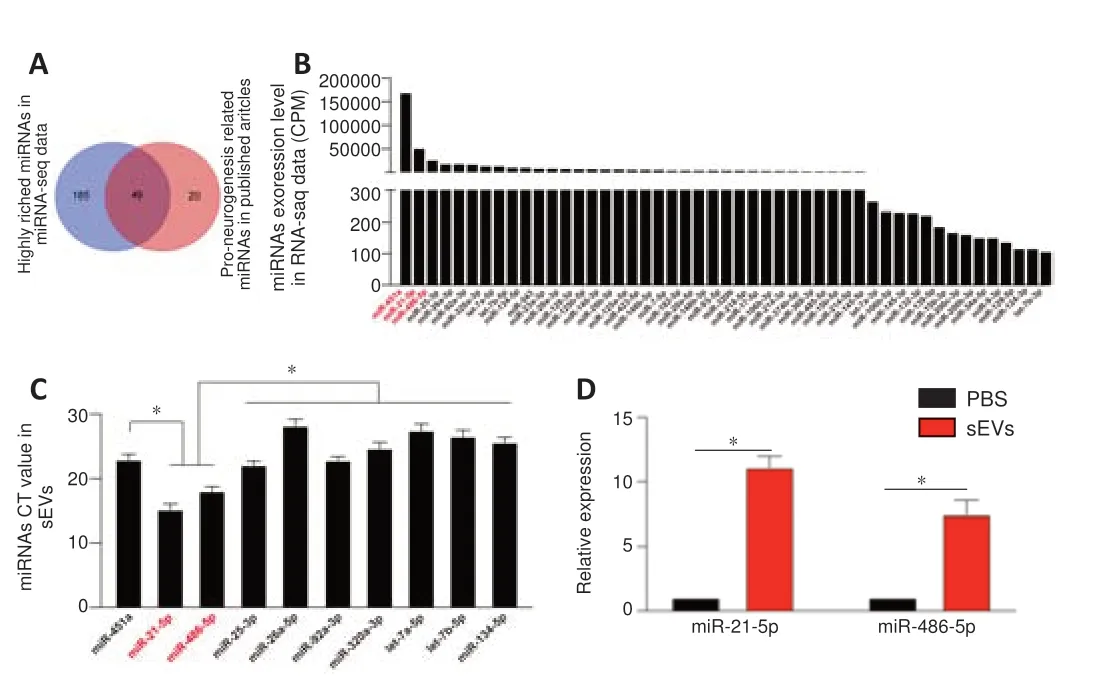
Figure 7 |miRNA expression profile in iMSC-sEVs. (A) Comparison of highly enriched miRNAs in iMSC-sEVs and neurogenesis-promotingrelated miRNAs in literature search. (B) Counts per million values of co-expressed miRNAs (n = 3). (C) Ct values of the 10 most highly expressed miRNAs were validated by qPCR (n = 3). Expression levels of miR-21-5p and miR-486-5p were significantly higher than those of the other miRNAs. (D) qPCR analysis and quantification of miR-21-5p and miR-486-5p in PBS- or iMSC-sEV-treated DM-tGCI/R H-NSCs (n = 3 per group). All data presented as mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). CPM: Counts per million; DM: diabetes mellitus; H-NSCs: hippocampal neural stem cells; (iMSC-)sEVs: induced pluripotent stem cell-derived mesenchymal stem cellsderived small extracellular vesicles; qPCR: real-time quantitative polymerase chain reaction analysis; tGCI/R: transient global cerebral ischemia/reperfusion.
We tested this hypothesis by transferring inhibitors of miR-21-5p and miR-486-5p into iMSC-sEVs to block their function. DiO-labeled iMSCsEVs overlapped with Cy3-labeled miRNA inhibitors in cells, suggesting that the miRNA inhibitors were successfully loaded into iMSC-sEVs and transferred to DM-tGCI/R H-NSCs (Figure 8A
). The proliferation- and neuronal differentiation-promoting functions of iMSC-sEVs in DM-tGCI/R H-NSCs were abolished after incubation with miRNA inhibitor-containing iMSC-sEVs (sEVs-IN group) compared with iMSC-sEVs without miRNA inhibitors (sEVs-NC group), although the percentages of proliferating H-NSCs and differentiated neurons were higher than in the PBS group (EdU/DAPI:P
= 0.0001 for NCvs
. IN,P
= 0.0039 for INvs
. PBS; β-III tubulin+:P
= 0.0001 for NCvs
. IN,P
= 0.0007 for INvs
. PBS;Figure 8B
andC
). We also injected iMSC-sEVs containing miRNA inhibitors intravenously into DM-tGCI/R mice. The escape latency and swimming distance for mice in the sEVs-IN group were longer than in sEVs-NC mice, but shorter than in mice in the PBS group (escape latency:P
= 0.0038 for NCvs
. IN,P
= 0.0001 for INvs.
PBS; swimming distance:P
= 0.0003 for NCvs
. IN,P
= 0.0001 for INvs.
PBS;Figure 8D
). The time spent in the target quadrant for mice in the sEVs-IN group was shorter than that in the sEVs-NC group, but longer than that in the PBS group (P
= 0.0007 for NCvs
. IN,P
= 0.0003 for INvs
. PBS). Mice in the sEVs-IN group had fewer platform crossings compared with the sEVs-NC group, but more than in the PBS group (P
= 0.0019 for NCvs
. IN,P
= 0.0015 for INvs
. PBS). Moreover, the numbers of Sox2/GFAPcells and DCXcells in the SGZ in sEVs-IN mice were lower than in sEVs-NC mice but higher than in the PBS group (Sox2/GFAP:P
= 0.0006 for NCvs
. IN,P
= 0.0002 for INvs
. PBS; DCX:P
= 0.0001 for NCvs
. IN,P
= 0.0001 for INvs
. PBS). These results thus indicated that iMSC-sEVs promoted DM-tGCI/R H-NSC self-renewal and neurogenesis, resulting in cognitive recovery in DMtGCI/R mice, partly via the transfer of miR-21-5p and miR-486-5p.Pan et al. (2021) demonstrated that miR-21-5p directly targeted Epha4 in NSCs to promote neurogenesis, and Yang et al. (2020) demonstrated that miR-21-5p inhibited CDKN2C to promote cell proliferation. Moreover, miR-486-5p has been demonstrated to inhibit FoxO1, which is specifically expressed in NSCs but becomes undetectable during the transition to the neuroblast stage (Kim et al., 2015). We therefore determined if iMSC-sEVs promoted hippocampal neurogenesis by transferring miR-21-5p and miR-486-5p to regulate the expression of Epha4, CDKN2C, and FoxO1 in H-NSCs. We determined the expression levels of Epha4, CDKN2C, and FoxO1 in H-NSCs from mice in the Non, PBS, and sEVs groups. Administration of sEVs significantly decreased gene and protein expression levels of Epha4, CDKN2C, and FoxO1, as shown by qPCR and western blot (Epha4:P
= 0.0001 for sEVsvs.
PBS; CDKN2C:P
= 0.0001 for sEVsvs
. PBS; FoxO1:P
= 0.0001 for sEVsvs.
PBS), respectively (Figure 9A
andB
). Expression levels of Epha4, CDKN2C, and FoxO1 were also significantly increased in H-NSCs from sEVs-IN mice compared with sEVs-NC mice (Epha4:P
= 0.0001 for NCvs
. IN; CDKN2C:P
= 0.0001 for NCvs
. IN; FoxO1:P
= 0.0001 for NCvs
. IN). However, expression levels of these proteins were lower in H-NSCs from mice in the sEVs-IN group compared with the PBS group (Epha4:P
= 0.0001 for INvs.
PBS; CDKN2C:P
= 0.0001 for INvs.
PBS; FoxO1:P
= 0.0001 for INvs
. PBS;Figure 9C
andD
). Finally, we detected the expression levels of these genes and proteins in H-NSCs isolated from DMsham + PBS, DM-sham + sEVs, DM-tGCI/R + PBS, and DM-tGCI/R + sEVs mice.Epha4
,CDKN2C
, andFoxO1
gene and protein expression levels were highest in H-NSCs from DM-tGCI/R + PBS mice, while the administration of iMSC-sEVs (DM-sham + sEVs and DM-tGCI/R + sEVs groups) significantly decreased the expression levels of these genes and proteins in H-NSCs compared with H-NSCs in the DM-sham + PBS and DM-tGCI/R + PBS groups (Epha4:P
= 0.0001 for DM-tGCI/R + PBSvs
. DM-tGCI/R + sEVs; CDKN2C:P
= 0.0001 for DM-tGCI/R + PBSvs
. DM-tGCI/R + sEVs; FoxO1:P
= 0.0001 for DM-tGCI/R + PBSvs.
DMtGCI/R + sEVs;Figure 9E
andF
). Overall, these results demonstrated that iMSC-sEVs transferred miR-21-5p and miR-486-5p to H-NSCs in DM-tGCI/R mice to inhibit the expression of Epha4, CDKN2C, and FoxO1, leading to H-NSC self-renewal and enhanced neurogenesis, resulting in cognitive recovery.
Figure 8 |iMSC-sEVs transfer miR-21-5P and miR-486-5P to promote H-NSC proliferation and neuronal differentiation. (A) IF staining of DiO-labeled iMSC-sEVs (green) overlapped with Cy3-labeled miRNA inhibitors (red) internalized by H-NSCs isolated from DM-tGCI/R mice (n = 3). miRNA inhibitors were successfully loaded into iMSC-sEVs and transferred to DM-tGCI/R H-NSCs. (B) IF staining of EdU incorporation into isolated H-NSCs and quantification of EdU+/DAPI+ cells among DAPI+ cells (n = 6/group). The proliferation-promoting function of iMSC-sEVs on DM-tGCI/R H-NSCs was abolished when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. (C) IF staining of neuronal differentiation in isolated H-NSCs and quantification of β-III tubulin+ (Alexa Fluor 488, green)/DAPI+ cells among DAPI+ cells (n = 6/group). The neuronal differentiation-promoting function of iMSC-sEVs on DM-tGCI/R H-NSCs was abolished when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. (D) Spatial learning and memory abilities in DM-tGCI/R mice were tested by Morris water maze (n = 10/group). Spatial learning and memory abilities were damaged when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. (E) IF staining of Sox2+ (Alexa Fluor 488, green) and GFAP+ (Alexa Fluor 594, red) in the hippocampus and estimated number sof Sox2+/GFAP+ cells (n = 6/group). The proliferation-promoting function of iMSC-sEVs on DM-tGCI/R H-NSCs was abolished when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. (F) IF staining of hippocampal proliferative immature neurons (DCX+, Alexa Fluor 488, green) and estimated numbers (n = 6/group). The neuronal differentiationpromoting function of iMSC-sEVs on DM-tGCI/R H-NSCs was abolished when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. Scale bars: 50 µm in A, E, F; 100 µm in B, C. All data presented as mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). DCX: Doublecortin; Dio: 3,3′-dioctadecyloxacarbocyanine perchlorate; DM: diabetes mellitus; Edu: 5-ethynyl-2′-deoxyuridine; GFAP: glial fibrillary acidic protein; H-NSCs: hippocampal neural stem cells; IF: immunofluorescence; (iMSC-)sEVs: induced pluripotent stem cell-derived mesenchymal stem cells-derived small extracellular vesicles; IN: inhibitor; miRNA: microRNA; NC: negative control; tGCI/R: transient global cerebral ischemia/reperfusion.

Figure 9 |iMSC-sEVs transfer miRNAs to inhibit Epha4, CDKN2C, and FoxO1 to promote hippocampal neurogenesis in DM-tGCI/R mice. (A) qPCR and (B) western blot analyses of Epha4, CDKN2C, and FoxO1 in DM-tGCI/R H-NSCs. Administration of sEVs significantly decreased gene and protein expression levels of Epha4, CDKN2C, and FoxO1. (C) qPCR and (D) western blot analyses of Epha4, CDKN2C, and FoxO1 in DM-tGCI/R H-NSCs from PBS, sEVs-NC, and sEVs-IN groups. The inhibitory effects of iMSC-sEVs on Epha4, CDKN2C, and FoxO1 in DM-tGCI/R H-NSCs were largely abolished when miR-21-5p and miR-486-5p were inhibited in iMSC-sEVs. (E) qPCR and (F) western blot analyses of Epha4, CDKN2C, and FoxO1 in H-NSCs isolated from DM-tGCI/R mice. Administration of sEVs significantly decreased gene and protein expression levels of Epha4, CDKN2C, and FoxO1 in H-NSCs isolated from DM-tGCI/R mice. All data presented as mean ± SEM (n = 6/group). *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). CDKN2C: Cyclin-dependent kinase inhibitor 2C; DM: diabetes mellitus; Epha4: Eph receptor A4, FoxO1: forkhead box O1; H-NSCs: hippocampal neural stem cells; IF: immunofluorescence; (iMSC-)sEVs: induced pluripotent stem cell-derived mesenchymal stem cells-derived small extracellular vesicles; IN: inhibitor; miRNA: microRNA; NC: negative control; PBS: phosphate buffer saline; qPCR: real-time quantitative polymerase chain reaction analysis; tGCI/R: transient global cerebral ischemia/reperfusion.
Discussion
POCD is one of the most common complications in surgical patients. DM further increases the risk and severity of cognitive dysfunction following surgery, which can in turn seriously impair patient quality of life and utilize social resources. However, no effective medications have yet been developed to prevent or treat DM-POCD. In this study, we found obvious cognitive impairment in DM mice suffering from tGCI/R injury, as well as significant H-NSC loss and reduced neurogenesis. Treatment with iMSC-sEVs effectively promoted H-NSC proliferation and neurogenesis and reversed cognitive impairment in DM mice suffering from tGCI/R injury. Furthermore, iMSCsEVs exerted neurogenesis-promoting effects by transferring highly enriched miRNAs, including miR-21-5p and miR-486-5p, to inhibit Epha4, CDKN2C, and FoxO1. To the best of our knowledge, these data provide the first evidence showing that iMSC-sEVs may promote hippocampal neurogenesis to restore cognitive function in DM-POCD.
POCD causes deficits in attention, concentration, executive function, and memory, and can develop over a period of weeks, months, or even years (Monk and Price, 2011; Steinmetz and Rasmussen, 2016). Neuroinflammation, immune activation, and oxidative stress contribute to disruptions in synaptic plasticity and glutamate signaling and are regarded as crucial mechanisms underpinning the development of cognitive dysfunction following surgery (Cibelli et al., 2010; Terrando et al., 2010). DM is associated with decrements in cognitive function and changes in brain structure; for example, individuals with DM have demonstrated mild to moderate reductions in cognitive function, and type 2 DM has also been associated with a 50% increased risk of dementia (Moheet et al., 2015). Lower total brain volume, more infarcts, greater white matter hyperintensity volume, and lower gray matter volume may substantially mediate the association between DM and cognitive dysfunction (Callisaya et al., 2019). Multiple studies have demonstrated that individuals with DM appear to be at increased risk of developing cognitive dysfunction after surgery (Thourani et al., 1999; Kadoi et al., 2005). The increasing prevalence of DM (Saeedi et al., 2019) and associated rise in the number of diabetic surgical patients has thus led to an increase in DMPOCD (Daiello et al., 2019). In this study, we applied the transient bilateral common carotid artery occlusion surgery in DM mice to imitate DM-POCD in human situation (Jaspers et al., 1990), because it can induce 50% reduction of cerebral blood flow and produce persistent cognitive function impairment, which was similar to the underlying mechanism of POCD (van Harten et al., 2012; Hovens et al., 2016). We found that POCD was more severe in DM mice compared with normal mice, suggesting that DM increased the severity as well as the prevalence of POCD. There is thus a need to explore effective new strategies to prevent and treat DM-POCD. Potential neuroprotective agents, including antioxidants, antiinflammatory agents, and modifiers of glutamate signaling, have shown preclinical promise for POCD (Skvarc et al., 2018). For example, N-acetylcysteine exerted protective effects against cognitive dysfunction via its anti-inflammatory activity (Skvarc et al., 2016). However, most potential therapies for POCD have encountered problems in terms of their clinical applications, and their efficacies for DM-POCD remain unclear. The development of other potential therapies for DM-POCD should thus be a priority.
Hippocampal neurogenesis plays a crucial role in maintaining and restoring hippocampus-dependent functions. Decreased hippocampal neurogenesis seriously impairs cognitive function, including age-related cognitive decline and neurodegenerative disorders. Restoring H-NSCs and promoting their neurogenesis were shown to improve damaged hippocampal structure and promote functional repair, partly by increasing the production of functional granule neurons and increasing their integration into existing hippocampal circuits (van Praag et al., 2002; Skvarc et al., 2016; Toda and Gage, 2018; Sun et al., 2021). The greater depletion of H-NSCs and reduction of hippocampal neurogenesis in DM compared with normal mice were in accordance with the observed cognitive decline, indicating that reduced neurogenesis in the hippocampus is an important mechanism for cognitive decline in DM-POCD, and that promoting hippocampal neurogenesis may in turn be a promising therapeutic strategy for DM-POCD.
iPSCs are reprogrammed cells with features similar to embryonic stem cells that can be propagated indefinitely in the primitive undifferentiated state and can also differentiate into different tissues or cell types (Takahashi and Yamanaka, 2006). MSCs have been widely studied in relation to stem cell therapy, mainly because of their high self-renewal capacity, high plasticity, low immunogenicity, and effective therapeutic function (Mundra et al., 2013). Specifically, iMSCs are promising agents for stem cell therapy because they can be continuously differentiated from patient iPSCs, thus providing an adequate cell source and avoiding immune rejection, and because they exhibit greater proliferative capacity than primary cultures of adult MSCs (Diederichs and Tuan, 2014; Hu et al., 2015). Recent accumulating evidence has shown that sEVs secreted by stem cells are an important component of their therapeutic action (Hao et al., 2017). For example, MSC-sEVs have shown attractive therapeutic potential in liver, heart, kidney, bone, brain, and spinal cord diseases and in cancer (Zhao et al., 2019; Zhou et al., 2022). Embryonic stem cell-derived sEVs exhibited powerful regeneration-promoting functions in brain damage (Hu et al., 2020, 2021) and skin injury (Chen et al., 2019), and iMSC-sEVs demonstrated beneficial effects in promoting angiogenesis (Hu et al., 2015), skin cell proliferation (Kim et al., 2018), and bone regeneration (Zhu et al., 2017). Exogenous sEVs have also been shown to cross the blood-brain barrier and target brain cells (Wang et al., 2020; Heidarzadeh et al., 2021). For example, intravenously injected RVG-targeted sEVs (containing GAPDH small interfering RNA) were shown to transfer to neurons in the brain to knockdown specific genes, without affecting other tissues (Alvarez-Erviti et al., 2011). We therefore considered that intravenously injected iMSC-sEVs could be taken up by H-NSCs, and might exhibit powerful neurogenesis-promoting and cognitive-recovery functions in diabetic POCD. The current results showed that chronic delivery of iMSC-sEVs could recover the compromised self-renewal and neurogenesis capacities of H-NSCs in DM-POCD, resulting in cognitive recovery. These results indicated that the application of iMSC-sEVs may be a promising strategy for curing DM-POCD.
sEVs act as a delivery system partly by transferring miRNAs to recipient cells to alter their gene expression and bioactivity (Lara-Barba et al., 2021). miRNAs are also important in regulating NSC (Shi et al., 2010) and cognitive functions (Swarbrick et al., 2019). We therefore detected the variety and expression levels of miRNAs in iMSC-sEVs and found that miR-21-5p and miR-486-5p were highly expressed in iMSC-sEVs and were also involved in neurogenesis regulation (Dori et al., 2020; Pan et al., 2021). Furthermore, when these two miRNAs were blocked by their inhibitors, iMSC-sEVs largely failed to promote H-NSC proliferation and neurogenesis, as well as cognitive recovery, in DM mice suffering from POCD. These findings suggested that miR-21-5p and miR-486-5p acted as crucial mediators in iMSC-sEVs to promote hippocampal neurogenesis in DM-POCD. miR-21-5p can directly target and inhibit EphA4 to promote NSC differentiation into neurons (Pan et al., 2021) and can also inhibit CDKN2C to promote cell proliferation (Yang et al., 2020), while miR-486-5p has been demonstrated to inhibit FoxO1, which is specifically expressed in NSCs and becomes undetectable during transition to the neuroblast stage (Kim et al., 2015). We therefore also detected the expression levels of these proteins in H-NSCs and found downregulation of EphA4, CDKN2C, and FoxO1 in iMSC-sEV-treated DM-POCD H-NSCs, while the inhibitory effects of iMSC-sEVs on these proteins failed when miR-21-5p and miR-486-5p were blocked in iMSC-sEVs. These results indicated that iMSC-sEVs transferred miR-21-5p and miR-486-5p to inhibit EphA4, CDKN2C, and FoxO1 in DM-POCD H-NSCs to promote their proliferation and neuronal differentiation. Notably however, the effects of iMSC-sEVs on EphA4, CDKN2C, and FoxO1 inhibition and on H-NSC proliferation and neurogenesis were not entirely abolished by blocking miR-21-5p and miR-486-5p in iMSC-sEVs, suggesting that additional miRNAs may also be involved in these processes. miRNAs such as miR-93 (Chen et al., 2016), miR-22-3p (Kong et al., 2021), and miR-27a (Wang et al., 2018) also inhibited EphA4, CDKN2C, and FoxO1, respectively (Additional Table 1
) and were expressed in iMSC-sEVs, but at low levels; however, these miRNAs may also promote hippocampal neurogenesis in DM mice suffering from POCD.Our research also had certain limitations. First, we did not explore cognitive function and hippocampal neurogenesis or the therapeutic effect of iMSCsEVs in female DM-POCD mice. In addition, the experimental period of DMPOCD in this study was 1 month, but given that POCD-related changes in cognitive function may occur over a long period, further studies are needed to extend the experimental period to verify our results. Finally, some results need to be confirmed using other experimental indexes; for example, we defined H-NSCs as Sox2/GFAPco-expressing cells, but H-NSCs may also be represented by Sox2/Nestinco-expression. There are no significant sex differences in the incidences of DM and postoperative cognitive impairment; however, hormonal changes in female mice during the physiological cycle may affect brain function, and male mice generally have better physical health indicators than female mice, and we therefore selected male mice for this experiment.
In summary, our results demonstrated that DM mice subjected to tGCI/R showed serious depletion of H-NSCs and reduced neurogenesis, ultimately resulting in cognitive dysfunction. We showed that iMSC-sEVs can promote cognitive recovery in DM-POCD, partly by transferring highly enriched miRNAs, miR-21-5p and miR-486-5p, to inhibit EphA4, CDKN2C, and FoxO1 in H-NSCs, leading to increased proliferation and neurogenesis in the hippocampus. The application of iMSC-sEVs may thus provide a novel cell-free therapeutic tool for diabetic patients with POCD.
Author contributions:
Study conception and design and animal study: YZZ, RJX; H-NSCs culture: JS, YC; data acquisition and analysis: JS, YC, HLL; manuscript draft: HLL; manuscript revision: GWH, GHX. All authors read and agreed to the final manuscript of the manuscript.
Conflicts of interest:
The authors declare that there are no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Marimélia A. Porcionatto, Universidade Federal de São Paulo, Brazil; Yvonne Couch, John Radcliffe Hospital, UK.
Additional files:
Expression level of microRNAs in extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells.
microRNAs related to pro-neurogenesis in literature search (PubMed database).
Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Inflammation and retinal degenerative diseases
- Synaptic alterations as a common phase in neurological and neurodevelopmental diseases: JNK is a key mediator in synaptic changes
- Brain-derived neurotrophic factor in main neurodegenerative diseases
- The best of both worlds: mastering nerve regeneration combining biological and nanotechnological tools
- Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage
- Chlorogenic acid alleviates hypoxic-ischemic brain injury in neonatal mice

