NH2-MIL-125 (Ti) Derived Flower-Like Fine TiO2 Nanoparticles Implanted in N-doped Porous Carbon as an Anode with High Activity and Long Cycle Life for Lithium-Ion Batteries
Yue Yang , Jiawei Zhu , Pengyan Wang , Haimi Liu , Weihao Zeng , Lei Chen , Zhixiang Chen ,Shichun Mu ,*
1 State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology,Wuhan 430070, China.
2 Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory, Xianhu Hydrogen Valley,Foshan 528200, Guangdong Province, China.
Abstract: Owing to their advantages such as safe operation, high power density, long cycle life, and low self-discharge rate, lithium-ion batteries (LIBs)have attracted attention for applications ranging from portable electronics to electric vehicles (EVs)/hybrid EVs (HEVs). However, the striking exothermic reaction and growth of lithium dendrites during lithiation-delithiation cycles for commercial graphite anodes are hidden safety risks associated with LIBs.Titanium dioxide (TiO2) is considered as an important material for LIBs because of its high safety and excellent cycling stability. In addition, TiO2 anode used in lithium-ion storage system has a relatively high voltage (~1.5 V vs. Li/Li+), and thus, it meets the strict safety standards of commercial LIBs. However, the unsatisfactory conductivity and ion diffusion rate prevent the further application of TiO2 in LIBs. To date, the combination of graphene, carbon nanotubes (CNTs), carbon quantum dots (QDs) and porous carbon with TiO2 has attracted significant research attention.Nevertheless, it is still challenging to introduce a unique nanostructure design by organically compounding TiO2 with N-doped porous carbon matrix. Herein, N-doped porous carbon incorporating fine TiO2 nanoparticles (NPs) with a flower-like structure (denoted as FL-TiO2/NPC) is successfully prepared using flower-like NH2-MIL-125(Ti) as the hard template. The as-prepared Ti-based framework shows a flower-like structure, which is assembled with two-dimensional (2D) corrugated porous nanosheets. On the one hand, the corrugated carbon nanosheets incorporating fine TiO2 particles can offer a magnifying contact area between electrode matrix and electrolyte. On the other hand, the N-doped porous carbon plays a crucial role in improving the conductivity and structural integrity of the whole matrix. Therefore, the as-prepared FLTiO2/NPC can deliver an excellent reversible lithium storage capacity of 384.2 mAh·g-1 at the current density of 0.5 A·g-1 after 300 cycles and 279.1 mAh·g-1 at 1 A·g-1 after 500 cycles. Furthermore, even when tested at 2 A·g-1, FL-TiO2/NPC can deliver a reversible capacity of 256.5 mAh·g-1 with a coulombic efficiency of 100% after 2000 cycles. The superior electrochemical performance and the structural toughness of LIBs originate from the unique flower-like structure. We believe that the proposed synthesis strategy will provide a new idea for the preparation of metal oxides/N-doped porous carbon composites with high lithium storage performance.
Key Words: TiO2 nanoparticles; N-doped porous carbon nanosheet; Flower-like structure; NH2-MIL-125 (Ti);Anode; Lithium-ion battery
1 Introduction
As non-renewable resources are gradually consumed, trying to find low cost and clean energy conversion is more and more urgent and important. Lithium-ion batteries (LIBs) are important components in applications from portable electronics to electric vehicles (EVs)/hybrid EVs (HEVs)1-6. TiO2has entered the field of vision of researchers due to its abundant reserves on the earth,good safety and environmental friendliness. Moreover, TiO2has relatively stable chemical properties, and its morphology is easy to regulate7,8. However, the application of TiO2still confronts fast capacity decay and sluggish reaction kinetics associated to their inherent poor electrical conductivity (~10-12S·cm-1) and low ion diffusion coefficients during lithiation-delithiation cycles9-12. Various strategies are applied to modify the structure of TiO2or TiO2/C-based composite materials to improve the electrochemical performance of LIBs and Sodium-ion Batteries(SIBs). For example, self-assembled nanocrystalline-rutile/anatase TiO2with graphene hybrid nanostructures13, TiO2hollow nanostructures14, hierarchical anatase TiO2spheres constructed with exposed (001) facet ultrathin nanosheets15mesoporous anatase TiO2beads16nanometer-sized rutile TiO217TiO2-B nanowires18mesoporous TiO2nanocrystals with graphene aerogels composites19and so on, the above mentioned works have achieved superior electrochemical performance of LIBs.
On the other hand, because of their various morphologies and structures, different kinds of metal cations, Metal-organic frameworks (MOFs) are widely used in the fields of heterogeneous catalysis20, overall water splitting21,22, gas storage and separation23, solar fuels24and so on. Recently, a lot of MOF derivatives with unique structure are used as the anode material for LIBs or SIBs. For instance, the spindle-like porous α-Fe2O325, porous hollow ZnFe2O4/ZnO/Carbon layer octahedral26, MOF-derived core-shell ZnO@ZnO quantum dots(QDs)/C27, porous CuO hollow octahedral28, ultrafine CoS2NPs/N-doped porous carbon29, porous hollow Co9S8NPs/graphitic carbon nanocage composites30, ZnO and Fe2O3coated with three-dimensional graphene31, mesoporous nanostructured Co3O432-46etc. Although the above works are full of novelty and have made some breakthroughs in the field of LIBs and SIBs.However, the design and synthesis of new structure MOFs are still faced with great challenges.
Take the above-mentioned difficulties and needs into mind.Herein, N-doped porous carbon incorporating fine TiO2particles(FL-TiO2/NPC) with flower-like structure is successfully prepared by using NH2-MIL-125(Ti) as a hard template. In NH2-MIL-125(Ti) crystals, metal and oxygen atoms are arranged at the periodical atom level. Therefore, the Ti-MOF can be converted into N-doped porous carbon incorporating fine TiO2nanoparticles without migrating long-range atomic. Most importantly, our synthesis method can provide new ideas and routes for the preparation of other metal-organic framework with special morphologies (such as Fe, Co, Ni-MOFs).
2 Experiment section
2.1 Chemicals
Titanium tetraisopropanolate (C12H28O4Ti, Aladdin, China,95%, CAS:546-68-9) and 2-aminoterephthalic acid (C8H7NO4,Aladdin, China, 99%, CAS:10312-55-7) were purchased from Aladdin Industrial Corporation. The resistivity of DI H2O used in this work was 18.25 MΩ·cm-1.
2.2 Synthesis of flower-like NH2-MIL-125 (Ti)
Typically, 0.28 g of 2-aminoterephthalic acid was dissolved in DMF (20 mL) and ethanol (20 mL) mixed solvent, sonicated for 30 min. When 2-aminoterephthalic acid was completely dissolved into mixed solvent, TTIP (0.3 mL) was added to the above solution and sonicated for 5 min. Then the mixture was transferred into a 100 mL Teflon-lined steel autoclave and placed in an oven at 150 °C for 24 h under static conditions. The product was collected by repeated centrifugation (5000 r·min-1, 5 min)and washed with DMF and ethanol for 3 times respectively, then dried in an oven under vacuum at 60 °C overnight.
2.3 Synthesis of FL-TiO2/NPC and P-TiO2
The as-prepared NH2-MIL-125 (Ti) was heated with a rate of 2 °C·min-1by heating up and maintaining at 600 °C for 3 h in a tube furnace under Ar atmosphere. The prepared composite was named as FL-TiO2/NPC. When other calcination conditions remained the same, Ar is replaced by air, and the obtained product was recorded as pure TiO2(P-TiO2).
3 Results and discussion
3.1 Synthesis and structure of FL-TiO2/NPC and Pure TiO2 (P-TiO2)
Fig. 1 illustrates the detailed synthesis route of FL-TiO2/NPC.Firstly, flower-like NH2-MIL-125 (Ti) was synthesized by using tetraisopropyl titanate (TTIP) and 2-aminoterephithalic acid(NH2-PTA) as titanium source and organic linker. After being hydrothermal at 150 °C for 24 h and calcined at 600 °C in argon atmosphere, the organic linker will be pyrolyzed to N-doped porous carbon nanosheets. As a result, the fine TiO2NPs embedded in N-doped porous carbon matrix with a flower-like structure. Other synthesis conditions remain the same, and the calcined atmosphere is changed from argon to air to pure TiO2(denoted as P-TiO2).
The morphologies of flower-like NH2-MIL-125 (Ti), FLTiO2/NPC and P-TiO2were characterized by scanning electron microscope (SEM). As shown in Fig. 2a, flower-like NH2-MIL-125 (Ti) show a smooth leaf and flower-like shape, and there was no reunion or collapse. After calcination in inert gas atmosphere,the synthesized FL-TiO2/NPC can perfectly inherit flower-like shape of the NH2-MIL-125 (Ti). (Fig. S1c, d) As shown in Fig.2b, compared with NH2-MIL-125 (Ti), the overall appearance of FL-TiO2/NPC shows more roughness and wrinkles. The thickness of the N-doped porous carbon nanosheets is further verified by atomic force microscopy (AFM) analysis (Fig. 2e).The characterization results show that the thickness is only about 1.5 nm, which is also in good agreement with the characterization results of SEM and TEM. On the contrary, if NH2-MIL-125 (Ti) is calcined directly in the air atmosphere,most of the TiO2particles will be reunited together (Fig. 2c).These agglomerated TiO2particles will form blocks and have no regular morphology. Low magnification SEM images of FLTiO2/NPC (Fig. S2a, b) and P-TiO2(Fig. S2c, d) are showed in Supporting Information. There is no doubt that the carbon matrix plays an important role in the maintenance of the whole flowerlike structure in the process of calcination. In the process of calcination in the air atmosphere, the carbon matrix will be burned gradually, which leads to the collapse and agglomeration of the whole structure due to severe shrinkage.
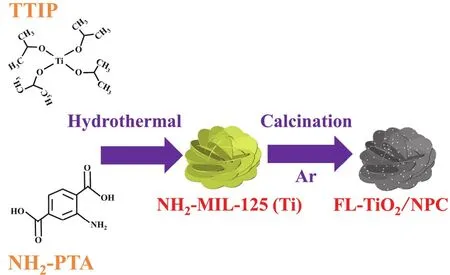
Fig. 1 Schematic exhibiting the synthetic process of flower-like TiO2/N-doped porous carbon nanosheets (FL-TiO2/NPC).
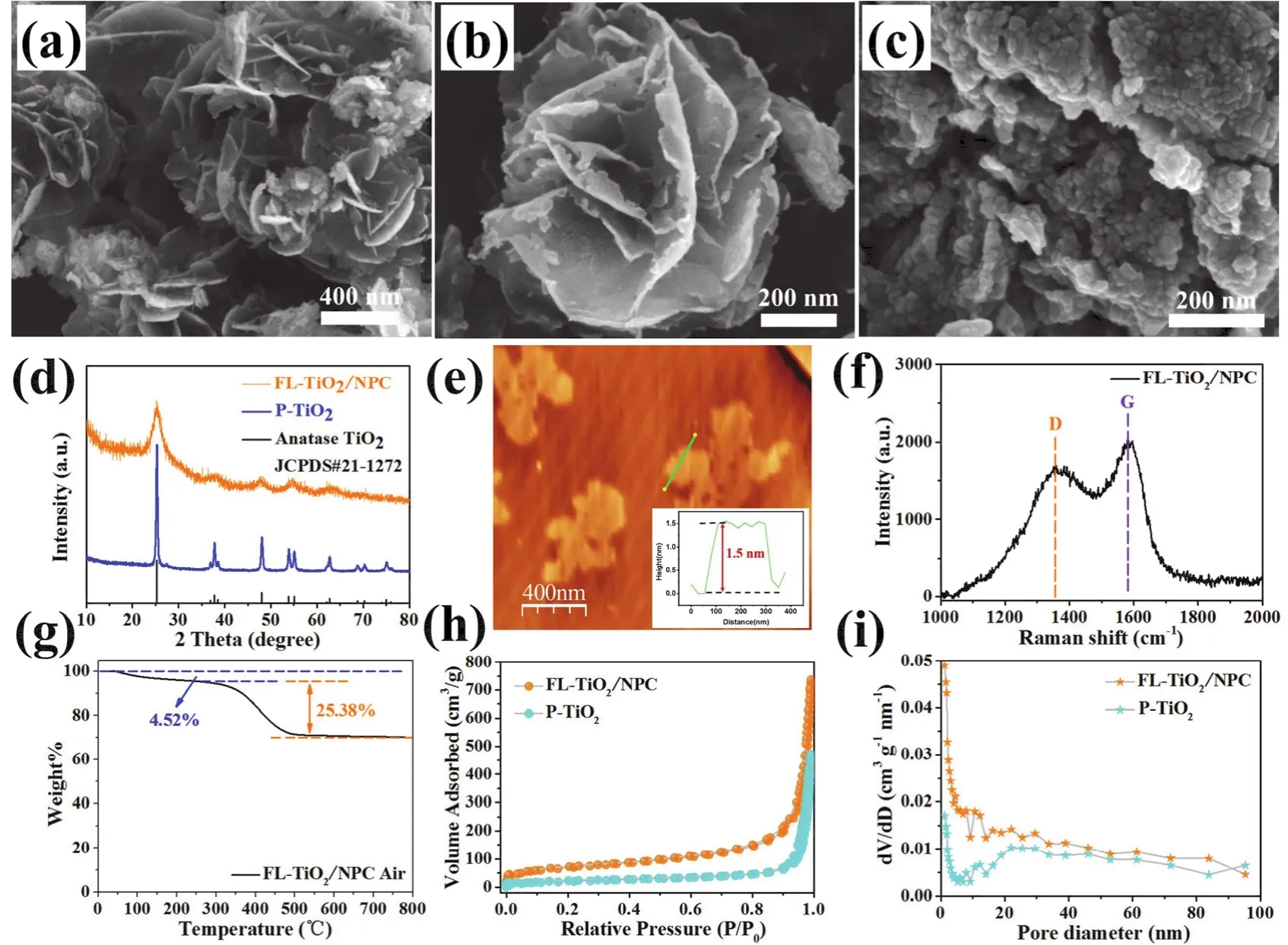
Fig. 2 SEM images of NH2-MIL-125 (Ti) (a), FL-TiO2/NPC (b), P-TiO2 (c). XRD patterns (d) of FL-TiO2/NPC and P-TiO2. AFM image of FL-TiO2/NPC and the height profile of the corresponding line (e). Raman spectra (f) and TGA curve (g) of FL-TiO2/NPC.N2 adsorption/desorption isotherms (h) and pore size distribution curves (i) of FL-TiO2/NPC and P-TiO2.
As shown in Fig. 2d, the XRD patterns of FL-TiO2/NPC and P-TiO2are well consistent with the standard card of anatase TiO2(JCPDS card NO. 21-1272). Moreover, the N-doped porous carbon nanosheets can suppress the growth of the TiO2nanoparticles, FL-TiO2/NPC has weaker and wider peak shapes,which is in accordance with previous reports47. There are no characteristic diffraction peaks of carbon for the FL-TiO2/NPC,which is probably because carbon is amorphous and the peak intensity is weak. Moreover, the XRD pattern and FTIR spectrum of NH2-MIL-125 (Ti) are also exhibited in Fig. S1a and Fig. S1b. The Raman spectra of FL-TiO2/NPC is exhibited as Fig. 2f, the two peaks of FL-TiO2/NPC are indexed as D band(~1358.6 cm-1) and G band (~1581.8 cm-1), in addition, the calculated ID/IGratio is 0.84, suggesting that a partially graphitic structure existing in the porous carbon nanosheets of FLTiO2/NPC. Thermogravimetric analysis is used to detect the carbon content in FL-TiO2/NPC. As shown in Fig. 2g, a mass weight loss of 4.52% below 250 °C is caused by the absorbed water of composites. When it continues to be calcined from 250 to 800 °C in the air, the mass weight loss of 25.38% can be thought of as the percentage of carbon in the whole sample.
The N2adsorption/desorption isotherms were performed to measure the SSA and pore size of FL-TiO2/NPC and P-TiO2. As shown in Fig. 2h, i, The SSA of FL-TiO2/NPC (238.3 m2·g-1) is a little more than three times larger than that of P-TiO2(71.2 m2·g-1). The N2adsorption/desorption isotherms of FLTiO2/NPC and P-TiO2can be identified as Type IV isotherms with a typically mesoporous hysteresis loop. Besides, the pore sizes distribution of FL-TiO2/NPC and P-TiO2are all in the range of micropores, mesopores and macropores. We can find the answer to explain this result from the SEM images of FLTiO2/NPC and P-TiO2. Almost all TiO2particles agglomerate in large scale to form blocks for P-TiO2, which will reduce the specific surface area of the whole material and produce more stacked pore structures.
To achieve further insight into the distinctive shape of FLTiO2/NPC, transmission electron microscopy (TEM) is used. It can be seen very clearly from Fig. 3a that the individual FLTiO2/NPC morphology is assembled from the ultrathin 2D porous carbon nanosheets. This observation is in good agreement with the SEM images of FL-TiO2/NPC. The higher magnification TEM image (Fig. 3b) of the edge of FL-TiO2/NPC can be seen that a large number of fine TiO2NPs with a diameter less than 10 nm are uniformly dispersed in the porous carbon nanosheets. The corresponding selected-area diffraction patterns image is shown as Fig. 3c, the concentric rings corresponding to the (101), (103), and (200) planes of the anatase TiO2phase.Moreover, the high-resolution TEM (HRTEM) image (Fig. 3d)reveals the clear crystalline lattice fringes with d-spacing values of 0.353 nm, corresponding to the (101) planes of anatase TiO2.In order to further explore the composition of various elements of FL-TiO2/NPC, HAADF-STEM and EDX analysis were carried out. As shown in Fig. 3e-i, the homogeneous distribution of C (f), N (g), O (h), and Ti (i) of FL-TiO2/NPC can be clearly distinguished. The EDX results further proved the existence and structural dispersion of TiO2and N-doped carbon. Whether it is ultrathin N-doped porous nanosheets structure, or fine uniformly dispersed TiO2particles. This unique structure ensures that the electrolyte can fully contact the active sites and the effective transport of lithium ions/electrons during the charge and discharge of LIBs. More importantly, carbon matrix materials can not only ensure the integrity of the overall structure, but also effectively restrain volume expansion in the process of charge and discharge, providing reliable cycle stability for electrode materials.
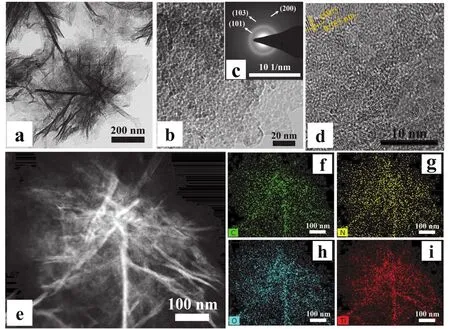
Fig. 3 TEM image (a) and HRTEM images (b, d) of FL-TiO2/NPC. The corresponding SAED pattern (c) and EDS mapping images of FL-TiO2/NPC (h) with C (green), N (yellow), O (cyan) and Ti (red) elements from a selected area.
The element chemical states of FL-TiO2/NPC was further investigated by XPS. As shown in Fig. S3, the C, N, O, and Ti elements of FL-TiO2/NPC are verified. The full XPS spectrum of P-TiO2is also shown as Fig. S4. The high-resolution XPS spectrum of Ti (Fig. 4a) represents the peaks at 458.7 and 464.4 eV, which are in accordance with Ti 2p3/2and Ti 2p1/2,respectively. The overlapped high-resolution C 1s peak was fitted by three components centered at 284.7, 285.4, and 288.7 eV, which corresponding to the C―C or C=C, C―N and C=O bonds, respectively (Fig. 4c). In the highly deconvoluted O 1s spectrum of FL-TiO2/NPC (Fig. 4b), the three fitted peaks at binding energies of 529.7, 530.3, and 531.6 eV are assigned to the bonds of Ti―O, C―O, and Ti―O―H bond, respectively48.As to the high-resolution N 1s XPS spectra (Fig. 4d), the three peaks located at around 398.5, 400.3, and 401.5 eV could be assigned to the Pyridinic-N, Pyrrolic-N, and Quaternary-N bonds, respectively49. The above XPS results further prove the existence of N-doped carbon and TiO2.
3.2 Electrochemical performance of FL-TiO2/NPC and P-TiO2 electrodes for LIBs
As shown in Fig. 5a, the cyclic voltammograms (CV) curves of FL-TiO2/NPC and P-TiO2(Fig. S4a) were measured during the initial three cycles in the voltage range of 0.1-3.0 V at a scan rate of 0.1 mV·s-1. For the initial CV cycle, a distinct reduction peak at 1.72 V and an oxidation peak at 2.03 V can be found, this pair of oxidation/reduction peaks in the first cycle are related to lithiation-delithiation for anatase TiO2. Moreover, in the initial discharging process, another pair of small reduction humps at around 1.47 and 0.75 V can be attributed to the pseudocapacitive Li+storage and the formation of solid electrolyte interphase(SEI) film, respectively50. In the following two cycles, the major reduction peak at 1.75 V and the oxidation peak at 2.03 V remain unchanged, indicating well cycling stability of FL-TiO2/NPC anode composites. The EIS spectra of FL-TiO2/NPC and P-TiO2were measured before cycling. Fig. 5b shows the Nyquist curves of FL-TiO2/NPC and P-TiO2. After fitting, the Rctof FLTiO2/NPC and P-TiO2composites are 29.7 and 701.7 Ω. The figure in Fig. 5b corresponds to the enlarged Nyquist curve diagram of FL-TiO2/NPC. The AC impedance results of FLTiO2/NPC and P-TiO2further prove that the introduction of N-doped porous carbon nanosheets can greatly enhance the conductivity of the whole complex, which is undoubtedly conducive to the transport of lithium ions and electrons in the process of lithiation-delithiation.
Fig. 5c shows the first five GDC curves of FL-TiO2/NPC at 0.2 A·g-1. In accordance with the CV curves, a small platform appeared around 1.7 V during first discharge and a small voltage platform emerged around 2.0 V during first charge, respectively.The first discharge and charge capacity of 773.5/468.1 and 373.1/179.7 mAh·g-1is delivered by FL-TiO2/NPC and P-TiO2electrodes (Fig. S4b), with the coulombic efficiency of 60.52%and 48.16%. The initial irreversible capacity loss which is usually associated with the side reactions with the electrolyte and formation of SEI films.
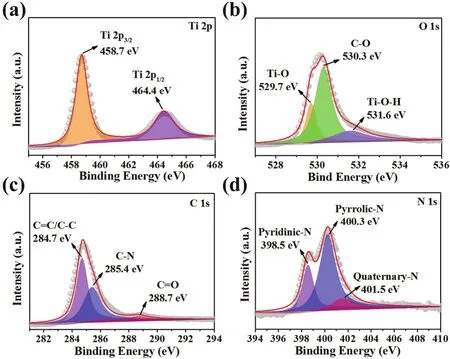
Fig. 4 High-resolution XPS spectra of Ti 2p (a), O 1s (b), C 1s (c), and N 1s (d) from FL-TiO2/NPC.
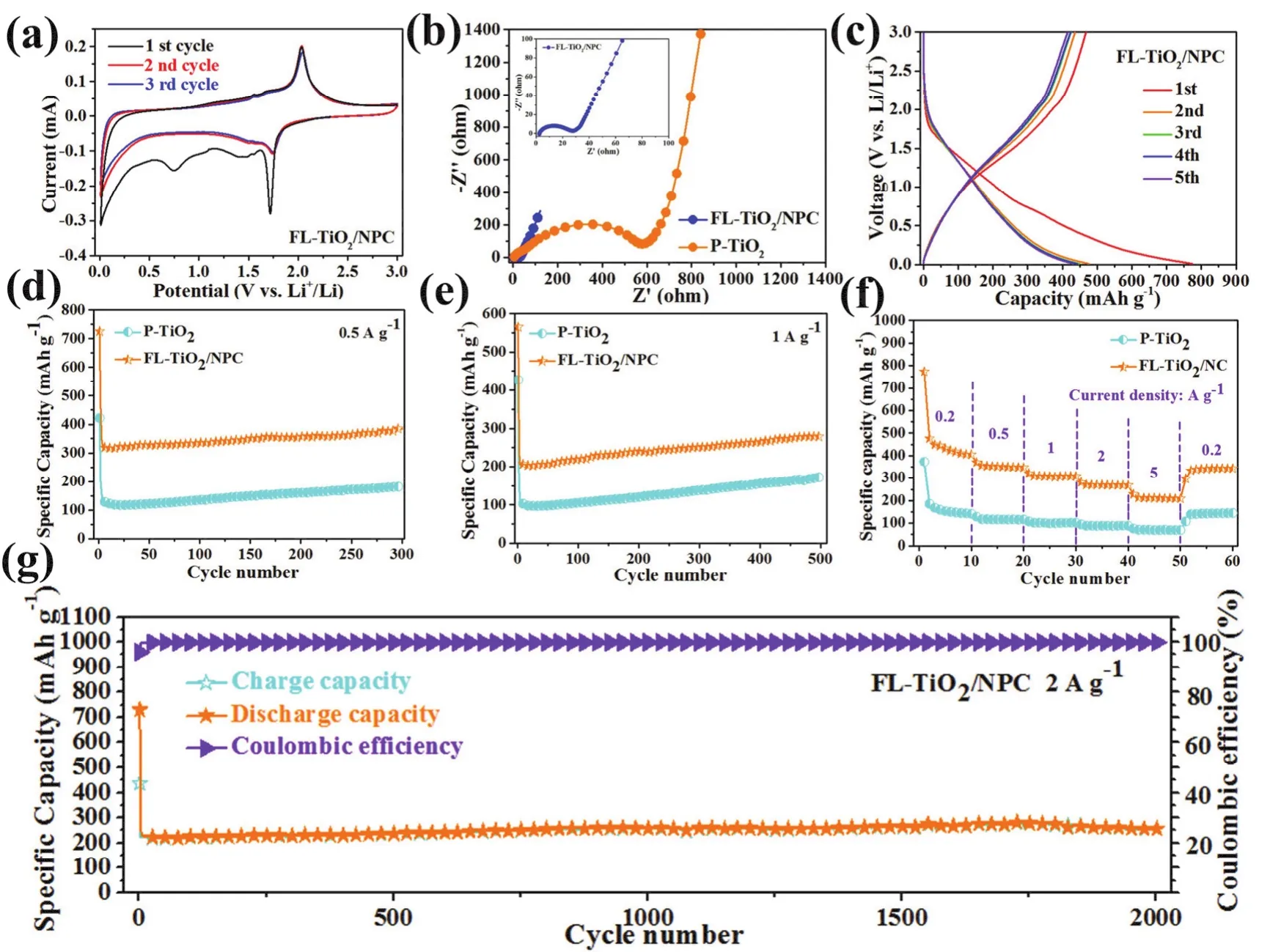
Fig. 5 CV plots (a) of FL-TiO2/NPC for the first three cycles at a scan rate of 0.1 mV·S-1 in the voltage range of 0.01-3 V. Nyquist plots (b) of FL-TiO2/NPC and P-TiO2 electrodes before cycling. Discharge/charge curves of voltage range at a current density of 0.2 A·g-1 for the first five cycles(c) of FL-TiO2/NPC. Long-term cycling performance (d, e) of FL-TiO2/NPC. Rate capability (f) of FL-TiO2/NPC and P-TiO2 electrodes.Long-term cycling performance at high current (g) of FL-TiO2/NPC.
The long-term cycling performances of FL-TiO2/NPC and PTiO2at 0.5 A·g-1are present in Fig. 5d. The first two cycles for FL-TiO2/NPC and P-TiO2is electrode activation process at 0.1 A·g-1. As shown in Fig. 5d, the initial discharge capacity of FLTiO2/NPC is 725.6 mAh·g-1, much higher than that of P-TiO2(421.7 mAh·g-1). After 300 cycles, the capacity of FL-TiO2can still remain 384.2 mAh·g-1, the same phenomenon can be further verified in Fig. 5e. When tested at 1 A·g-1, after 500 cycles, the capacity of FL-TiO2/NPC can still remain 279.1 mAh·g-1. On the contrary, the capacity of P-TiO2can only remain 171.4 mAh·g-1after 500 cycles. Obviously, discharge capacities of FLTiO2/NPC are much larger than that of P-TiO2at 0.5 and 1 A·g-1.Additionally, the capacity of FL-TiO2/NPC and P-TiO2increase gradually for long-term cycling at 0.5 and 1 A·g-1. This phenomenon is commonly found for anode materials like Co3O4,TiO2and NiO, etc. With the continuous infiltration of electrolyte,more and more active sites participate in the reaction of lithiation-delithiation.
To further explore the electrochemical properties of FLTiO2/NPC and P-TiO2, the rate performance was investigated(Fig. 5f). The average specific capacities of FL-TiO2/NPC are 405.5, 351.1, 307.6, 269.7, 210.7 mAh·g-1at 0.2, 0.5, 1, 2, 5 A·g-1, respectively. When the current density is switched back to 0.2 A·g-1, the average capacity of FL-TiO2/NPC can still reach 341.2 mAh·g-1. On the contrary, when tested at 5 A·g-1, the capacity of P-TiO2is only 69.4 mAh·g-1. When the mass loading of active material electrodes is 1.6 mg·cm-2, the long-term cycling performance of FL-TiO2/NPC can reach 335.8 mAh·g-1at 0.1 A·g-1for 50 cycles and 276.9 mAh·g-1at 0.5 A·g-1for 200 cycles (Figs. S5 and S6). Fig. 5g exhibits the long-term cycling performance at high current density. When tested at high current densities of 2 A·g-1with 2000 cycles, FL-TiO2/NPC still could achieve 256.5 mAh·g-1after 2000 cycles with nearly 100%coulombic efficiency, which indicated that FL-TiO2/NPC owns an excellent cyclic stability and good electrochemical activity.The long-term cycling performance of FL-TiO2/NPC is further compared with some TiO2/N-doped carbon anodes for LIBs(Table S1). Obviously, FL-TiO2/NPC is superior to the previously reported materials. This demonstrates that FLTiO2/NPC with a unique flower-like structure, can enhance the conductivity and contact area between electrolyte and electrode,and shorten the transmission path of Li ions and electrons,leading to excellent electrochemical performance.
3.3 Pseudocapacitive contribution of FL-TiO2/NPC electrodes for LIBs
In order to better explore the excellent performances of FLTiO2/NPC composites, electrochemical kinetics under different scan rates were investigated by CV measurements. Fig. 6a and Fig. S5a show the CV plots of FL-TiO2/NPC and P-TiO2electrode at different scan rates from 0.2 to 10 mV·S-1. In general, the pseudocapacitance of lithium ions behavior could be calculate based on the relative equations:
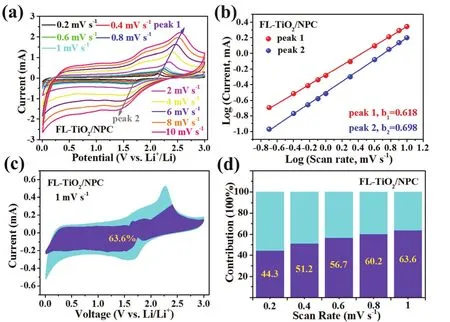
Fig. 6 CV curves of the FL-TiO2/NPC at different scan rate of 0.2-10 mV·s-1 (a). Calculation of the b values by plotting lgi versus lgv (b).The contribution of diffusion (cyan) and pseudocapacitive-controlled capacity (purple) at the scan rate of 1 mV·s-1 (c). Contribution percentage of pseudocapacitive-controlled capacity at different scan rates of 0.2-1 mV·s-1 (d). (The cyan correspond to diffusion capacity and the purple correspond to pseudocapacitive-controlled capacity.)

When the value of b approaches 0.5, the leading factor of diffusion-controlled lithium ions storage is the internal lithiation-delithiation reaction. On the contrary, when the value of b approaches 1, the capacity contribution is dominated by charge storage process. In general, the intensity and width of major peaks increase along with the higher scanning rate.However, there is no relative high polarization phenomenon for FL-TiO2/NPC. After calculation and fitting, the values of b1and b2are 0.618 and 0.698 (Fig. 6b). By contrast, the b1and b2values of P-TiO2are 0.518 and 0.522. (Fig. S5b), this shows that FLTiO2/NPC has higher pseudocapacitive lithium storage51. As shown in Fig. 6c, the yellow shadow area is related to the pseudocapacitive contribution (63.6%) when the scan rate of the CV graphics of FL-TiO2/NPC reach 1 mV·s-1.
From the equation, we can see that the capacity of FLTiO2/NPC is composed of pseudocapacitive contribution and diffusion-controlled contribution. Moreover, we can calculate the two parts as the following equation:

where k1·v corresponds the capacitive-controlled reactions,while k2·v0.5is related to the diffusion-controlled reactions. By sorting out the equations, we can obtain the value of k1and k2at the same time (k1refers to the slope, k2refers to the intercept).When the CV scanning rate increases gradually, the proportion of capacitive-controlled reactions also increased at the same time(Fig. 6d). As a result, a pseudocapacitive contribution (63.6%)can be achieved when the scan rate of the CV graphics reach 1 mV·s-1. This result undoubtedly shows that when the current at high current density, the pseudocapacitive contribution plays an important role in the contribution of capacity.
4 Conclusions
In our synthesis strategy, the unique flower-like structure and the introduction of N-doped porous carbon nanosheets are very important for FL-TiO2/NPC to achieve impressive electrochemical performance. Because of the fine TiO2NPs and the overall flower-like structure, the electrolyte can fully contact and permeate with the electrode material, and can effectively restrain the volume expansion of the electrode composites in the process of lithiation-delithiation. On the other hand, the introduction of N-doped porous carbon nanosheets can improve the conductivity, integrity and specific area of the overall composites, which is beneficial to promote the transport of lithium ions and electrons during the charge and discharge process. As a result, the prepared FL-TiO2/NPC composites can acquire attractive reversible capacity (256.5 mAh·g-1after 2000 cycles, 2 A·g-1) with nearly 100% coulombic efficiency and superior rate capability (210.7 mAh·g-1at 5 A·g-1), the excellent electrochemical performance and unique porous flower-like structure of FL-TiO2/NPC make it a new anode material for secondary LIBs with a strong application potential.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Strategies to Improve the Energy Density of Non-Aqueous Organic Redox Flow Batteries
- 一体化电极电催化二氧化碳还原研究进展
- 锂离子电池氧化亚硅负极结构优化和界面改性研究进展
- Challenges and Opportunities for Seawater Electrolysis: A Mini-Review on Advanced Materials in Chlorine-Involved Electrochemistry
- 锂离子电池隔膜的功能化改性及表征技术
- 三维大孔/介孔碳-碳化钛复合材料用于无枝晶锂金属负极

