Dynamics of sexual development in teleosts with a note on Mugil cephalus
J. Logamanya Tilak, Angeline Samuel, A. Kalarani, R. Moses Inbaraj
Endocrinology Unit, Department of Zoology, Madras Christian College, Tambaram, Chennai, 600059, India
Keywords:
Sex determination
SRY
sdY
Sex differentiation
Mugil cephalus
A B S T R A C T
The process of sex determination (SD) and sex differentiation (Sd) with associated sex-specific behaviour in teleosts is brought about by genetic factors and environmental factors under the in fluence of chemical messengers. SD is initiated by inherited genes, which in turn in fluence Sd by the production of hormones. To understand the plasticity of SD in fishes, the functional role of genes must be clearly elucidated. During early development, the dimorphic expression of male and female are mediated by the differentially sexualized brain. In mammals, SD region (SDR) on the Y chromosome (SRY) can be considered as male-specific copy of SOX3 and in the absence of SRY in teleosts suggests SD might be regulated by alternate genes. DM (doublesex/mab)-related genes, AMH (anti-mullerian hormone), TGF-b (transforming growth factor beta), GSDF (gonadal soma derived growth factor) and other genes (around 18) located in chromosomes of teleosts are found to be responsible for SD. The SD gene, sdY (sexually dimorphic on the Y chromosome) has been detected in more than 15 salmonid species, and in medaka and tilapia SD system is governed by heterogametic mechanism. In mullet, Mugil cephalus Dor et al. identified 27 SDR genes and suggested to be potential candidate genes for SD. Recently whole-genome sequencing data were produced from mullet and assembled into a draft genome sequence in which >30 loci are potentially associated with SD. Further analysis is required to know the involvement of each sex-biased gene for its functional in fluence on sex. Investigators attempted to know the sex-linkage group in tilapia and found that 2 linkage group i.e. LG1 and LG22 show SD loci, whereas in mullet it was documented that LG8 is the SD loci.Rigorous study with all male population(YY) or knockout of male marker gene, might give conclusive understanding on SD in future. The review highlights the absence of genomic understanding of SD and SDR genes for futuristic direction of research.
1.Introduction
The central switch of the control regulation of the formation of male and female in fishes are still not understood completely, although few sex determining and differentiating genes were identified in different fishes with its specific location in chromosomes. The neurohormones produced from the brain such has steroids, amines and peptides has an operative mechanism in converting the primordial germ cells of the gonad to form either testis or ovary (Oyola & Handa, 2017; Rey et al.,2020). However, when the germ cells unite to form an embryo, the sex determination (SD) is effected with its internal self-expressional pattern which was inherited through the germs of male and female much before the complete formation of central governing system of brain.
Sex determining genes predominantly decide the fate of the bipotential gonads, causing them to develop either into testis or ovary. The role ofSRYin humans andsoxin other therians and its presence in specific parts of the brain have been determined by numerous studies and it has been accepted,sryandsoxgenes have conserved sequence with similar orthologs in various species (Clepet et al. 1993, Lahr et al.,1995, Mayer et al., 1998, 2000; Wilson & Koopman, 2002). While birds and mammals have stable conserved sex chromosomes, only 10% fishes show heteromorphic allosomes, distinguished as XX/XY or ZW/ZZ(Feller et al., 2021). “Master” genes such assox,dmy/dmrt1bY,gsdfY,amhy,amhrIIandsdYwere identified in fishes species namelyOryzias latipes(Nanda et al., 2002), O. curvinotus(Matsuda et al., 2003),Nothobranchius furzeri(Valenzano et al., 2009), Takifugu rubripes(Kamiya et al., 2012), O. luzonensis(Myosho et al., 2012), Odontesthes hatcheri(Hattori et al., 2012), Oncorhynchus mykiss (Yano, Guyomard,et al., 2012) andO. dancena(Takehana et al., 2014), Oreochromis niloticus(Li et al., 2015). InMugil cephalus, Dor et al. (2016; 2020) have successfully identified a sex determination loci and mapped SD genes on LG9 chromosome. This has shed light on genes that could possibly function as SD gene (Fig. 1).
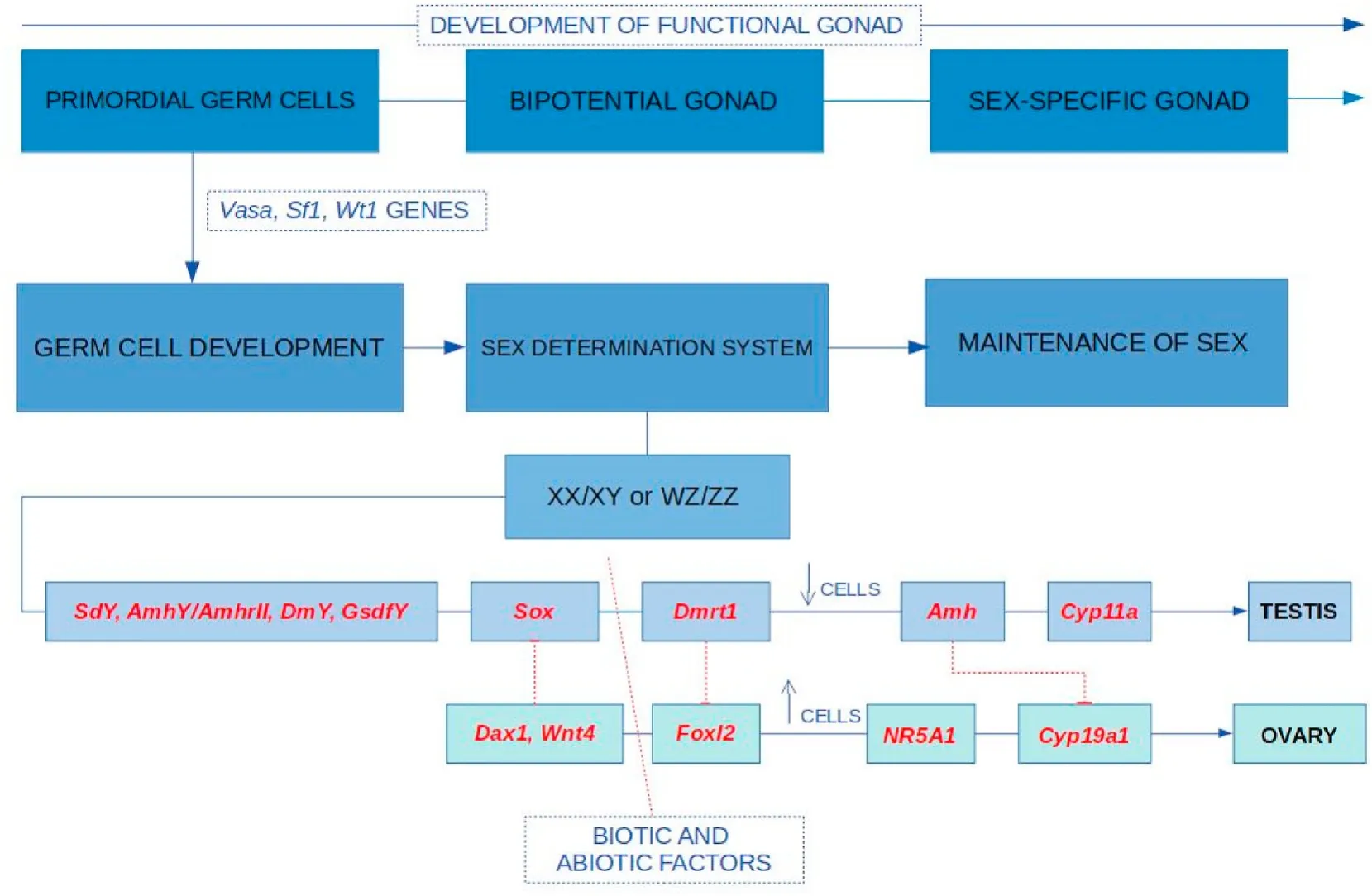
Fig. 1.Schematic diagram showing the sex determination and sex differentiation in teleost. Primordial germ cell under the in fluence of vasa, wt1 and sf1 genes lead to sex determination system. sdY (Yano et al., 2012a), amhY (Hattori et al., 2012), dmY (Shirak et al., 2006), gsdfY (Myosho et al., 2012) promotes the sox gene upregulation leading to development of testes (Takehana et al., 2014). Dmrt plays a role in the secondary sex determination (Devlin & Nagahama, 2002). Amh helps in gonad differentiation (Shan et al., 2021), it blocks cyp19a1b from brain to inhibit conversion of androgen to estrogen. Dax gene expresses female characteristics(Swain et al., 1998) with specific gene like cyp11a for development of female sexual characters (Martins et al., 2007) and wnt for development of ovary (Nusse &Varmus, 2012). Foxl2 upregulation in fluences estrogen and downregulation leads to testicular development (Wu & Chang, 2009. Dotted line and solid lines indicates inhibition and promotion of gene expression, respectively. (↓) - Decrease in cell number; (↑) -Increase in cell number.
The sexual differentiation of the gonad is due to systematic and accurate coordination of the brain and gonad interactions through various signal mechanism. Although the inherent factors imprinted in genetic level, the governing control mechanism operated through brain. The sexual differentiation of brain controls the formation of gonads through hormones. Many investigators reported that theamh,dmrt,cyp19a,gsdfand sox9 (Rajendiran et al., 2021) are playing a crucial role for forming the testis and ovary. The dimorphic behaviour of male and female are mediated by the differentially sexualized brain and the hormones (Lee et al., 2018; Rosenfeld, 2017). In the absence of testosterone, feminization of brain and in its presence, masculinization of brain is evidenced(Carruth et al., 2002; Peterson et al., 2013; Xu et al., 2012). Aromatase enzyme production levels for sex differentiation by the expression of genescyp19aandcyp19bleading to development of female gonads has been studied in zebra fish,Danio rerio. Similarly, the environmental exogenous factors like temperature, pH, salinity, and other chemical treatments lead to sex reversal against the inherited genetical imprint.The flow of information in the form of proteins translated from genes to the primordial germ cells (PGC) result in sex determination and the feedback of PGCs lead to sex differentiation by the action of the hormones produced from the hypothalamus.
Neuropeptides produced by the hypothalamus are key regulators of sex change in fishes. Arginine vasotocin (AVT) is a modulator of behaviour and is produced by the brain in response to the social environment. In response to social cues, without the in fluence of the gonads or sex hormones, the behaviour of the animal undergoes changes.Kisspeptin is a neuropeptide that promotes the secretion of GnRH, in directly aiding in sexual maturation by the production of sex hormones(Godwin, 2010; Ohga et al., 2018).
There are lot of areas which are still unclear in SD, development,growth and steroidogenic regulation of brain and gonadal development(Tenugu et al., 2021). The factors and molecules modulating the process of onset of steroidogenesis, its regulation during maturation and during unfavorable conditions is less investigated. The molecular machinery involved in brain sexual differentiation and regulation, genes and gene networks that play an important role in the androgen-mediated behavioural masculinization during early development are areas of in finite potential for further studies. The lack of information in endocrinological and genetic research especially in sex differentiation and regulation and the factors that in fluence brain sexual differentiation and the need to understand gene expression changes during different stages of maturity of fish is of great scientific concern are dealt with the present review with a special note onM. cephalus
M. cephalusis a migratory fish inhabiting rivers, estuaries, marine littoral as well as the open ocean during different stages of its life cycle.Being cosmopolitan in distribution, this species of the Mugilidae family is actively fished for consumption across all inhabited continents. The flathead mullet is a high-priced product and the cultivation of this species led to its introduction in non-native water bodies for its valuable roe also termed as “grey gold” in Asian and Mediterranean countries.Despite its popularity, purposeful cultivation of this fish has not been successful. The collection of fry is still dependent on conventional fry collection from the wild and selective sex cultivation by steroid administration was banned, making cultivation of high growth rate female fishes impossible. Thus genetic studies on sex and sex reversal in flathead mullet in order to produce an environmentally safe brood are necessary.
2.Sex determination in teleost
While SD and sex differentiation is fairly straightforward in birds and mammals, SD in teleost fishes is non-conservative, with both genetic factors as well as environmental factors playing major roles in the differentiation of gonads. These factors lead to various means of sexual reproduction in fishes including unisexuality, hermaphroditism and gonochorism (Heule et al., 2014).
The development of male, female or hermaphroditic organisms is regulated by a molecular signalling cascade in the developing gonads in fluenced by environmental or genetic cues. In birds and mammals, the difference in genes initiates SD signalling, with one morphologically distinct chromosome containing a single locus that acts as the master switch termed as chromosomal sex determination or genetic sex determination (CSD/GSD) (Liew et al., 2012). Polygenic sex determination(PSD) is governed by several independent loci that arise from modified allosomal chromosomes. PSD was discovered first in fish (Xiphophorusspecies) and later in insects, mammals and plants, with SD loci on autosomal chromosomes. PSD results in combination and epistatic effect at many loci and the dominant allele determines sex but this dominance varies in different genotypes (Moore & Roberts, 2013). Therefore in a PSD system, genes that control sex determination and gonad differentiation are distributed throughout the genome and sex of the individual is displayed based on the sum of genetic effects or alleles over many loci(Bulmer & Bull, 1982).
Though the ‘master’ genes predominantly control SD, sex steroidal hormones produced in response to environmental factors activate or deactivate any number of genes, shifting the fate of the undifferentiated gonadal tissue and therefore, sex determination and differentiation is due to mixed genetic and environmental factors (Tao et al., 2018).Environmental sex determination (ESD) factors include changes in temperature, pH, density and oxygen concentration. Temperature induced masculinization studies inO. niloticus(Yao et al., 2021) andDicentrarchus labrax,European sea bass (Díaz & Piferrer, 2015) lead to the finding that environmental factors affect gene expression. From these studies, it can be elucidated that: one, some fishes exhibit polygenic sex determination (PSD), wherein, SD genes are distributed throughout the genome and two, sex-biased gene expression is predominantly seen in fishes.
As substantial difference in gene expression was observed in SD of teleost fishes (Rajendiran et al., 2021), transcriptome analysis has led to the discernment of variation in gene expression at various stages of development. Studies on differences in the gene expression of gonads(Santos et al., 2007; Small et al., 2009) and brain (Arslan-Ergul &Adams, 2014; Wong et al., 2014) in sexually differentiated adults of zebra fish have not shown the molecular mechanism in brain sexual differentiation hence proving that the genes in the autosomal chromosomes activates the sexual differentiation than thesrygene (Wallis et al.,2007).
Transcriptome analysis is a major upcoming tool that can paint a clear picture of genes involved in process of SD. One of the major discoveries of transcriptome analysis is the identification of several mammalian genes such as transforming growth factor beta (TGF-β), a superfamily of proteins that play a major role in cell growth and differentiation. The proteins of this superfamily and the genes coding them were proved to be factors that in fluenced SD (Ma et al.,2016). Despite the presence of several SD mammalian genes in the teleost genome, their function in fishes have not been fully understood.
2.1.Process of sex determination
The process is initiated by the fertilisation of egg with subsequent repeated mitotic divisions leading to the formation of a blastula, the cells of which have the potential to develop into various organs. From this stage, the cells continue to divide, migrating to different regions in order to form rudiments of organs according to its fate map. The germinal or urogenital ridge is the origin of the gonads seen as a thickening of the mesoderm (Balinsky, 1975). The development of these rudimentary gonads and the formation of primordial germ cells is governed by genes.The role of autosomal genes in formation of PGCs, the migration of PGCs to the germinal ridge and in development of sex-specific gametes has been extensively studied inDrosophila,Caenorhabditis elegansand mice(Nikolic et al., 2016). The genesstella(dppa3/PGC7),vasa,fragilis,lhx9,sf1andwt1were observed to play a crucial role in PGC differentiation and migration to form a bipotential gonad (Gilbert, 2000; Lange et al.,2003; Wongtrakoongate et al., 2013). In fishes, the development of PGCs was studied in zebra fish, tilapia, rainbow trout and medaka and it was observed that thevasagene was expressed specifically in PGCs, differentiating them from the surrounding somatic cells (Nagahama, 1999;Yoon et al., 1997; Yoshizaki et al., 2000). The LIM-homeodomain developmental transcription factor (lhx9) was identified inO. latipesas a gene responsible for the development of the forebrain (Alunni et al.,2004). InO. niloticus, the silencing of thesf1gene was shown to affect gonad development (Cao et al., 2021) and in fluences the production of aromatase which decides the fate of the developing gonad (Yoshiura et al., 2003). Thewt1gene of mammals has been conserved as two co-orthologs,wt1aandwt1bin zebra fish and medaka, the expression of which is needed for the maintenance of PGC (Bollig et al., 2006; Klüver et al., 2009).
The PGCs do not undergo further differentiation or determination,that is, they are transformed into spermatogonia or oogonia only under the in fluence of the developing gonads and the hormones produced by it(Devlin & Nagahama, 2002). In most animal models includingDrosophila,C.elegans, mice and human, the allosomal chromosomes dictate the development of gonads from somatic cells surrounding the PGCs whereas in teleosts, a fixed “master” gene is absent and therefore its role is taken on by several other genes (Bachtrog et al., 2014). These genes, which are present as autosomal genes in mammals, are also seen in fishes, acting as the master sex determination genes.
2.2.Primary sex determination
The XX/XY system was identified inPoecilia reticulata(Winge, 1922),O. aureus(Don & Avtalion, 1988),Parodon hilarii(Moreira-Filho et al.,1993),O. latipes(Matsuda et al., 2003),Cynoglossus semilaevis(Zhuang et al., 2006),O. niloticus(Eshel et al., 2014) andPseudobagrus ussuriensis(Pan et al., 2021) show the WZ/ZZ SD-system. Thesrygene orsryhomologs present on the Y or Z chromosomes of organisms govern the organisation of testis in vertebrates.Sryhomologs (sry-like genes) were discovered in XX mice (Koopman et al., 1990) and in several salmon species (Yano, Guyomard, et al., 2012), proving thatsryorthologs are present in autosomal genes.
2.2.1.sdY
Sex in salmonid fishes is governed by the XX/XY system with a master sex-determining gene,sdY(sexually dimorphic on the Y-chromosome). ThesdYwas identified in vertebrates (Sinclair et al.,1990) andO.mykissas male conserved gene on Y chromosome and the gene is highly conserved across most salmon species, the gene being male specific with thesdYcopy in females inactivated (Yano, Guyomard, et al.,2012). The inactivation ofsdYin females can be attributed to environmental factors such as estrogen and temperature (Cavileer et al., 2015).However, in a few fishes of subfamily CoregoninaesdYexpression was observed in both male and female. In these fishes, the sequence study revealed that thesdYproteins have homology withirf9sequence and merge with autosomal (Yano, Nicol, et al., 2012) and hence it cannot be considered as a sex marker gene. The recent review (Rajendiran et al.,2021) also highlights that thesdYgene may not be the Y specific location conserved towards sex determination in the light of other fish species.The phenotypes of fishes were in fluenced by one or more autosomal copies of thesdYgene in addition to thesdYon the Y chromosome but the autosomal copies appear to have lost their role in sex determination(Ayllon et al., 2020).
2.2.2.Sox
Soxor thesry-related high mobility group box and the doublesex and mab-3-related transcription factor 1 ordmrt1are highly conserved in invertebrate as well as vertebrate species (Hu et al., 2021; Marchand et al., 2000). Thesoxgenes was first discovered in 1990 and were named so for sharing a motif with the sex determiningsrygene (Lovell-Badge,2010). Thesoxgene is identical to the mammaliansrygene with up to 50% identity. Takehana et al. (2014) studied the effect ofsoxgene inO. dancenaand elucidated that thesoxgene upregulation led to development of testis. Thesrygene does not act alone in the formation of testis. A cascade of genes are expressed in male-fated individuals initiated by thesry. These genes are autosomal and in fluence differentiation and growth of gonads and development of sex-specific secondary characteristics (Gilbert, 2000).sox9expression controls the bipotential determination of male and female gonad formation in assistance with extra cellular signals offgf9andwnt4(Kim & Capel, 2006). Based on sequence homology and protein structure,soxgenes are classified assox1-32 under 12 subfamilies (Phochanukul & Russell, 2010).
Eight subfamilies have been reported in fish:sox9in gonad development was reported inO. latipes(Yokoi et al., 2002),Paralichthys olivaceus(Wen et al., 2011),Acipenser baerii(Berbejillo et al., 2013) andAstyanax altiparanae(Adol fiet al., 2015). Thesox9aandsox9bproteins play roles in maintenance of germ cells in later developmental stages.sox3protein plays a role in male gonad maturation inAcanthopagrus schlegeli(Shin et al., 2009) andClarias batrachus(Rajakumar & Senthilkumaran, 2014). Thesox2gene ofScophthalmus maximus,was found to be a sex determining gene in a ZZ/ZW SD system (Martínez et al.,2021). InO. niloticus,sox11a,soxH, andsox17play a role in maturation of gametes and gonad development (Wei et al., 2016). InMisgurnus anguillicaudatusandP. olivaceus,sox8aregulates gonadal differentiation and maintenance (Xia et al., 2011; Yu et al., 2019). The expression patterns ofsoxgenes, its in fluence as a signalling factor in sex development is yet to be discovered inM. cephalus.
2.2.3.dax
Thedaxgenes were first observed in cases of congenital adrenal hyperplasia in human and was later identified for its role in sex determination in connection withsf-1gene (Bhagavath & Layman, 2013).The dosage dependent region on X (Dax1) gene, a mammalian sex determination gene, has been identified in a highly conserved form in fishes including European seabass andO. niloticus, playing a major role in development of female gonads (Martins et al., 2007; Wang et al.,2002).Dax1antagonises the function of theSrygene by downregulating theSox9andSF-1gene expression. In mammals, duplication of theDax1gene on the active X chromosome in an XY genotype, leads to suppression ofSrygene and the individual develops female characteristics(Swain et al., 1998).
In teleosts, thedax1gene sequence is seen to be an isolate of the human X-chromosome DSS (dosage-sensitive sex reversal) region.Dax1is therefore a female-specific SD gene that in turn regulates the expression of several genes essential for development of female sexual characters such asstar,cyp11a,cyp17,cyp19, müllerian inhibiting substance (mis), estrogens receptors α and β and androgen receptor (Martins et al., 2007). The upregulation of these proteins result in protein-protein interaction to suppress or maintain steroidogenic enzymes and steroid receptors. Thedax1gene is upregulated by thewt-1protein along with co-activatorlhx9.
Thesrygene anddax1gene compete to activate or deactivate thesf-1gene: activation ofsf-1leads to transcription ofsox9while deactivation results in the expression of the gene,wnt4(Gilbert, 2000; Mizusaki et al.,2003). GnRH regulates the expression ofsf-1, which in fluences the expression of GtH IIβ and estrogen receptors in later stages of development of salmons (Melamed et al., 1998). Thesf-1, at the point of gonadal development in sex changing fish,Trimma okinawae, is seen to decrease in male while increasing in female individuals from which it can be conferred thatsf-1plays a role in oocyte growth and maturation(Kobayashi et al., 2005).
2.2.4.wnt
Thewnt(wingless-type MMTV integration site family) gene codes for glycoproteins that are seen to be crucial in the formation of ovary in female fated individuals and was first observed inDrosophilafor body pattern defects (Nüsslein-Volhard & Wieschaus, 1980; Nusse & Varmus,2012). In SD,wnt4is a key protein in the regulation of the wnt/β-catenin signal pathway. The presence of β-catenin disrupts the expression ofsox9and testis formation which in turn enhances the expression ofdax1to form ovaries (Maatouk et al., 2008). The lack ofwnt4gene in XX mice showed the expression of male relatedamhgene (Gilbert, 2000).wnt4in fishes has been identified inDanio rerio(Liu et al., 2000)O. latipes(Li et al., 2012),A. schlegelii(Wu & Chang, 2009(Hu et al., 2014),O. mykiss(Nicol et al., 2012) andScatophagus argus(Chen et al., 2016). The two paralogs ofwnt4gene in teleosts arewnt4aandwnt4b, both involved in the development of female gonad, late ovarian growth and sex change in protandrous fish species (Hu et al., 2014). Thewntgene pathway might give further insight in the estuarine mullet towards the female gonadal differentiation.
2.3.Secondary sex determination
Following primary and secondary SD occurs by hormonal regulation,with hormones produced both from the newly formed gonads as well as the brain. The secondary SD takes place in two phases: first at the embryo stage and later at maturation or puberty (Gilbert, 2000). Primary SD results in the formation of sertoli cells by the action ofsox9along with the upregulation offibroblast growth factor 9 (fgf9) which suppresseswnt4expression.sox9expression is maintained by a feed-forward regulatory loop initiated by thesry. Upregulation ofsox9is affected by lipocalin-type prostaglandin D2 synthase (Ptgds), an enzyme that catalyses the production of prostaglandin D2 (PGD2), a paracrine factor that induces sertoli cell differentiation. Therefore, a feedback loop is maintained between the expression ofsox9that upregulatesfgf9to suppress feminization and the production ofPGD2upregulatessox9(Adams & McLaren, 2002; Huang et al., 2017). In the development of oocytes,wnt4produced β-catenin controls follistatin signalling that results in accumulation of retinoic acid needed to activatestra8expression which facilitates the switch from mitosis to meiosis to form oocytes (Mi et al., 2009).
2.3.1.amh
At this phase, secondary SD is initiated by sex-specific genes such asamh(anti-Müllerian hormone), a transforming growth factor-beta (TGF-beta) superfamily protein, is secreted from the differentiated sertoli cells in the male gonad. The presence of the gene and its role in female mullerian duct regression in humans was reported in 1947 (Jost, 1947;Lehman et al.,2012).amhandgsdf(gonadal soma-derived factor) that play a role in gonad differentiation and spermiogenesis respectively were identified in several teleost fishes (Shan et al., 2021). Thesox9protein is an activator of theamhgene or Mullerian-inhibiting substance(mis), binding to the promotor of theamhgene along withsf-1andwt-1proteins that act as upregulators ofamhby binding to thesox9protein.Though teleosts lack Müllerian ducts, their genome contains an ortholog of theamhgene which has been reported in 21 fish species (Pfennig et al., 2015). A duplicate copy of theamhon the Y chromosome (amhY)has been said to play a role in sex determination ofO. hatcheriandO. bonariensis(Hattori et al., 2012; Yamamoto et al., 2014).amhexpression in male embryo was proved to increase during sex determination inO. mykiss(Marchand et al., 2000),P. olivaceus(Yoshinaga et al., 2004),Dicentrarchus labrax(Halm et al., 2007),A. schlegelii(Wu,Chiu, et al., 2010),O. hatcheri(Hattori et al., 2012),Gadus morhua(Haugen et al., 2012),Anoplopoma fimbria(Smith et al., 2013),O. niloticus(Eshel et al., 2014) andD. rerio(Webster et al.,2017) but theamhrII(amh receptor) gene was expressed only inO. latipes(Klüver et al., 2007),A. schlegeli(Wu, Chiu, et al., 2010) andT. rubripes(Kamiya et al., 2012). Therefore, the role ofamhis not distinct in fishes but mutantamhinD. rerioshowed the female-biased sex determination in embryos and the development of large testis in males and development of sterility in female, proving the necessity ofamhin male sex determination and germ cell accumulation while inhibiting oocyte development or survival (Yan et al., 2019).
The presence ofamhin oocytes of Atlantic salmon and medaka showed that the role ofamhis decided based on the number of germ cells: lower number of germ cells maintained by thedmrtgene lead to male SD whereas large number of cells trigger aromatase production or the expression ofcyp19a1agene (Guiguen et al., 2010; Herpin et al.,2007; Morinaga et al., 2007; Tanakaet al., 2007). Therefore,sox9inducedamhproduction in males inhibit the conversion of androgen to estrogen by blocking the expression ofcyp19a1agene which encodes aromatase. In the downregulation ofamh, aromatase enzyme activity was found in all vertebrates, including primitive fishes and amphioxus(Han et al., 2019) The genes producing aromatase belong to the cytochrome P450 superfamily and is responsible for plasma sex steroid levels. In fishes, thecyp19a1gene exists in two forms: thecyp19a1aform in gonads and thecyp19a1bform in the brain. The expression ofcyp19a1aincreases drastically during ovarian differentiation in many fishes including Japanese medaka, rainbow trout,O. niloticus, European seabass, and southern flounder (Chen et al., 2017).In undifferentiated orange-spotted grouper (Epinephelus coioides), overexpression ofamhinduced male gonad development (Han et al., 2019) and a significant increase in the expression ofamhandamhrIIwas seen in male-fated embryos (Pfennig et al., 2015).
2.3.2.dmrt/dmY
Sex phenotype establishment is through the maintenance of the formed gonads. This is carried out by autosomal genes, Doublesex and mab-3 related transcription factor 1 (dmrt1) and Forkhead box L2(foxl2), playing antagonistic roles in the development of functional gametes in the gonads of male and female organisms respectively(Uhlenhaut et al., 2009). The doublesex/mab3 (DM) gene family shares similarities with theDrosophila(dsx) andCaenorhabditis elegans(mab3)homologs (Ottolenghi et al., 2002). A mutation in the mammalianDmrtgene causes sex reversal from male to female and sertoli cells in males continually proliferated but failed to differentiate and thus die (Huang et al., 2017).
In zebra fish, the genedmrt1was expressed in juveniles that differentiated into male adults and it was deduced thatdmrt1is necessary for male sexual development (Webster et al.,2017; King et al., 2020). In tilapia,dmrt1was found in both testis and ovary though it was eventually upregulated only in the testis by the action ofsoxgene. The function of thedmrt1gene in rainbow trout (Marchand et al., 2000),medaka (Kobayashi et al., 2004) and tilapia (Jiang et al., 2016) was studied by expression analysis on exposure to external steroids and it was elucidated thatdmrtexpression is in fluenced by hormones produced and thus plays a role only in secondary sex determination in these fishes(Devlin & Nagahama, 2002).
However, inO. latipeswhich has the XY system of SD, a DM domain gene on the Y chromosome,dmYwas shown to be necessary for male germline development and is a recent duplication of thedmrt1gene, a new sex chromosome analogous to the mammalianSRY(Matsuda et al.,2003; Shirak et al., 2006). This gene, though present in all wild typeO. latipesindividuals and its closely related species,O. curvinotus, is not present in any other species leading to the hypothesis that thedmYgene has lost its SD function and a new gene acting as a master SD gene has appeared (Tanaka et al.,2007).
2.3.3.gsdf
The theory of evolution of sex chromosomes in closely related species was supported by the discovery ofgsdfgene in the SD loci of Y chromosome ofO. luzonensis(Myosho et al., 2012). This gene was termed as GsdfY and was seen to be a master switch gene for this species. While thegsdfgene is present in most teleosts, they are not found within the SD loci but play a role in the SD cascade as a downstream product ofdmrt1/dmYgene expression. Bothsoxanddmrt1can activate thegsdfgene to initiate spermiogeneis (Hu et al., 2021; Jiang et al., 2016) and therefore, playing a role in sex differentiation (Takehana et al., 2014).Gsdf, an autosomal gene, is a key factor in sex differentiation and its role male sex differentiation inO. niloticus,O.latipesandC. semilaeviswas established(Kaneko et al., 2015; Zhang et al., 2016; Zhu et al., 2018).
2.3.4.foxl2
The absence ofdmrtor its mutation reduces the production ofamhand promotes over-expression of thefoxl2gene (Webster et al., 2017).Thefoxl2gene was initially reported inDrosophilaembryos in 1989 and later thought to be present in only a few vertebrate groups. Paralogs of the gene were seen in teleosts and further broad evolutionary analysis proved the presence of the gene as a subgroup of the teleost copy in birds and marsupials (Geraldo et al., 2013). In mice, the mutation offoxl2leads to complete female-male sex reversal where the germline transformed to sertoli and leydig-like cells.foxl2, along with nuclear receptor 5A (nr5a1), acts as a transcription regulator ofcyp19a1, playing a crucial role in estrogen biosynthesis asfoxl2aand somatic differentiation in the ovary asfoxl2band therefore is one of the earliest markers for ovarian determination in teleosts (Baron et al., 2005; Fan et al., 2019). Similar to thedmrt1gene, the regulation offoxl2expression can be in fluenced by steroid treatment with estrogen upregulatingfoxl2expression and aromatase inhibitors leading to down-regulation offoxl2, with testicular development in predetermined female embryo (Wang et al., 2007).Thus, a positive feedback loop betweencyp19a1andfoxl2expression was discovered in gilthead seabream (Wong et al., 2006), Nile tilapia(Wang et al., 2007), European seabass (Navarro-Martín et al., 2011),Japanese flounder (Si et al., 2016) and rainbow trout (Xu et al., 2016).
2.4.Sex determination in Mugil cephalus
Mullets show gonochoristic character, developing indirectly from non-functional intersex or hermaphroditic gonads as male and female individuals with functional testis or ovary in a wild population (Devlin &Nagahama, 2002; McDonough et al., 2005). Irrespective of age, gonadal development was seen to be directly proportional to growth. McDonough et al. (2005) correlated total length and age of the fish to the development of gonads through histological studies. Spermatogenesis was observed in fishes of length>250 mm and maturity was observed at lengths>325 mm while oogenesis began at lengths>290 mm and all individuals over 400 mm were mature. Fifty percent maturity was seen at 275 mm and 325 mm in male and female individuals respectively.Histology and plasma steroid level analysis deduced that sex differentiation in mullet occurred at about 1 year of age (Chang et al., 1995). The gonadal development ofM.cephaluscan be divided into: undifferentiated stage (<6 months of age), differentiating stage (7–14 months) and differentiated stage (>15 months). The body weight had no in fluence on the GSI and ovarian differentiation preceded that of testis. Following differentiation, the diameters of the oogonia and spermatogonia showed considerable increase in size, reaching complete maturation (Chang et al., 1995).
InM. cephalusthere are no records of primary and secondary SD that were studied in other group of teleosts. While cytogenetic analysis ofM. cephalus, reported that the karyotype consists of 24 chromosome pairs with no distinct allosomes (Gornung et al., 2001). To form species-specific linkage maps in order to identify loci for economically important traits, forming genetic stock and for marker-based breeding programs, Dor et al. (2016; 2020) attempted to construct a first generation genetic linkage map of the SD region using a homology-based(RNA sequence) approach. Syntenic gene relationship was established between Nile tilapia and flathead mullet based on phylogenetic relation of whole mitochondrial sequences. Synteny was observed between LG9 ofM. cephalusand tLG8 of tilapia, with conserved synteny in LG1 of seabass and human chromosome 2 and 10. The sex of offspring was determined by XY system as alleles were inherited from the father and the SD loci was mapped on LG9. Moreover, an attempt to know the sex-linkage group in mullet revealed that LG9 is the SD loci whereas in tilapia 2 linkage group i.e. LG1 and LG22 show SD loci (Dor et al., 2020).In mandarin fish (Siniperca chuatsi) among the 24-linkage group mapped, LG23 was considered a sex-related linkage group (Sun et al., 2017),similarly LG23 was identified as a SD loci in GIFT tilapia (Taslima et al..,2020), among 13 linkage group studied L10 was the potential SD loci inO. melastigma(Lee et al., 2019).
Dor et al. (2016; 2020) identified 27 sex determination region (SDR)genes by comparing the male and female genomic sequences ofM. cephalusin LG9, and suggested it to be the possible candidate genes for SD. On successful sequencing of SDR on the LG9 chromosome, sheds light on genes such asbccip,dhx32a,dock1, andfshr(GTH-RI) as possible sex determination genes in the flathead mullet,M. cephalus. Among the 27 genes identified,DOCK1 and DHX32A genes are seen to be associated with other genes playing a crucial role in SD (Dor et al., 2020). Further,Curzon et al. (2021) convincingly proved that c.1759T>G variant infshrhas been found to be concordant with male determination and it also further con firmed in mullet (Ferraresso et al., 2021). The role of putative SD genes include:Dock1downregulation in ovary leading to PCOS(polycystic ovarian syndrome) in rat model and development of growth-related traits in human and pig (Ubba et al., 2017);Dhx32a, a protein from the DEAD/H-box RNA helicase family, is found in two copies in teleosts and is seen to be highly expressed in cat fish females(Tian et al., 2017) while in mice, it is expressed from 14 days to 1 month embryos (Valenzano et al.,2009);Bccipin teleosts was shown to be upregulated in male rainbow trout embryos, playing a key role in neural development (Hale et al., 2011) while in human, it was seen to have a role in DNA repair (Liu et al.,2013);Gth-rI(fshr) plays a role in gonad development and despite expression level in experimental larval stages being low, further studies performed con firms its position as a master SD gene even thoughfshrhas never been reported before as a sex-determining locus (Ferraresso et al., 2021). Validation of 27 SDR genes with respect to SD and the role of “Master” SD inM.cephalusare the areas of research that remains unexplored.
3.Sex differentiation and sex reversal
SD mechanism does not initiate sex differentiation but interacts at intermediary stages to direct gonad cell formation, therefore, sexspecific steroid cells and the difference in hormone production is apparent before the formation of morphologically distinct gonads. Sex differentiation or formation of functional gonads and gametes, under endocrine control, is a complex interplay between the brain and gonad,with the production of gonadotropins from the pituitary from brain and steroids from gonad (Devlin & Nagahama, 2002). On the contrary brain sexualisation is not permanent in fishes at early developmental stages as seen in mammals and birds (Le Page et al., 2010). In many gonochoristic species sex reversals can be brought about by environmental and social factors or through hormone treatment due to brain plasticity. Though sex differentiation and sex reversal are two different phenomena their regulation seems to overlap (Horiguchi et al., 2017). Moreover, there is a distinct expression of sex determining genes during both sex differentiation and sex reversal process. This part of the review highlights the mechanism of sex differentiation and sex reversal.
3.1.Steroidal regulation
The sexual differentiation of the brain is due to systematic and accurate coordination of the sex steroid hormones (Rosenfeld, 2017). Sex steroids mainly investigated include estradiol-17β, testosterone and 11-ketotestosterone with estradiol- 17β levels higher in females than in males, maintaining ovarian development and the latter promoting testicular differentiation. The cholesterol side-chain cleavage enzyme,P450scc cleaves steroid precursor, cholesterol to pregnenolone and other functional steroid hormones. The formation of estradiol is facilitated by the upregulation of aromatase genes in the brain and gonads of female fishes. The expression of the aromatase gene was studied in several models such as zebra fish, tilapia, trout, medaka and gold fish,resulting in the identification of multiple aromatase genes in certain species (Trant et al., 2001). The aromatase genes of the brain and gonad,cyp19aandcyp19b, differ in expression levels at various stages of development, suggesting that the gene may have varied function in sex differentiation based on sex of the individual. The expression of aromatase gene in mammals reduces drastically following birth but in fishes, the expression of bothcyp19aandcyp19bis maintained even in adults, with its down regulation leading to testicular differentiation and is therefore linked to TSD (Temperature-dependent sex determination)(Ospina-Alvarez & Piferrer, 2008). The production of steroid hormones requires viable germ cells and a positive feedback mechanism to the brain is regulated by gonadal steroid levels. Aromatase enzyme production levels by expression analysis of thecyp19aandcyp19bin zebra fish in early developmental stages (0–41 days post fertilisation)(Trant et al., 2001) revealed that hormone production began with the formation of gametes, before gonadal differentiation. Similar observations were seen in trout and salmon, with testosterone levels at a peak prior to significant gonad differentiation (Fitzpatrick et al., 1993). In northern snakehead (Channa argus) the testicular differentiation was affected byamhwhile estrogen modulated its expression (Luo et al.,2020).
The gene expression levels and steroid hormone levels that fluctuate during sex reversal is similar to initial sex differentiation. Sex reversal study in juvenile black porgy,A. schlegeli, revealed that the ovary of the hermaphroditic gonadal tissue remained inactive even following estrogen treatment but with the removal of testicular tissues, methylation levels ofcyp19a1decreased, leading to ovary activation (Wu, Tomy,et al., 2010). In a change from male to female, there is a decrease in 11-ketotestosterone levels followed by increase in estrogen level with upregulation of male-specific genes such asdmrt1, amhandsox9.The change of female to male is characterised by sudden downregulation ofcyp19a1gene and estradiol levels and a gradual rise in 11-ketotestosterone levels with upregulation ofcyp19a1a, foxl2, wnt4(Todd et al.,2016). Treatment with 17α Methyltestosterone altered the brain transcriptome of male and female (Stephanie et al.,2018). This was proved in zebra fishes by comparison of control male and female fishes to fishes exposed to 17α-methyltestoterone. The fishes exposed to the hormone showed masculinization of the brain, that is, male dominant gene expression was observed (Lee et al., 2018). The dmrt1gene expression is altered by sex steroids inE. coioides(Lyu et al., 2018). Studies related to the effect of endocrine disruptors on the development of gonads and the eventual sexual phenotypes was found successful (Le Page et al., 2010;Godwin, 2010; Liu et al.,2016) inD. rerio,a dependable model for endocrine studies (Todd et al., 2016; Wong et al., 2014).
3.2.Environmental regulation
Fishes exhibit sexual plasticity, that is, the ability of changing phenotypic sex as well as a change in gonad differentiation. This is therefore based not only on chromosomes but also on external factors and it can be concluded that neurogenic activity is not restricted to developmental stages as in mammals but extends through adulthood in teleosts (Le Page et al., 2010). Epigenetics has a pivotal role in regulation of genes involved in sex differentiation and sexual behaviour through environmental factors. Steroid hormone spike during initial differentiation and at time of maturation trigger epigenetic changes in brain regions, resulting in sex-specific brain response (Rosenfeld, 2017).Recent evidence in long non-coding RNAs that are involved in epigenetic regulatory mechanisms (Mercer et al.,2009) is found to regulate thedmrt2expression in Chinese tongue sole (C. semilaevis) (Feng et al.,2021). An increase indmrt1gene methylation in female barramundi was seen during sex reversal (Domingos et al., 2018). In the European sea bass, high temperature regulates epigenetic modification of thecyp19a1leading to masculinization of the brain in females in 60 days post fertilised embryos (Navarro-Martín et al., 2011). Similarly in tilapia(O. mossambicus) the water temperature caused alteration in brain aromatase activity in the ten days post hatch embryo (Tsai et al., 2003)
The connection of environmental stimuli to epigenetic and hormonal changes leading to sex reversal is speculated to be the in fluence of cortisol, a stress hormone. Changes in the environment and social structure imitate stress situations and an invariable increase in cortisol levels, leading to masculinization of the brain as TSD in general facilitates development of female individuals at lower/optimal temperatures and males only at higher temperature exposure at a critical stage during early development (Conover, 2004). The induction of brain masculinization by cortisol treatment has been reported in several species:rainbow trout (Van den Hurk & Van Oordt, 1985), pejerrey (Hattori et al., 2009), Japanese flounder (Yamaguchi et al., 2010), southern flounder (Mankiewicz et al., 2013) wrasse (Nozu & Nakamura, 2015)and medaka (Adol fiet al., 2019). Similar studies reported that hypoxia,acidic pH, density in social groups and change in environment colour caused stress-induced cortisol mediated sex change (Shen & Wang,2018a). Temperature dependent upregulation ofamhwas observed inOdontesthes bonariensis(Fernandino et al., 2008),O. niloticus(Poonlaphdecha et al., 2013) andSchizothorax kozlovi(He et al., 2018),foxl2that plays a vital role in ovarian tissue differentiation was affected by temperature during the early development of bluegill,Lepomis macrochirus(Shen et al., 2018b). InO. niloticushigh temperature induces masculinization through transcriptional variation ofdmrt1andgsdf.(Teng et al., 2020).
Under the in fluence of external stimuli or social structure, norepinephrine (NE) and arginine vasopressin/vasotocin (AVP/AVT) stimulate sex change whereas dopamine (DA) and serotonin (5-HT) inhibit sex reversal. The interaction of AVP/AVT, a key neuropeptide, with androgens produced mediates male-typical social behaviour. In the sex changing fish, Bluehead wrasse, sex is effected by social cues. Larger females develop into males with the gonads having no in fluence on this sex reversal and in such cases, the level of AVP/AVT increases irrespective of gonad absence when larger females are present along with subordinate females and the large individuals exhibit male typical behaviour (Semsar & Godwin, 2003). The level of gonadotropin releasing hormone (GnRH) and gonadotropin (GtH) are increased and decreased by NE/5-HT and DA respectively in gold fish (Larson et al.,2003). NE increases GtH2 levels to initiate and complete sex reversal by binding to alpha-adrenergic receptors of brain and pituitary. The pituitary, which lies outside the blood-brain barrier, is directly affected by DA to inhibit spontaneous GtH release (Peter, 1986).
Another important component of the HPG axis is the Kiss1/Kiss1r system which affects gonad differentiation by regulating production of GtH and GnRH and their absence results in incomplete differentiation of gonads. The feedback mechanism of regulating sex steroids is through the Kiss1/Kiss1r system having both estrogen and androgen receptors in the Kiss1 expressing neurons. It was found that the structure and function of kiss1r was conserved and similar in mammals and fishes includingO. niloticus, cobia (Rachycentron canadum), senegalese sole(Solea senegalensis) (Mechaly et al., 2009) and grey mullet (M. cephalus).The presence of twokissgenes,kiss1, kiss2and kiss1r was con firmed in medaka (Kanda et al., 2008), gold fish (Yang et al., 2010), seabass(Escobar et al., 2013) and zebra fish (Ogawa & Parhar, 2018) with kiss1 protein facilitating formation and release of LH and FSH through GnRH neurons. The levels of GnRH could be correlated to sex change in dusky anemone fish, orange-spotted grouper and bluehead wrasse (Kauffman et al., 2007, 2009; Shi et al., 2010).
3.3.Sex differentiation and sex reversal in Mugil cephalus
InM. cephalus, no clear sexual dimorphism was observed in plasma steroid levels but a surge in both estradiol and testosterone was observed at 9–12 months or the gonad differentiating stage whereas complete gonad differentiation was observed only post 15 months (Chang et al.,1995). The plasma testosterone levels and gonadal aromatase activity increased considerably in induced sex differentiation experiment on 4-month-oldM. cephalus(Chang et al., 1999). The study on the effect of endocrine disruptors like ethynylestradiol in juvenileM. cephaluswhere the sex differentiation initiated, revealed that it resulted in atypical gonadal morphogenesis and advanced differentiation of ovarian tissues(Aoki et al.,2011).
Breeding ofM. cephaluswas accomplished through induced spawning at 32% using human Chorionic Gonadotropin (HCG) by Kuo (1982)in mature females. Hormonal treatment with estradiol and methyltestosterone of rearedM.cephalusshowed successful sex reversal with milt characteristics and increase in body weight similar to untreated fish. Sexual plasticity was con firmed by reversal of sex in treated fishes with discontinued hormonal treatment (Meiri-Ashkenazi et al., 2011).
4.Conclusion
The review highlights various factors and genes responsible for the processes of sex determination, sex differentiation and sex reversal but it clearly shows the huge lacuna in understanding the mechanism behind each of the process. Understanding them would be a huge boon to aqua culturist in creating sex-controlled aquaculture practices. Since steroid administration towards sex selected culture was banned, making cultivation of high growth rate female fishes impossible. Thus, to produce an environmentally safe brood, further studies are required to con firm the role of identified genes, selective expression studies and expression modification in response to environmental cues which in turn can be used to identify key brain molecular pathways involved in reproduction and in the development of biomarkers of reproductive performance and for successful breeding of this species. Gene networks can be deciphered using the global gene quantification technology. The relationship between a phenotype and its governing genotype that relays information regarding cell fate and development can be analysed using transcriptome analysis of a complete set of transcripts, that is, coding and non-coding RNA which varies based on the external environmental factors and developmental stage or physiological condition of the organism (Lindberg & Lundeberg, 2010; Okazaki et al., 2002; Qian et al.,2014). Transcriptome analysis can thus be used to identify and target the genes that regulates the sexual differentiation during development,providing proof to ascertain progressive events in reproduction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
Acknowledgements
Authors acknowledge the research grant from Department of Science and Technology - SERB No: CRG/2020/0004363, Govt. of India.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Understanding the impact of stress on teleostean reproduction
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
