The ghrelinergic system in zebra fish gonads is suppressed during food unavailability
Azdeh Htef, Jithine Jykumr Rjeswri, Surj Unnippn,*
aLaboratory of Integrative Neuroendocrinology, Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan,Saskatoon, Saskatchewan, S7N 5B4, Canada
bToxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B3, Canada
Keywords:
Ghrelin
Ghrelin receptor
Ghrelin-O-acyltransferase
Fasting
Ovary
Testis
Follicle
Zebra fish
A B S T R A C T
Ghrelin is an appetite stimulatory peptide that regulates reproduction in vertebrates. The acylation of ghrelin by ghrelin-O-acyltransferase (Goat) is essential for most of its functions. The ghrelinergic system (ghrelin, ghrelin receptor [Ghsr] and Goat) is present in teleost fish ovary and testis. In this research, we determined the abundance of the ghrelinergic system mRNAs in different stages of follicle (oocyte) development in zebra fish ovary.The highest levels of preproghrelin mRNA were observed in the first stage of follicular development (previtellogenesis). Significantly low levels of preproghrelin mRNA were observed in stages 3II, 4 and 5 of the follicle development. Ghsr mRNA abundance remained unchanged at early stages of follicle development and decreased in stage 5 compared to other developmental stages. A significant decrease in preproghrelin and Ghsr mRNAs in the testis, and ghsr mRNA in the ovary of zebra fish deprived of food for 3 days were found. At 7 days food deprivation, preproghrelin, ghsr and goat mRNA in the ovary and preproghrelin and goat in the testis of zebra fish were significantly reduced. These results show that metabolic status modulates gonadal ghrelinergic system in zebra fish and provide support for a role for ghrelin in fish reproduction.
1.Introduction
There is a well-characterized association between energy balance and reproduction in vertebrates (Barreiro & Tena-Sempere, 2004; Bertucci et al., 2016; Shepperd et al., 2012; Sheridan, 2021; Volkoff et al.,2005, 2009). Nutrient status has a significant impact on reproductive functions including sex hormone production, gametogenesis, and early embryonic development. In fish, vitellogenin and its derived yolk proteins serve as nutrients for the embryos during development (Arukwe &Goksøyr, 2003; Finn & Fyhn, 2010). Hormones help to integrate metabolism and reproduction in fish (Hatef & Unniappan, 2019; Shahjahan et al., 2014; Volkoff et al., 2005, 2009). For instance, nesfatin-1, an inhibitor of feeding has a suppressive role on the hypothalamo-pituitary–gonadal (HPG) axis in gold fish (Gonzalez et al., 2010,2012; Rajeswari & Unniappan, 2020). Concurrent changes in feeding,migration, spawning and reproduction in fish during seasonal variations further suggest integration of nutrition and reproduction (Volkoff et al.,2009).
Ghrelin is the only known stomach-derived appetite stimulatory hormone that acts through the growth hormone secretagogue receptor(Ghsr) or ghrelin receptor (Kojima et al., 1999). Post-translational processing of its precursor peptide preproghrelin by prohormone convertase (PC) 1/3 results in mature ghrelin production. The post-translational acyl modification usually seen in the third serine residue is necessary to activate the ghrelin receptor (Jönsson, 2013).Ghrelin-O-acyl transferase (Goat) is the enzyme responsible for the acylation of ghrelin (Gutierrez et al., 2008; Hatef et al., 2015; Shlimun &Unniappan, 2011; Yang et al., 2008). The ghrelinergic system composed of ghrelin, ghrelin receptor and Goat is found both centrally and peripherally in several fishes (Amole & Unniappan, 2009; Hatef et al.,2015; Kaiya et al., 2008; Manning et al., 2008; Parhar et al., 2003;Shepperd et al., 2012; Unniappan et al., 2002; Unniappan & Peter,2005). Ghrelin injection to the brain or to the periphery stimulates food intake in gold fish (Matsuda et al., 2006; Miura et al., 2006, 2007;Unniappan et al., 2002, 2004) and Mozambique tilapia (Riley et al.,2005). In fish, food deprivation increases ghrelinergic system abundance in the gut and brain, and refeeding decreases it to basal levels (Amole &Unniappan, 2009; Blanco et al., 2016; Hatef et al., 2015; Kaiya et al.,2013).
Ghrelin stimulates Lh secretion from the dispersed pituitary cells of gold fishin vitro. Intracerebroventricular (ICV) or intraperitoneal (IP)injections of ghrelin in gold fish increased circulating Lh levels(Unniappan & Peter, 2004). In female tilapia, ghrelin mRNA expression was more abundant prior to the rapid growth stage, suggesting a role for ghrelin in tilapia reproduction (Parhar et al., 2003). In halibut, ghrelin expression is increased prior to exogenous feeding, and remains elevated during metamorphosis and stomach differentiation (Manning et al.,2008). Ghrelin, Ghsr and Goat are modulated by gonadal steroids(estradiol and testosterone) in gold fish (Bertucci et al., 2016). Ghrelin administration increased ovarian follicle sizein vivoinBarbus sharpeyi(Mabudi et al., 2011). In contrast, our research found a suppressive effect for ghrelin on oocyte maturationin vitroin zebra fish (Shepperd et al., 2012). Together, the above studies support a species-specific role for ghrelin in fish reproduction. Recently we reported modulation of the ghrelinergic system in the ovary and testis of reproductively active gold fish under metabolic challenges (Rajeswari et al., 2019). Our result indicates sex- and organ-specific patterns of ghrelinergic system expression in gold fish. Food deprivation for 28 days (chronic energy unavailability) leads to a major increase in preproghrelin, Ghsr-1a and Goat in the ovary of gold fish (mRNA and protein abundance) and significant mRNA upregulation in the hypothalamus of male gold fish(Rajeswari et al., 2019). In the present study, we determined the expression pattern ofpreproghrelinandgoatin various oocyte development stages in zebra fish(Danio rerio).The expression of ghrelinergic system in the gonads of sexually mature male and female zebra fish under acute metabolic stress (food deprivation) was also studied.
2.Material and methods
2.1.Animals
Study protocols employing zebra fish adhered to the Canadian Council of Animal Care standards and was reviewed and approved by the animal care committee of the University of Saskatchewan (Protocol Number 2012-0082). Zebra fish (Danio rerio; age: ~8 months; length:~2 cm; and body weight: 1–1.5 g) were purchased from an approved vendor (Aquatic Imports, Calgary, Alberta, Canada). A 12 h light:12 h dark cycle and a water temperature of 28 ℃ was used for the fish holding tanks. A 2% body weight ration (commercial flake diet, Nutra fin Max, Rolf C. Hagen, Inc.) was provided at 11:00 a.m. daily. Fish were acclimated for at least 2 weeks before the commencement of experiments. Fish were humanely euthanized in a two-step process where they were anesthetized in tricaine methanesulfonate (Syndel Laboratories,Vancouver, BC, Canada) first, followed by spinal transection.
2.2.Preproghrelin and ghrelin receptor mRNA expression in various stages of zebra fish follicles
Female zebra fish (age ~8 month) were euthanized as described earlier and ovaries were removed. Pooled ovaries were placed in a Petri dish containing modified Cortland’s medium (Wu et al., 2000). Fish follicles have been classified into five distinct stages (Selman et al.,1993) based on their size. Stage l is up to 0.14 mm and transparent, and stage II is 0.15–0.34 mm with a halo. Both stages are known as previtellogenic follicles. Stage III (0.35–0.69 mm), known as the growth phase, is when vitellogenesis occurs. It was shown that small follicles from stage III (0.35–0.51 mm) do not respond to hormone treatment for maturation, whereas stage III-2 (0.52–0.68 mm) follicles are very sensitive to maturation-inducing hormone (Mih) (Pang & Ge, 2002; Selman et al., 1993; Wu et al., 2000). Follicle is opaque at stage IV (0.69–0.73 mm) where oocyte maturation/germinal vesicle breakdown (GVBD)occurs. Stage V (>0.73 mm) follicles are mature and transparent (Clelland et al., 2006). The ovaries were teared, and follicles were separated under a dissection microscope using forceps and transfer pipettes without trypsinization and grouped based on size as described above.Follicles were pooled from 4 to 5 fish were used for the experiment. A 24 well culture plate was seeded with 15–20 follicles/well with 1 mL of culture medium (4 well for each follicle stage) and used to quantify the mRNA expression in each developmental stage. Each experiment was repeated twice. Total RNA was obtained from each sample, cDNAs made, and RT-qPCR was conducted as described in section 2.4.
2.3.Acute food deprivation induced changes in preproghrelin, ghrelin receptor and goat mRNA expression in zebra fish ovary and testis
Four groups of fish (n =6/group) were used for this study. All animals were fed daily for 2 weeks before the experiment. From the first day of the experiment commencement, two groups continued to have access to food at the regular feeding time (11 a.m.), while 2 groups remained unfed. On days 3 and 7 of the experiment, one fed group and one fooddeprived group were euthanized, and testis and ovary were collected.Total RNA was extracted and cDNAs were synthesized and RT-qPCR was conducted as described in section 2.4.
2.4.Total RNA extraction, cDNA synthesis and qPCR quantification
The total RNA extraction from the ovary and testes samples from zebra fish was obtained as previously described (Hatef et al., 2015;Rajeswari et al., 2019) employing TRIzol (Invitrogen Canada Inc., Burlington, Ontario, Canada). All RNA samples underwent DNase 1 treatment (Thermo Fisher Scientific, Carlsbad, USA). The concentration and purity of total RNA was estimated from the OD absorbance measured at 260/280 nm using a Nanodrop 2000 (Thermo Fisher Scientific, USA).iScript cDNA synthesis kit (Bio-Rad Laboratories, Mississauga, ON,Canada) was used for cDNA synthesis. qPCR forpreproghrelin, ghrelin receptor(ghsr, GHS-Ra or GHS-R1a,the most prominent and widely studied ghrelin receptor) andgoatwere conducted on a CFX Connect(BioRad) using iQ SYBR Green Supermix (BioRad). The PCR conditions used were: 3 min at 95 ℃ and then 40 cycles of 10 s at 95 ℃, followed by 30 s at 60 ℃. Eukaryotic elongation factor 2 alpha 1 (eef2a1) was used as the internal control for each tissue sample. Livak method was used for qPCR data analyses (Livak & Schmittgen, 2001). Primers used are listed in Table 1.

Table 1List of forward and reverse primers based on zebra fish mRNA sequences used for RT-qPCR analysis.
2.5.Statistics
In vitrodata were analyzed using one-way ANOVA and Tukey’s multiple comparison test . Student’s t-test was used for food deprivation study data analyses. All data are presented as mean +SEM.P <0.05 were considered statistically significant. PRISM version 5 was used for all analyses (GraphPad Inc., USA).
3.Results
3.1.Preproghrelin and ghrelin receptor mRNA expression in zebra fish follicles
PreproghrelinmRNA in ovarian follicles was significantly more abundant at stages 1, 2 and 3I (Fig. 1A), compared to what was observed in stage of 3II, 4 and 5. Only 13.6% ofpreproghrelinmRNA expression was observed in the last stage follicle (5th stage) compared to stage one(Fig. 1A).GhsrmRNA abundance was significantly lower in stage 3II, 4,and 5 of follicle development compared to stage 1, 2 and 3I (Fig. 1B).
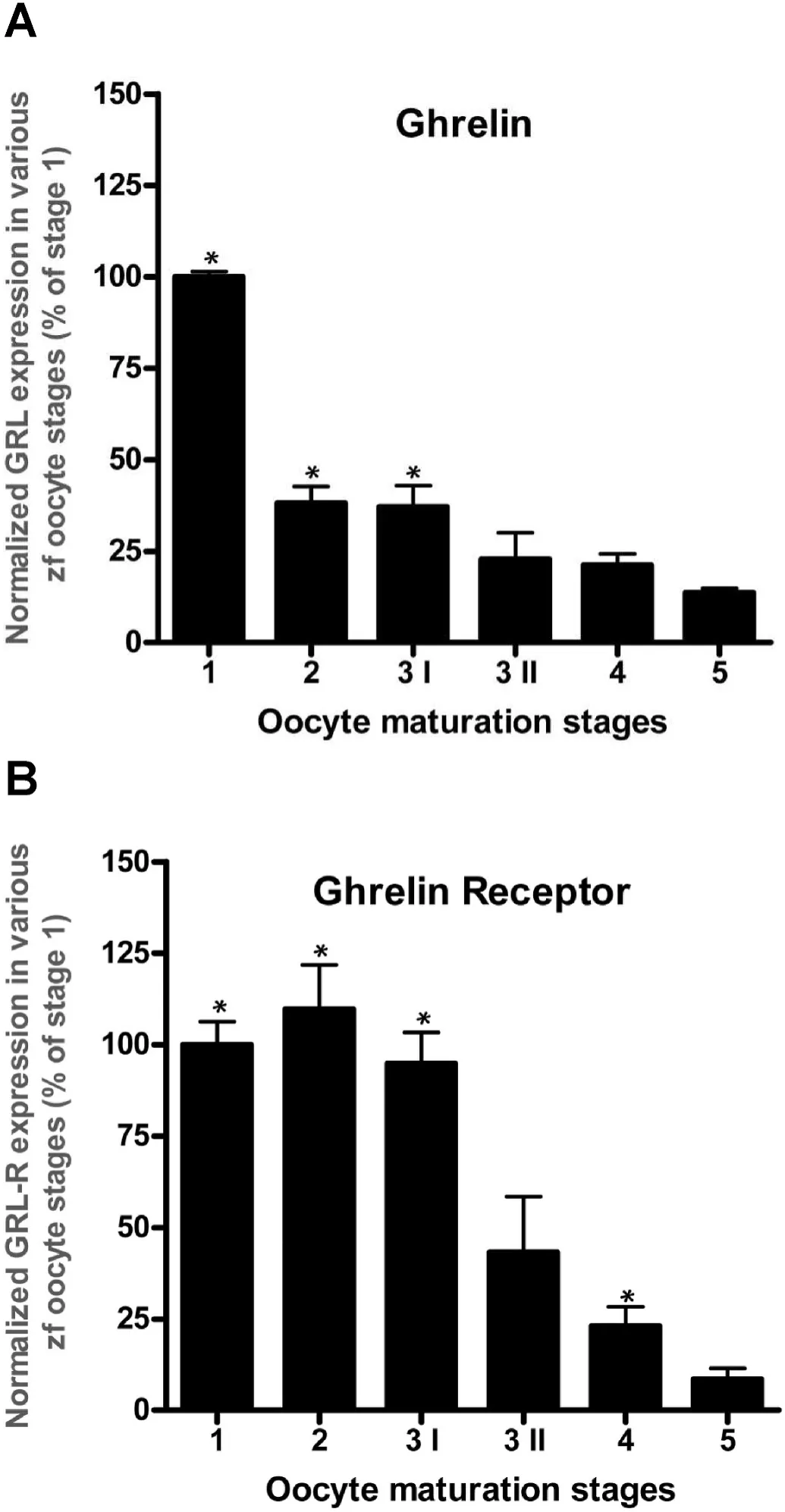
Fig. 1.Changes in the expression of ghrelin (A) and ghrelin receptor (B) mRNA in zebra fish follicles in five progressive developmental stages. Data are normalized to the housekeeping gene and expressed as a percentage of stage 1 follicles. Asterisks (*) represent significant differences in the expression with respect to the first stage of development. (P <0.05, n =6 zebra fish/group).
3.2.Food deprivation changed preproghrelin, ghrelin receptor and goat mRNA abundance in zebra fish ovary
A significant reduction inpreproghrelinandgoatmRNA abundance in the ovary was found in zebra fish on day 7 (Fig. 2A–B), but not on day 3(Fig. 2A–B) of food deprivation. Meanwhile,ghsrmRNA in the ovary was significantly downregulated only on day 3 (Fig. 2C).
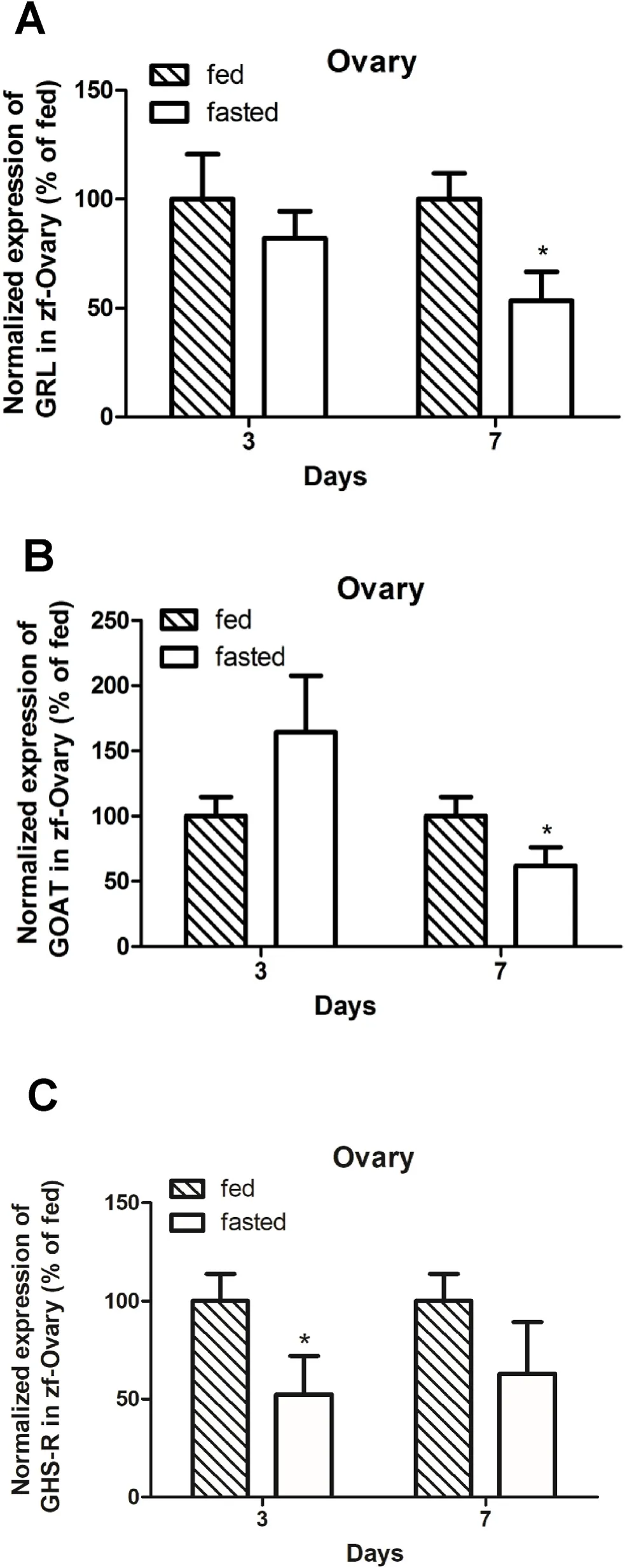
Fig. 2.Ghrelinergic system mRNA pro file in zebra fish ovary on days 3 and 7 post-food deprivation. Preproghrelin mRNA abundance was significantly decreased in the ovary of zebra fish deprived of food for 7 days (Fig. 2A). Food deprivation for 7 days also reduced the expression of goat mRNA in zebra fish ovary (Fig. 2B). Ghrelin receptor (ghsr) mRNA was significantly decreased in the ovary of zebra fish deprived of food for 3 days (Fig. 2C). Results are shown as mean +SEM of data (n =6 zebra fish/group). Student’s t-test was used for the statistical analysis. Asterisks (*) represent significant differences compared with fed group at the same time point (P <0.05).
3.3.Food deprivation changed preproghrelin, ghrelin receptor and GOAT mRNA expression in zebra fish testis
Preproghrelin, goatandghsrmRNA in the testis was significantly downregulated on day 7 of food deprivation (Fig. 3A–C). In addition,bothpreproghrelinandghsrmRNAs (Fig. 3A and D), notgoatmRNA(Fig. 3B) was significantly attenuated in the testis of zebra fish on day 7 of food deprivation.

Fig. 3.Ghrelinergic system pro file in zebra fish testis on days 3 and 7 post deprivation. Preproghrelin mRNA abundance was significantly decreased in the testis of zebra fish deprived of food for 3 or 7 days (Fig. 3A). Food deprivation for 7 days also reduced the expression of goat mRNA in zebra fish testis (Fig. 3B).Ghrelin receptor (ghsr) mRNA was significantly decreased in the testis of zebra fish deprived of food for 3 or 7 days (Fig. 3C). Results are shown as mean+SEM of data (n =6 zebra fish/group). Student’s t-test was used for the statistical analysis. Asterisks represent significant differences compared with fed group at the same time point (P <0.05).
4.Discussion
Our results revealed a decrease inpreproghrelinandghsrmRNA abundance with the progression of follicle maturation in zebra fish. It is possible that ghrelin has an inhibitory effect on follicle maturation at the final stages of follicle development. As the follicles develop, the endogenous ghrelin and its receptor levels fall to facilitate follicle maturation by attenuating the action of locally produced ghrelin. Previous research from our lab found that ghrelin inhibits stage IV GVBD and oocyte maturation (in vitro) in zebra fish (Shepperd et al., 2012).This agrees with our current findings that show a lower expression of ghrelinergic system during later stages of follicle maturation. A ghrelin receptor antagonist, D-lys3-GHRP-6, abolished the inhibitory effects of ghrelin on stage IV GVBD (Shepperd et al., 2012). This further con firms a direct role for ghrelin via its receptor on zebra fish follicles. The fact thatghsrmRNA decreased alongsidepreproghrelinmRNA further supports a general suppression of an oocyte maturation inhibitor at critical stages of follicle development. Overall, locally produced ghrelin and its receptor are downregulated as the oocytes progress through developmental stages. We have not studiedgoatexpression in the different stages of the follicle development. However, we expect a similar developmental expression considering the negative functions of ghrelin on oocyte maturation. Ghrelin mRNA expression is expressed in sheep follicles and the highest expression was observed in the development of the corpora lutea (CL) and lower expression in the regressing CL (Du et al., 2009). Ghrelin receptor expression was detected in the ovaries of humans (Gnanapavan et al., 2002), chicken (Sirotkin et al., 2006),spotted grouper (Chen et al., 2008) and pigs (Rak-Mardyla & Gregoraszczuk, 2010). This suggests a direct regulatory role of ghrelin on vertebrate follicles. Ghrelin stimulated ovarian estradiol secretion and aromatase activity in porcine ovarian follicles at prepubertal stage (Rak& Gregoraszczuk, 2008). The wide presence and development-specific expression of ghrelin and ghrelin receptor suggest that ghrelin could act as a nutritional and metabolic cue in fluencing reproductive maturation and function in vertebrate ovaries.
It is evident that metabolic hormones play an important role in the regulation of reproduction in fish (Hatef & Unniappan, 2019; Shahjahan et al., 2014). Interestingly, previous studies have shown an increase inpreproghrelinandgoatmRNAs in the brain and gut of unfed zebra fish(Amole & Unniappan, 2009; Hatef et al., 2015). It was also reported that circulating ghrelin in gold fish increase in response to fasting (Unniappan et al., 2004). Fish reproduction is very linked to energy balance (Mircea et al., 2007), and thereby metabolic abnormalities that can induce changes in the expression levels of ghrelin, ghrelin receptor and Goat could affect reproduction. At lower energy levels, when the amount of circulating ghrelin is possibly high, the ghrelin receptor expression in the ovary is decreased, reducing the functionality of ghrelin.
In our previous study, the ghrelinergic system was found upregulated in gold fish ovary after chronic food deprivation (Rajeswari et al., 2019).We speculated that this elevation probably helps suppress reproductive functions during food de ficiency and save energy for other body functions involving survival. In our current study, acute fasting decreased ghrelinergic system in zebra fish ovary. This difference in results might be due to several reasons including the duration of food deprivation (28 days in gold fish versus 3 or 7 days in zebra fish) and the differences in reproductive phases. Zebra fish breeds throughout the year (Spence et al., 2008) with females generally producing eggs once every one to three days. Meanwhile, gold fish is a seasonal breeder and the sexually mature period coincides spring and summer where more food is available. While such differences exist between species, locally produced ghrelin, and its receptor as well as the modifying enzyme are responsive to metabolic challenges. Ghrelin was found to suppress germinal vesicle breakdown in zebra fish follicles (Shepperd et al., 2012). If similar functions for ghrelin exist in gold fish, a possible reason for the upregulation of the reproductive inhibitor ghrelin is to spare energy by suppressing reproduction during energy challenges. The ghrelinergic system likely contributes to reproductive changes during metabolic challenges in zebra fish.
5.Conclusions
This study, while descriptive in nature, revealed important information interlinking the metabolic and reproductive roles of ghrelin in zebra fish. Developmentally, ghrelin and its receptor are more abundant in the early stages of ovarian follicles. A limitation of this research is the lack of information on Goat in different stages of the follicles, which warrants further studies. Future studies to determine the effects of food deprivation on oocyte maturation and roles ghrelin plays during such metabolic challenges warrant consideration. Further, ghrelin and its role in the reproduction of male fish remains unclear. The presence of the ghrelinergic system in the testis and its adaptation during food deprivation provides another reason to investigate ghrelin as a reproductive regulator in male zebra fish. Our study did not measure protein levels,and this is required in future research, especially those aiming to tease out locally produced ghrelin.
Funding
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada and the University of Saskatchewan through the Centennial Enhancement Chair in Comparative Endocrinology to Suraj Unniappan. Azadeh Hatef was a recipient of post-doctoral fellowships from the Canadian Institutes of Health Research (CIHR) and the Saskatchewan Health Research Foundation (SHRF). Jithine Jayakumar Rajeswari was a recipient of the WCVM-CGPS Ph.D. Scholarship.
Author contributions
Conceptualization: SU, AH, JJR; Data curation: AH, JJR; Funding acquisition: SU; Investigation: AH, JJR; Methodology: SU, AH, JJR;Project administration: SU, AH, JJR; Resources: SU; Software: SU; Supervision: SU; Validation: SU, AH, JJR; Visualization: AH, JJR; Roles/Writing – original draft: SU, AH, JJR; Roles/Writing – Review and editing: SU, AH, JJR.
Declaration of interest
None. Authors have no conflicts to disclose.
Declaration of competing interest
Declarations of interest: none.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Understanding the impact of stress on teleostean reproduction
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
