Kisspeptin stimulates oocyte maturation, and food deprivation modulates the abundance of kisspeptin system in zebra fish gonads
Azdeh Htef, Jithine Jykumr Rjeswri, Surj Unnippn ,*
aLaboratory of Integrative Neuroendocrinology, Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan,Saskatoon, Saskatchewan, S7N 5B4, Canada
bToxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B4, Canada
Keywords:
Oocyte
Kisspeptin
Food deprivation
GVBD
Gonadotropin
Testis
Ovary
Zebra fish
Oocyte maturation
A B S T R A C T
Kisspeptin is a hormone involved in the neuroendocrine control of reproduction in fish. We hypothesized that kisspeptin stimulates oocyte maturation and modulates other reproductive hormones in zebra fish; and the gonadal kisspeptin system in zebra fish is affected during energy unavailability. The main goals of this research were to test in vitro effects of kisspeptin on oocyte maturation and mRNA abundance in zebra fish ovarian follicles and determine how short-term feed restriction affects kisspeptin and its receptors in zebra fish testis and ovary.This study demonstrates the presence of kisspeptin and its receptors in zebra fish (Danio rerio) gonads and its direct action on ovarian follicles in vitro. Kisspeptin (10 ng/mL) induced oocyte maturation, as indicated by germinal vesicle break down at 18- and 24- hours post-treatment. Kisspeptin significantly increased the abundance of mRNAs encoding reproductive hormones and its receptors in zebra fish oocytes. This suggests that kisspeptin-10 affects ovarian functions by modulating other hormones. Reproduction is a process that requires energy. Therefore, whether energy availability affects the kisspeptin system in zebra fish gonads was determined.Food deprivation modulated kisspeptin expression differently in zebra fish testis and ovary. Kiss2 and kiss1ra were upregulated while kiss1rb was downregulated in the testis post-food deprivation. Meanwhile, no changes in kiss in the ovary were found after food deprivation. However, kiss1rb was downregulated in unfed fish at 3- and 7-days post-food deprivation. Overall, our results suggest sex- and tissue-specific changes in the gonadal abundance of the kisspeptin system in zebra fish. The fine tuning of reproduction during energy fluctuations in fish is likely mediated via changes in hormones, including kisspeptin as shown in this research.
1.Introduction
Kisspeptin is a RFamide peptide derived from thekiss1gene (Lee et al., 1996). Kisspeptin acts through the G-protein-coupled receptor 54(Gpr54), which is also known as the kisspeptin receptor (KissR) (Ohtaki et al., 2001). The processing of kisspeptin precursor results in several shorter peptides including Kp54, Kp16, Kp14, Kp13, and Kp10 (Beltramo et al., 2014). They share a highly conserved C-terminal 10 amino acid sequence (Kiss10), which is considered to be the core sequence required for its binding to KissR (Kotani et al., 2001; Muir et al., 2001).The roles of kisspeptin-KissR signaling in puberty and Gnrh-Lh secretion were originally reported in mammals (de Roux et al., 2003; Seminara et al., 2003; Uenoyama et al., 2019). The presence of kisspeptin receptor(Kissr2) cDNA in the Gnrh neurons of Nile tilapia (Oreochromis niloticus)provided the first evidence for the kisspeptin system in fish (Parhar et al., 2004). In fish, kisspeptin system is composed of two genes, Kiss1 and Kiss2 (Akazome et al., 2010; Biran et al., 2008; Elizur, 2009; Kitahashi et al., 2009; Mechaly et al., 2011; Song et al., 2016; Tovar Bohórquez et al., 2017; van Aerle et al., 2008; Li et al., 2009; Song et al.,2015). However, puffer fish (Shahjahan et al., 2010), Japanese flounder(Song et al., 2016), three-spined stickleback (Felip et al., 2009), and tilapia (Ogawa & Parhar, 2013) only express Kiss2. Although four genes encoding kisspeptin receptors have been reported, most fish only have two receptors, Kissr1 and Kissr2 (Pasquier et al., 2012, 2014) with a few exceptions (Akazome et al., 2010; Pasquier et al., 2012, 2014). Overall,the Kiss system is present and is evolutionarily conserved in fish.
A role for the Kiss system in the control of reproduction is reported in fish. Peripheral administration of synthetic kisspeptin induced pubertal onset and gonadal recrudescence in adult chub mackerel (Selvaraj et al.,2013). In male and female cinnamon clown fish (Amphiprion melanopus),treatment with human Kp10 upregulated Gnrh, gonadotropins (Gths;Fsh and Lh), Gth receptors, estrogen receptor and vitellogenin mRNAs and protein (Kim et al., 2014). Kisspeptins stimulatedgnrhexpression and gonadotropin secretion (Lhb and Fshb) and these effects were dependent on fish species, sex, reproductive stage, and mode of administration employed (Biran et al., 2008; Felip et al., 2009; Nocillado et al., 2013; Park et al., 2016; Shi et al., 2010; Zmora et al., 2012, 2014,2015). In zebra fish and chub mackerel, Kiss2 but not Kiss1 stimulated Gnrh and Gth synthesis and release (Espigares et al., 2015; Felip et al.,2009; Kitahashi et al., 2009; Ohga et al., 2014). In adult female chub mackerel, mean oocyte diameters which represent the early vitellogenin oocytes, increased after peripheral injection of Kiss1-15 after 6 weeks(three times at an interval of 2 weeks). However, the gonadosomatic index (GSI) did not show differences with saline and Kiss2-12 treated fish (Selvaraj et al., 2015). The same trend has been reported in many other prepubertal female fish, including white bass after a 7 week period of Kiss1-10 or Kiss2-10 treatment (Beck et al., 2012), striped bass after 10 weeks of Kiss1-15 treatment (Zmora et al., 2014), and in immature Nile tilapia after 4 weeks of Kiss2-10 treatment (Park et al., 2016).However, recent findings from Kiss or KissR gene mutant zebra fish have provided evidence that kisspeptins are dispensable for reproduction in fish (Liu et al., 2017; Nakajo et al., 2018; Tang et al., 2015). Gene manipulation studies have yielded similar results that revealed the complex and dispensable nature of other major reproductive regulators including Gnrh and Gth in fish reproduction (Marvel et al., 2018;Whitlock et al., 2019; Zhang et al., 2015). All these results suggest that fish reproduction requires a complex orchestration of different hormones to meet species-specific physiological challenges.
Energy balance and reproduction are likely linked by kisspeptins(Tena-Sempere, 2006; Popa et al., 2008). For example, it is known that kiss1 expression decrease during negative energy balance and suppresses reproduction in the rat (Fernandez-Fernandez et al., 2006).Although a functional relationship between food intake and reproduction has been well-established among fishes (Volkoff, 2016), little is known about the putative role of kisspeptin system in linking energy balance and reproduction in fish. Previous studies have shown that Kiss2 is involved in food intake and growth in fish. It has been proposed that kisspeptin could link feeding and reproduction (Escobar et al., 2016;Mechaly et al., 2011, 2018). Fasting increases hypothalamickiss2and upregulate pituitarylhandfshmRNAs in Senegalese sole(Solea senegalensis) (Mechaly et al., 2011). Correspondingly, food restriction was shown to increasekiss2andkissr2mRNA expression in the hypothalamus andfshbandlhbin the pituitary of the European sea bass(Dicentrarchus labrax)during spermatogenesis (Escobar et al., 2016). To our knowledge, there are no reports that show how nutritional status regulates Kiss/KissR in the gonads.
We hypothesized that kisspeptin stimulates oocyte maturation in zebra fish ovarian follicles and that nutrient unavailability/food deprivation adversely affects the expression of Kiss/KissR mRNAs in the gonads of zebra fish. Our specific aims were to (i) assess the direct effects of synthetic KP-10 on oocyte maturation in zebra fish, (ii) study the effects of KP-10 on mRNAs encoding receptors of Lh and Fsh and other local reproductive factors, and (iii) to determine the abundance of Kiss and KissR in the gonads of food-deprived zebra fish.
2.Materials and methods
2.1.Animal Care
Adult male and female (one year old, ~1.5 g weight) zebra fish were purchased from the Aquatic Toxicology Centre at the University of Saskatchewan and maintained in aerated recirculating 10 L tanks at 26± 2 ℃, under a 12-h light, 12-h dark photoperiod cycle. All fish fed once daily (2% body weight ration) at a scheduled feeding time (12:00 h)with a commercial slow sinking pellets (Aqueon, Franklin, USA). All studies using fish complied with the Canadian Council for Animal Care regulations and was approved by the Animal Research Ethics Board of the University of Saskatchewan (Protocol # 2012-0082).
2.2.In vitro oocyte maturation assay
Sexually mature female zebra fish were anaesthetised in 0.5% tricaine methanesulfonate (MS-222; Syndel Laboratories, Vancouver, BC,Canada) and ovaries were collected in modified Leibovitz’s L-15 medium . Oocyte maturation studies followes protocols previously published from our lab (Rajeswari et al., 2020; Rajeswari & Unniappan,2020a) and by others (Clelland et al., 2007; Murugananthkumar et al.,2017; Senthilkumaran et al., 2015). Individual follicles were separated from the ovaries of 5–7 fish using a transfer pipette and stage III-2 follicles (0.55–0.62 mm diameter) that are sensitive to maturation inducing hormones (Mih) (Clelland et al., 2007; Rajeswari and Unniappan, 2020a; Selman et al., 1993) were separated under a dissection microscope. Approximately 10 follicles were placed into each well of a 12 well plate in 1 mL modified Leibovitz’s L-15 medium supplemented with 10 ng/mL Mih [17α, 20β dihydroxyprogesterone], a potent inducer of oocyte maturation (Clelland & Peng, 2009) or with 10 or 100 ng/mL zebra fish Kiss1 for 6, 18 and 24 h. Commercially synthesized core sequences of zebra fish Kiss1 peptide (YNLNSFGLRY-NH2;MW: 1245.41, GeneScript, Canada) were used for the study. The follicles were incubated in the medium at 27 ℃. No peptides were added to the control groups. Germinal vesicle breakdown (GVBD) was used as an indicator of oocyte maturation. This was visualized under a dissecting microscope through changes in the appearance of follicles from opaque to translucent (stage 5 of oocyte maturation) (Nagahama & Yamashita,2008; Selman et al., 1993; Suwa & Yamashita, 2007). The number of follicles undergone GVBD was divided by the total number of follicles per well and was scored at 6-, 18- and 24 h post-incubation to assess the maturation rate. Each experiment was repeated 3 times with three wells per treatment in each experiment. Approximately 90 oocytes were used per treatment group. After 24 h incubation, oocytes were collected,frozen in liquid nitrogen and stored at -80 ℃ until total RNA extraction.To evaluate the abundance of luteinizing hormone receptor (Lhr), follicle stimulating hormone receptor (Fshr), 20 beta-hydroxysteroid dehydrogenase (20β-Hsd), membrane progestin receptor alpha (mPrα) and membrane progestin receptor beta (mPrβ) mRNAs after incubation with kisspeptin or Mih, total RNA extraction, cDNA synthesis and RT-qPCR were performed as described before (Hatef & Unniappan, 2017; Rajeswari & Unniappan, 2020b) and outlined under section 2.4. For mRNA abundance measurement, we selected one time point (24 h) where the effects of kisspeptin on oocyte maturation was significant.
2.3.Food deprivation and kiss/kissr mRNAs in zebra fish gonads
In this study, we investigated changes inkiss1, kiss2, kiss1ra and kiss1rbmRNA abundance in the testis and ovary of zebra fish after food deprivation over a 7-day period. Zebra fish (n =12) were acclimated at the regular feeding time (12 p.m.) for 2 weeks. After two weeks under this feeding schedule, 2 groups of fish stopped receiving food (fooddeprived group), while 2 other groups (fed group) continued to have access to food at the regular feeding time (12 p.m.). On days 3 and 7 of the experiment, one fed group (at 2 h post-feeding, 2 p.m.) and one fooddeprived group were anaesthetised by immersion in MS-222 followed by spinal transection. Brain and gonads were collected, flash-frozen in liquid nitrogen and stored at -80 ℃. Total RNA was extracted, cDNAs were synthesized, and RT-qPCRs forkiss1, kiss2, kiss1ra and kiss1rbwere conducted as described in 2.4.
2.4.Total RNA extraction, cDNA synthesis and RT-qPCR
Brie fly, total RNA extraction was performed using TRIzol Reagent(Product No:15596-026, Invitrogen, Burlington, ON, Canada). RNA concentrations were measured using a Nanodrop 2000 (Thermo, Vantaa, Finland), and RNA quality was verified using the A260nm/A280 nm(>1.8) and A230 nm/A260 nm (>2) ratios. cDNA was synthesized using the total RNA with iScript cDNA synthesis kit (Product No: 1708890,Bio-Rad, Mississauga, ON, Canada) following the manufacturer’s protocol. RT-PCR amplifications were performed on a final volume of 25 μL containing 0.4 mM deoxynucleotide triphosphates (dNTPs), 1.5 mM MgCl2, 1 unit of Taq DNA polymerase (product No: 18038018, Invitrogen, Carlsbad, CA, USA), and 0.4 μM of each specific primer and 1 μl of cDNA. PCR conditions included an initial denaturation step at 94 ℃ for 5 min, followed by denaturation: 94 ℃ (30 s), annealing: 55–60 ℃(30 s) and extension: 72 ℃ (45 s, 35 cycles), with final extension: 72 ℃(10 min). Zebra fish sequence-specific primers were used to amplify each gene (Table 1).
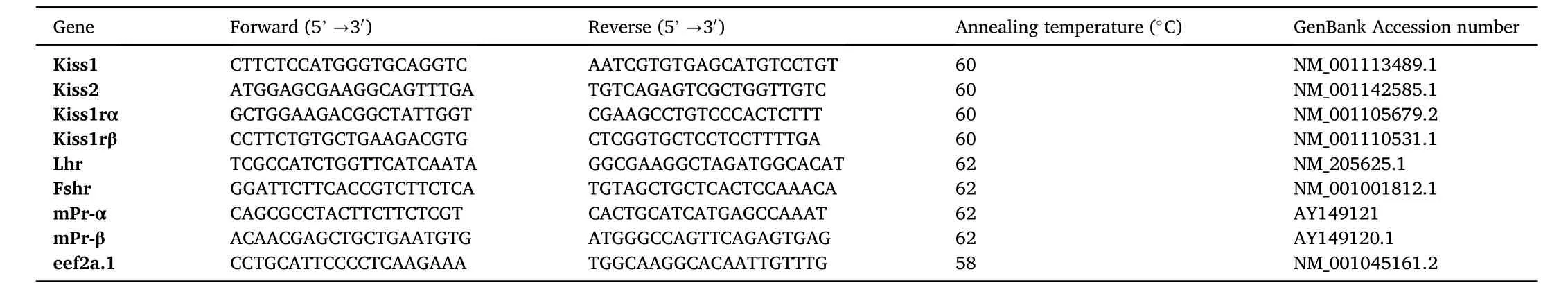
Table 1Details of Primers and Annealing Temperatures Used for RT and qRT-PCR.
For RT-qPCR, the reaction was set up using the Universal KAPA SYBR FAST qPCR Master Mix (2X) (Product No: KK4618, Kapa Biosystems,Wilmington, Massachusetts, USA) and performed on a CFX Connect(BioRad, Canada) qPCR machine. PCR conditions were, initial denaturation 95 ℃ (3 min), 35 cycle of denaturation: 95 ℃ (10 s), annealing(30 s): specific to each gene (Table 1), . The relative expression of target mRNAs was normalized against eukaryotic elongation factor 2 alpha 1(eef2a.1), as the reference gene, using a comparative cycle threshold(Ct) method (2-ΔΔCt) (Livak & Schmittgen, 2001). Results are expressed as a fold of abundance in controls/untreated samples.
2.5.Statistical analysis
Statistical significance was determined by Student’st-test (when two groups were compared) or one-way ANOVA (when more than two groups were compared). Homogeneity of variance and normal distribution of data were checked using Bartlett’s test before ANOVA. Tukey’s post-hoc test was used in conjunction with an ANOVA to find which means are significantly different from one another at each sampling time(P <0.05). All data are presented as mean +SEM. Graph Pad Prism version 5 (GraphPad Inc., IBM; USA) was used for statistical analysis and generation of graphs.
3.Results
3.1.Kiss/kissr mRNAs are present in zebra fish ovary and testis
An expected band size of approximately 150, 187, 127 and 179 base pairs respectively representingkiss1,kiss2,kiss1raorkiss1rbmRNA sequences were detected in samples containing cDNAs derived from the brain and gonads of zebra fish (Fig. 1). No amplicons were detected in the negative control.
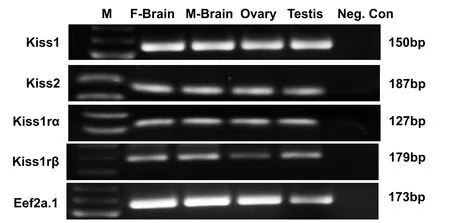
Fig. 1.Tissue distribution of kiss/kiss1r mRNA in zebra fish gonad and brain. Gel image showing RT-PCR amplicons for kiss1 (150 bp), kiss2 (187 bp), kiss1ra(127 bp), kiss1rb (179 bp) and eef2a.1 (173 bp) mRNAs in the female brain (F-Brain), male brain (M-Brain), ovary and testis. No amplicon was detected in the negative control (Neg. Con) PCR reaction without the template cDNA.
3.2.Kisspeptin upregulated key endocrine mRNAs in the ovarian follicles
Kisspeptin administered at a lower concentration (10 ng/mL)significantly increasedlhrmRNA abundance in ovarian follicles (Fig. 2).There were no significant changes inlhrmRNA abundance in oocytes incubated with Mih or 100 ng/mL kisspeptin compared to untreated controls. Incubation of follicles with kisspeptin or Mih did not changefshrmRNA abundance compared to controls (Fig. 2). Ovarian follicles treated with 10 ng/mL kisspeptin upregulated20βhsdmRNA abundance compared to controls (Fig. 2). However, no significant differences between 100 ng/mL kisspeptin, Mih and controls were observed (Fig. 2).BothmprαandmprβmRNA abundance was upregulated after incubation with 10 and 100 ng/mL kisspeptin and Mih compared to controls(Fig. 2).
3.3.Kisspeptin-10 stimulated oocyte maturation in zebra fish
Incubation of ovarian follicles with 10 ng/mL kisspeptin resulted in a significant increase in oocyte maturation at 18 and 24 h (Fig. 3B and C),but no such changes were found at 6 h (Fig. 3A). Meanwhile 100 ng/mL kisspeptin did not cause any change in oocyte maturation at 6 and 18 h compared to controls. However, a significant increase in oocyte maturation by 100 ng/mL kisspeptin was found at 24 h (Fig. 3C). Mih, the positive control caused the highest oocyte maturation rates at 6 (about 70%) (Fig. 3A) 18 (Fig. 3B) and 24 h (Fig. 3C).

Fig. 3.Kisspeptin stimulates germinal vesicle breakdown and oocyte maturation in zebra fish. Ovarian follicles incubated with kisspeptin at 10 ng/mL (KP-10) exhibited significantly higher maturation rate compared to the control at 18 and 24 h, but not 6 h (3B and 3C). Such an effect for 100 ng/mL(KP-100) was only found at 24 h (3C). Follicles incubated with Mih showed the significantly higher maturation rates at all time points tested (3A-C). The percentage of maturation increased with incubation time for oocytes incubated with kisspeptin, while those incubated with Mih seem to reach a peak after 6 h.Graphs represent pooled data from three independent study (n =3 wells/treatment, ~10 oocytes/well). One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical analysis. Asterisks (*) denote statistical differences between control and treatment groups. p <0.05 was considered statistical significance.
3.4.Food deprivation effects on kiss/kissr mRNA expression in the gonads of zebra fish
In the ovary,kiss1mRNA abundance was not significantly different between fed and unfed zebra fish at 3- and 7-day post-food deprivation(Fig. 4A–B). In the testis,kiss1mRNA significantly decreased in the 7-day food deprived group compared to fed group (Fig. 4D). However,no significant changes were observed in 3-day food deprived group(Fig. 4C). Thekiss2mRNA expression did not change after food deprivation in the ovary of zebra fish (5A and B). However,kiss2mRNA was found upregulated in the testis of 7-day food deprived fish compared to the fed group (Fig. 5D) and no significant changes were observed in 3-day food deprived group (Fig. 5C). In the ovary,kiss1ramRNA expression remained unchanged at 3- and 7-days post-food deprivation(Fig. 6A and B). In the testis,kiss1rawas upregulated at 3 days post-food deprivation (Fig. 6C). However, the testiskiss1ramRNA abundance was not significantly altered between fed and unfed zebra fish at day 7 postfood deprivation (Fig. 6D).kiss1rbmRNA was significantly reduced in the ovary of unfed zebra fish compared to those of fed fish at 3- and 7-days post-food deprivation (Fig. 7A and B).kiss1rbmRNA expression in the testis decreased significantly at 7 days post-food deprivation(Fig. 7D).
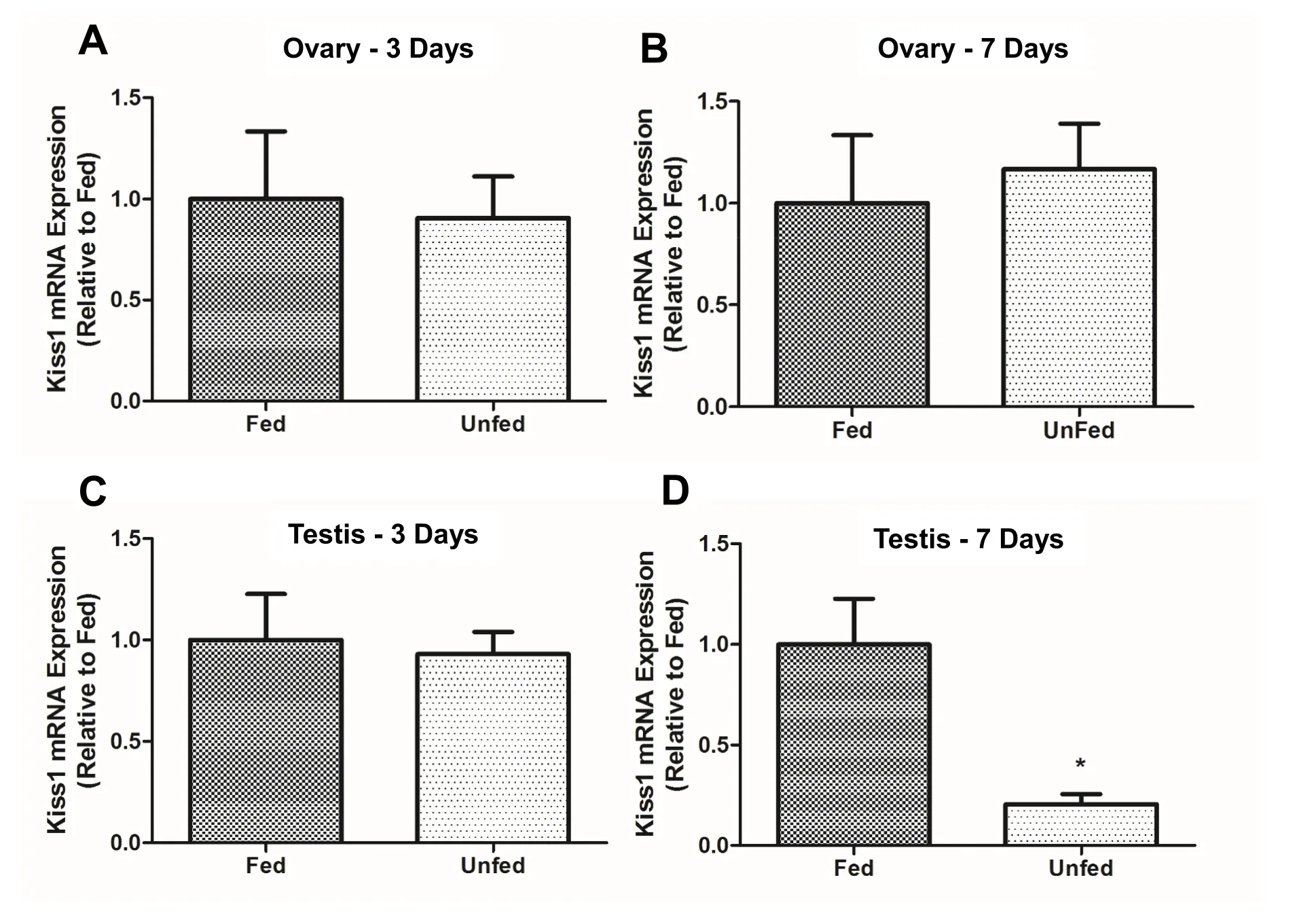
Fig. 4.Food-deprivation reduced the abundance of kiss1 in the testis of zebra fish. Food deprivation downregulated the expression of kiss1 mRNA in zebra fish testis at 7 days post deprivation (4D). No significant changes were observed in the testis (3-day food deprivation) (4C) and ovary (both 3- and 7-day food deprivation)of food-deprived groups compared to controls (4A-B). kiss1 mRNA expression was normalized to eef2a.1 and expressed relative to the fed group at each time point.Asterisks (*) represent significant differences between control(fed) and fasted group (p <0.05, n =6 zebra fish/group).
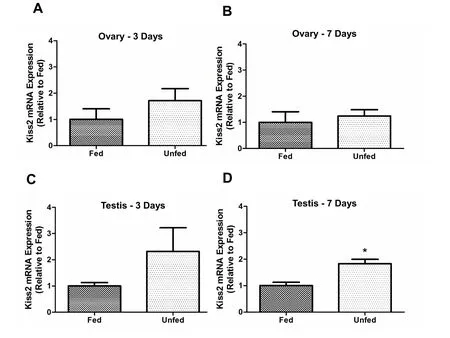
Fig. 5.Food-deprivation induced kiss2 mRNA abundance in zebra fish testis on 7-day post-food-deprivation. Kiss2 mRNA expression upregulated at 7 days post food deprivation in testis compared to fed fish. (5D). However, no significant changes were observed in the testis (3-day food deprivation) (5C) and ovary (both 3 and 7 day food deprivation) of fasted groups compared to control (5A-B) The kiss2 mRNA expression was normalized to eef2a.1 and expressed relative to the fed group at each time point. Asterisks (*) represent significant differences between control (fed) and fasted group (p <0.05, n =6 zebra fish/group).
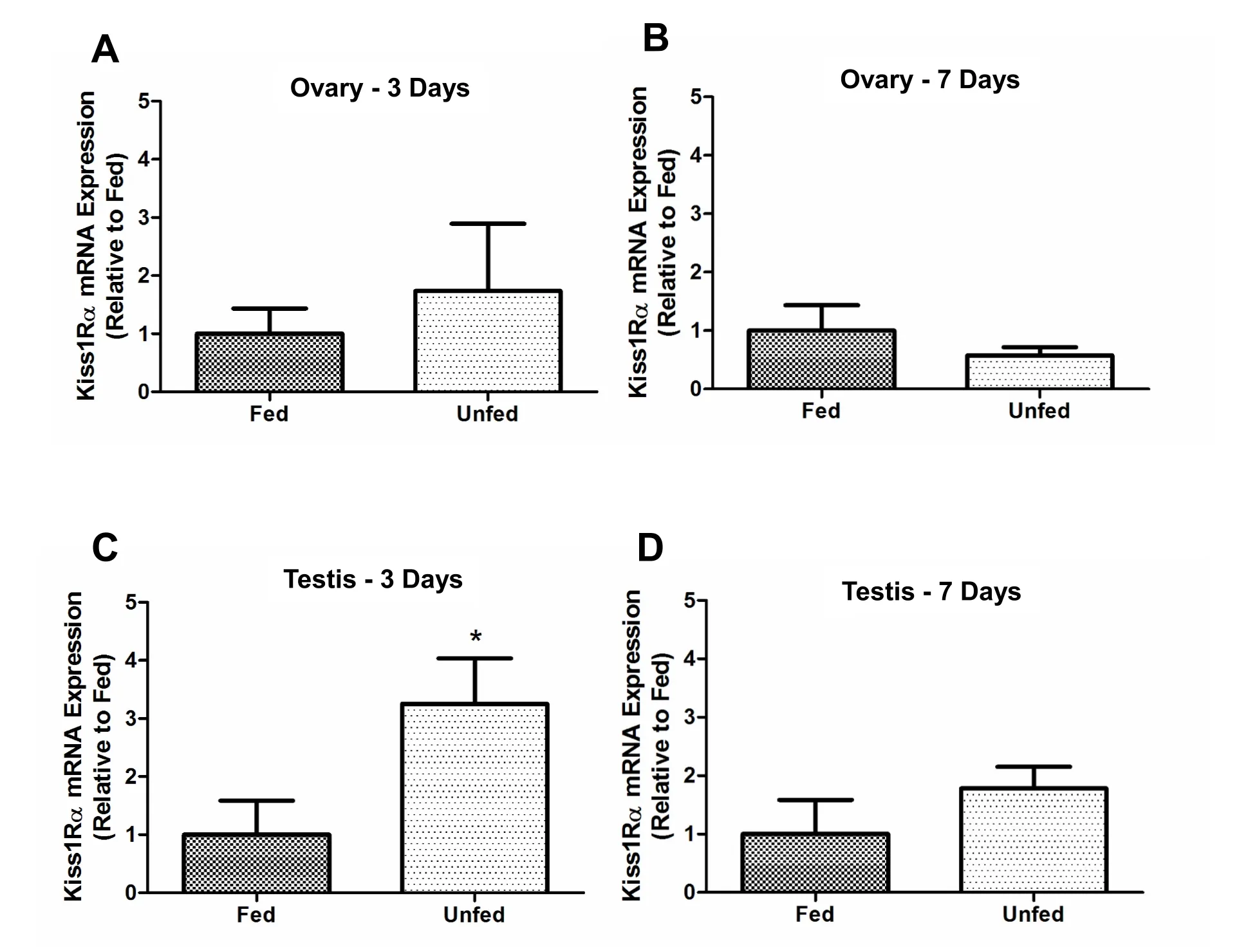
Fig. 6.Food deprivation upregulated kiss1ra mRNA abundance in zebra fish testis on 3-day post-food-deprivation. In testis, kiss1ra mRNA expression upregulated at 3 days post-food deprivations (6C) and no changes were observed on 7-day food deprived group (6D). In the Ovary, kiss1ra did not changed in unfed zebra fish compared to those of fed fish at 3- and 7-days post food deprivation (6A-B). The kiss1ra mRNA expression was normalized to eef2a.1 and expressed relative to the fed group at each time point. Asterisks (*) represent significant differences between control (fed) and fasted group (p <0.05, n =6 zebra fish/group).
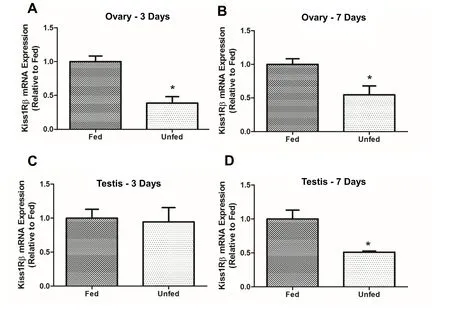
Fig. 7.Food deprivation affected kiss1rb mRNA expression in zebra fish gonads at 3- and 7-days post-food-deprivation. In the ovary, kiss1rb mRNA was downregulated in unfed zebra fish compared to those of fed fish at 3- and 7-days post-food deprivation (7A-B). However, kiss1rb mRNA expression decreased significantly in testis at 7 days post-food deprivation (7D) and no significant changes were observed in 3-day food deprived group (7C). The kiss1rb mRNA expression was normalized to eef2a.1 and expressed relative to the fed group at each time point. Asterisks (*) represent significant differences between control (fed) and fasted group (p <0.05, n =6 zebra fish/group).
4.Discussion
Our results demonstrate the presence ofkissand its receptors in the zebra fish tissues (brain, testis, and ovary) that are important for the regulation of reproduction in zebra fish. It also provides evidence for the sex (gonadal)-specific changes in the expression of kisspeptin system in response to feed restriction. The tissue distribution study shows thatkiss1,kiss2,kiss1raandkiss1rbmRNA are present in zebra fish ovary,testis, and brain. Kitahashi and colleagues reported that Kiss1 is expressed only in the testis, not in the ovary of zebra fish (Kitahashi et al.,2009). However, our results are similar with Biran et al. (2008) that reportedkiss1in both testis and ovary of zebra fish. It was also reported thatkiss1ra, notkiss1rbmRNA was expressed in the gonads of zebra fish,with highest abundance in the testis (Biran et al., 2008). The abundance of Kiss/Kissr system in the gonads of zebra fish suggest possible direct local actions of kisspeptin on gonadal gametogenesis and steroidogenesis. The expression of its receptors indicate that zebra fish gonadal cells are target of kisspeptin action. The roles and mode of action of locally produced kisspeptin on gonadal steroidogenesis and gametogenesis warrant further studies.
To study whether kisspeptin has any direct role in gamete development, we incubated zebra fish folliclesin vitrowith kisspeptin. Our results showed a stimulatory effect for kisspeptin on the maturation of oocytes in zebra fish. Kisspeptin exhibited a stronger and earlier effect in inducing oocyte maturation when it was administered at a lower concentration (10 ng/mL) compared to the higher dose (100 ng/mL) tested.The reason for the lack or attenuated response for the highest dose of kisspeptin used is likely due to receptor desensitization or downregulation. The positive control (Mih) shows the highest response in all three time points and is consistent with previous research (Gonzalez et al., 2012; Rajeswari et al., 2020; Rajeswari & Unniappan, 2020a;Shepperd et al., 2012). Kisspeptin is known to affect reproduction by acting on the brain by stimulating Gnrh neurons to release Lh and Fsh(Oakley et al., 2009) and affect gonadal maturation through their receptors, Fshr and Lhr that are important regulators of oocyte maturation.
Fsh signals the granulosa cells to convert testosterone to estradiol,which then facilitates the liver to release vitellogenin required for oocyte maturation. It is reported that serum levels of estradiol and testosterone are significantly elevated during final oocyte maturation (FOM) (Matsuyama et al., 2005) andkiss1andkiss2expression levels are highest during FOM in the spawning cycle in chub mackerel (Selvaraj et al.,2012). Lh signals the theca cells to convert 17 α-hydroxyprogesterone(17α-HP) into 17,20 β-dihydroxy-4-pregnen-3-ones or 17,20 βP (Mih).Mih signals through the receptors (Mprs) to activate maturation-promoting factor (Mpf), which induces oocyte maturation(Nagahama, 1987, 1994). Our result demonstrated that kisspeptin significantly upregulated the expression of Lhr, 20 βHd, mPrα and mPrβ mRNAs in zebra fish follicles. This suggests that kisspeptin affects oocyte maturation in zebra fish by in fluencing the local effectors of ovarian functions.
Our results show that the incubation of Kiss1 induced oocyte maturation after 18 h and these effects were comparable with Mih effects.Previous reports indicatein vivoadministration of kisspeptin promotes ovarian development in fish. For instance, after weeks of Kiss1or Kiss2 treatment, oocyte size increased in prepubertal striped bass (Zmora et al., 2014), Nile tilapia (Park et al., 2016) and chub mackerel (Selvaraj et al., 2013). It is possible that kisspeptin acts directly on the ovary to modulate the expression of thefshrandlhras we have seen in ourin vitroresults. This likely enables kisspeptin to better respond to Fsh and Lh and help induce oocyte maturation. High concentration of kisspeptin (100 ng/mL) did not cause any changes inlhrandfshr. However, a significant increase in oocyte maturation was found at 24 h. Further studies are required to understand the mechanism of action of kisspeptin at higher concentrations and the loss of effect when the dose increases.
The other factors that help in the oocyte maturation process and was found upregulated to kisspeptin in our study were mPrα and mPrβ.Previous studies have showed that mPrs are directly associated with oocyte maturation by mediating the effect of Mih (Thomas et al., 2004;Zhu et al., 2003). Our results also demonstrated that incubation of oocyte with 10 ng/mL kisspeptin significantly upregulated the abundance of 20β-Hsd. The 20β-Hsd is a key enzyme involved in the conversion of 17α-HP to Mih (17α-20 β) (Nagahama, 1994, 1997). In salmon, Lh increased 20β-Hsd in the granulosa cells of post-vitellogenic follicles prior to oocyte maturation (Nagahama & Adachi, 1985;Nagahama et al., 1983). We speculate that kisspeptin upregulation of 20β-Hsd caused Mih release and facilitated GVBD in our study. Taken together, our findings demonstrate that kisspeptin plays a physiological role in the zebra fish ovary to induce oocyte maturation. Whether kisspeptin produced in the gonads itself (autocrine/paracrine) contributes to oocyte maturation needs additional studies.
To determine the interplay between metabolic status and endogenous kisspeptin, the effects of food deprivation on gonadal kisspeptin system abundance in zebra fish was investigated. Fasting did not alterkissin the ovary and onlykissrbwas found decreased in unfed fish at day 3- and 7-days post-food-deprivation. In the testis, food deprivation caused a decrease inkiss1and increased the expression ofkiss2in 7-dayfood-deprived group compared to the fed group.kiss1rawas upregulated at 3 days post-food-deprivation andkiss1rbmRNA expression was downregulated in the testis at 7 days post food deprivation. The mostly positive changes in the local endogenous kisspeptin system in the gonads during energy restriction provides further evidence for its stimulatory role on zebra fish reproduction. Overall, our results suggest sex-specific differences in how nutritional challenges affect kisspeptin and its receptors in zebra fish gonads. It was reported that fasting increases hypothalamickiss2, and upregulates pituitarylhandfshmRNA in Senegalese sole(Solea senegalensis)(Mechaly et al., 2011). Similarly,food restriction was shown to increasekiss2andkissr2gene expression in the hypothalamus andfshbandlhbin the pituitary of the European sea bass(Dicentrarchus labrax)during spermatogenesis (Escobar et al.,2016) and in zebra fish (London & Volkoff, 2019). These results are in contrast to what has been observed in mammals (Fernandez-Fernandez et al., 2006). Such species- and tissue-specific differences must be considered while interpreting the information on energy availability,kisspeptin and reproduction in fish.
5.Conclusions
Taken together, these results suggest that zebra fish kisspeptin positively affects oocyte maturation by in fluencing the local effectors of ovarian functions. Kisspeptin regulates several factors, which are already known affect oocyte maturation (Clelland & Peng, 2009). While our research only considered mRNA abundance, not protein or activity,the outcomes agree with what has been reported previously. A future direction is to test whether kisspeptin affects sperm biology in zebra fish.In vivostudies, including I.P injections or infusion of kisspeptin may also provide more accurate information on the long-term effects of kisspeptin on oocyte and sperm maturation. While caveats exist, this research provides some new insights into how kisspeptin might help regulate reproduction by acting locally in the gonads of zebra fish.
Author contributions
Conceptualization: SU, AH, JJR; Data curation: AH, JJR; Funding acquisition: SU; Investigation: AH, JJR; Methodology: SU, AH, JJR;Project administration: SU, AH, JJR; Resources: SU; Software: SU; Supervision: SU; Validation: SU, AH, JJR; Visualization: AH, JJR; Roles/Writing – original draft: SU, AH, JJR; Roles/Writing – Review and editing: SU, AH, JJR.
Declaration of competing interest
None.
Acknowledgements
This research was supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to S.U.Research facilities used were funded by the Canada Foundation for Innovation-John R. Evans Leaders Fund, an Establishment Grant from the Saskatchewan Health Research Foundation (SHRF), and the University of Saskatchewan Centennial Enhancement Chair in Comparative Endocrinology to S.U. A.H. is a recipient of postdoctoral fellowships from the SHRF and the CIHR. JJR is a recipient of the WCVM-CGPS PhD fellowship.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Understanding the impact of stress on teleostean reproduction
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
