Seasonal dynamics, kinetics, and effects of 2-hydroxyestradiol-17β on some steroidogenic enzymes in the ovary of the cat fish Heteropneustes fossilis
Tapan Kumar Chourasia, Radha Chaube, Keerikkattil Paily Joy
Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, 221005, India
Keywords:
Cat fish ovary
Steroidogenic enzyme activity and kinetics
2-Hydroxyestradiol-17β
Steroidogenic shift
Human chorionic gonadotropin
A B S T R A C T
The synthesis of bioactive steroids is catalyzed by an array of enzymes of diverse properties and actions. In the present study, seasonal dynamics and kinetics of key steroidogenic enzymes, 17α-hydroxylase (Cyp17a), 3βhydroxysteroid dehydrogenase (Hsd3b), 20α-hydroxysteroid dehydrogenase (Hsd20a) and 20β-hydroxysteroid dehydrogenase (Hsd20b) were investigated in the female cat fish Heteropneustes fossilis. Further, the effects of the estrogen metabolite 2-hydroxyestradiol-17β (2-OHE2) and human chorionic gonadotropin (hCG) on activities of the above enzymes, cytochrome P450 aromatase (Cyp19a1), and steroid products including testosterone and cortisol were determined. The enzymes under investigation showed significant seasonal variations across the annual ovarian cycle with low activity in the gonad resting phase. The enzymes Hsd3b and Cyp17a showed high activity during early oogenesis but the activities of Hsd20a and Hsd20b were higher towards late oogenesis in the spawning phase. Hsd3b and Cyp17a elicited high apparent Km values (low substrate affinity) and high apparent Vmax in the vitellogenic phase compared to the postvitellogenic phase. Hsd20a did not elicit any significant differences in the kinetic parameters between the two phases. Hsd20b showed high apparent Km values (low substrate affinity) and high Vmax in the postvitellogenic phase. The incubation of ovarian slices with 2-OHE2 for 24 h increased dose-dependently Hsd3b, Cyp17a, Hsd20a and Hsd20b activities, similar to hCG. The levels of the corresponding C21 steroid products, progesterone (P4), 17α-hydroxyprogesterone (17-OHP4), 17, 20α-dihydroxy-4-pregnen-3-one (17,20α-DP) and 17,20β-dihydoxy-4-pregnen-3-one (17,20β-DP), and cortisol were elevated.However, 2-OHE2 decreased significantly the C19 and C18 steroids, testosterone and E2 levels, and Cyp19a activity. The co-incubation with hCG and 2-OHE2 produced a synergistic effect on the enzyme activities except that of CYP19a. The co-incubation reversed the inhibitory effect of 2-OHE2. The data show that 2-OHE2 exerts a dual role on steroidogenesis, stimulating the C21 pathway and inhibiting the C19–C18 pathway, resulting in the steroidogenic shift.
1.Introduction
A majority of fishes are seasonal breeders and exhibit annual or multiple reproductive cycles (reviews: Lam, 1983; Sundararaj, 1981, pp.1–82). Follicular growth (vitellogenesis), maturation and ovulation in females, and spermatogenesis and spermiation in males are in fluenced by the interactions of external environmental variables such as photoperiod, temperature, food, stress, etc., with the brain-pituitary- gonad(BPG) - endocrine axis to secrete sequentially gonadotropin-releasing hormone (GnRH), gonadotropins and steroids (reviews: Kah, 2009;Van der Kraak et al., 2009; Cardinaletti et al., 2010). Gonadotropins(FSH and LH) stimulate steroidogenesis and the steroid hormones are the tertiary regulators controlling specific events in gametogenesis and spawning. The ovarian cycle is conventionally divided into previtellogenic, vitellogenic (primary and secondary growth), and post vitellogenic maturational stages (Khan & Thomas, 1999). Estrogens especially estradiol-17β (E2) is the major estrogen synthesized in the vitellogenic phase (reviews: Fostier et al., 1983; Babin et al., 2007). With the completion of vitellogenesis, E2synthesis is decreased and under appropriate spawning conditions for the induction of a preovulatory LH surge, the post vitellogenic follicles initiate maturational activity(resumption of meiosis) under the in fluence of a maturation-inducing hormone (MIH). The MIH is a progestin derivative, either 17,20β-dihydroxy- 4-pregnen-3, 20-dione (17, 20β-DP) or 17, 20β, 21-trihydroxy-4-pregnen-3-one (20β-S) (Nagahama et al., 1985, 1997; Trant &Thomas, 1988). Thus, during the follicular growth and maturation, a shift steroidogenesis occurs from the estrogenic phase to the MIH phase(Nagahama et al., 1995; Young et al., 2005).
Steroidogenesis is controlled by an array of enzymes, many of which are conserved, and the steroidogenic pathways are characterized in a number of teleost species (Fostier et al., 1983; Young et al., 2005; Kime,1987; Tokarz et al., 2015; Tenugu et al., 2021). Though the steroidogenic organs have the capability to produce all classes of steroids, the pathways of functional steroids like androgens, estrogens, progestins and corticosteroids are dominant in an organ-specific manner. The enzymes can be broadly grouped into two categories; those modifying the steroid nucleus by side-chain cleavage, Δ5- Δ4- isomerization, hydrogenation, aromatization [cholesterol side-chain cleavage (Cyp11a1),3β-hydroxysteroid/Δ5- Δ4-isomerase (Hsd3b), 17α-hydroxylase/lyase(Cyp17a), and cytochrome P450 aromatase (Cyp19a1)], and those adding or modifying functional groups by hydroxylation, reduction or oxidation (Hsd17b and Hsd20b) (Young et al., 2005; Tokarz et al.,2015). The enzymes Hsd3b and Cyp17a are common to all steroidogenic organs and control major steroid conversions. Hsd3b is important in the formation of Δ4– ketosteroids from Δ5-hydroxysteroids and converts pregnenolone (P5) to progesterone (P4) and 17-OHP5to 17-OHP4.This step is crucial to the formation of C21corticosteroids and progestins, C19androgens and C18estrogens (Tenugu et al., 2021). Hsd3b exists as a single protein in fishes like Japanese eel (Kazeto et al., 2003) and cat fish(Singh & Singh, 1985; Bhat et al., 2018) or as two proteins in teleosts like Nile tilapia and black porgy (Senthilkumaran et al., 2009; Lin et al.,2015). Cyp17a is essential for the conversion of P5and P4to 17-OHP5and 17-OHP4, respectively, and subsequently in the synthesis of the down-stream major functional steroids like cortisol, progestins, androgens and estrogens. Cyp17a exhibits both hydroxylase and lyase activities in Japanese eel (Kazeto et al., 2000; Su et al., 2015) and African cat fish (Sreenivasulu & Senthilkumaran, 2009a) but exists in two forms in Nile tilapia and medaka (Zhou, Wang, Kobayashi, et al., 2007, b).Cyp17a1 possesses both hydroxylase and lyase activities while Cyp17a2 possesses only hydroxylase activity. Hsd20b is characterized in the ovary and testis of many species and is responsible for the formation of the maturation-inducing hormone 17,20β-dihydoxy-4-pregnen-3-one(MIH, 17,20β-DP), which plays a crucial role in final oocyte maturation(FOM) and spermiation (Nagahama et al., 1985; Scott & Canario, 1987;Nagahama, 1997; Young et al., 2005; Tokarz et al., 2015; Tenugu et al.,2021). The enzyme converts 17-OHP4to 17, 20β-DP in the granulosa and developing sperm. Hsd20a, on the other hand, catalyzes 17-OHP4to 17, 20α-dihydroxy-4-pregnen-3-one (17, 20 α-DP), which is an isomer of 17, 20β-DP. Though 17, 20α-DP has been identified in teleosts (Canario& Scott, 1989; Kime et al., 1993; Kime & Scott 1993, 1993, 1993;Schoonen et al., 1987; Thibaut & Porte, 2004; van den Hurk et al.,1987), studies on the physiological role of Hsd20a and its product in fish gonads are meager. The involvement of 17, 20α-DP in the induction of FOM and inhibition of estrogen hydroxylase activity was previously reported but these effects were low compared to 17,20β-DP in the cat fish(Chourasia & Joy, 2010).
Cyp19a is the most well studied steroidogenic enzyme due to its pivotal role in the synthesis of estrogens and participation in sex differentiation and ovarian growth (Devlin & Nagahama, 2002; Young et al., 2005; Guiguen et al., 2010; Tokarz et al., 2015; Tenugu et al.,2021). The enzyme is conserved in the vertebrate phylogeny and two forms, the ovarian type (Cyp19a1a) and brain type (Cyp19a1b), exist in the cat fish (Chaube et al., 2015). The teleost gonads are unique in that steroids undergo catabolism similar to liver and kidney (Kime, 1987;Schoonen et al., 1987). In the ovary of cat fishes and zebra fish, E2is converted into catecholestrogens (hydroxyestrogens) bycyp1a1andcyp1b1(estrogen hydroxylases), which are methylated to methoxyestrogens) by catechol-o-methyltransferase (Senthilkumaran & Joy, 2001;Mishra & Joy, 2006a; Chourasia et al., 2015; Chaube et al., 2017, 2021).The hydroxyestrogen 2-OHE2stimulates MIH and prostaglandin (PG)synthesis, and oocyte maturation (FOM) and ovulation (Senthilkumaran& Joy, 2001; Mishra & Joy, 2006a-d; Chourasia & Joy, 2012a, b). In both cat fish and zebra fish, 2-OHE2inhibited Cyp19a (P450 aromatase)activity but stimulated estrogen-2/4-hydroxylase activity (Chourasia et al., 2015; Chourasia & Joy, 2008b, 2010), resulting in E2depletion. It is postulated that formation of 2-OHE2signals the steroidogenic shift from estrogenic to progestin synthesis at the onset of FOM (see review:Joy & Chaube, 2013). It is not investigated whether 2-OHE2actions are limited only to the estrogen metabolism and functions, or affect steroidogenesis in general.
The steroidogenic enzyme transcripts and activity, and their product(steroids) levels vary with the gonad development and are regulated by the gonadotropins (Fostier et al., 1983; Kazeto et al., 2000, 2001, 2003;Nakamura, Kusakabe, Swanson, & Young, 2016). The prevalence of the Δ4pathway or Δ5pathway varies with respect to species, gender and developmental stages of the gonads (see review: Tenugu et al., 2021).The different steroidogenic patterns, the occurrence of one or more enzyme proteins and multiple substrate availability are factors that determine enzyme catalytic functions. To our knowledge, a few species have been investigated to analyze the kinetics of steroidogenic enzymes in teleost gonads (Eckstein & Azoury, 1979; Singh & Singh, 1985; Zhao et al., 2001; Chourasia & Joy, 2008b). Changes in kinetic properties are useful to explain the mechanisms underlying seasonal variations in the enzyme activity. The Michalis-Menten constant (Km) and maximum velocity (Vmax) for microsomal Cyp19a were reported in the cat fishH. fossilis(Chourasia & Joy, 2008b). Therefore in continuation of the previous study, we now report the seasonal dynamics and kinetics of Hsd3b, Cyp17a, Hsd20a and Hsd20b, and effects of 2-OHE2on these enzyme activities. Also, the data on the effects of human chorionic gonadotropin (hCG) on the enzyme activities were presented for comparisons. The experiments were carried out in vitellogenic (VT) and postvitellogenic (PV) ovaries, representing the peak vitellogenesis phase and the completed phase, respectively.
2.Material and methods
2.1.Chemicals
Steroids nomenclature is according to Steraloids Inc. (Newport, R. I.,USA) and the trivial names are used in parentheses. 1,3,5 (10) Estratriene-3,17β-diol (estradiol-17β, E2), 1,3,5 (10) estratriene-3,16α,17βtriol (estriol), 4-Androsten-17β-ol-3-one (testosterone, T), 4-Pregnen-11β,17,21-triol-3,20-dione (cortisol, F), 5-Pregnen-3β-ol-20-one (pregnenolone, P5), 4-pregnene-3,20-dione (progesterone, P4), 4-Pregnen-17α-ol-3,20-dione (17-hydroxyprogesterone, 17-OHP4), 4-Pregnen-17α,20β-diol-3-one (17α,20β-dihydroxyprogesterone, 17,20β-DP), 4-Pregnen-17α,20α-diol-3-one (17α,20α-dihydroxy-progesterone, 17,20α-DP), nicotinamide adenine dinucleotide phosphate reduced (NADPH)and bovine serum albumin (BSA) were purchased from Sigma Chemical Co., St. Louis, USA. The chemicals were of the highest purity grade set by American Chemical Society: ≥99% pure). 1, 3, 5, (10) estratriene-2, 3,17β-triol (2-hydroxyestradiol-17β, 2-OHE2) (CAS number: 362-05-0)was purchased from Steraloids Inc. Newport, R. I., USA. The ELISA kits cortisol (catalog no. DKO001), T (catalog no. DKO002), E2(catalog no. DKO003), 17-OHP4(catalog no. DKO004) and P4(catalog no.DKO006) were purchased from Dia Metra, Italy. Monobasic Nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate(G-6-P), and glucose-6-phosphate dehydrogenase were purchased from Sisco Research Laboratories Pvt. Ltd., India. Monobasic sodium phosphate, dibasic sodium phosphate, potassium chloride, sodium potassium tartrate, EDTA, sodium carbonate, sodium hydroxide, Folin Ciocalteu reagent, copper sulfate, acetonitrile (HPLC grade), glycerol and diethyl ether were purchased from E. Merck, New Delhi, India. Degassed and filtered nano pure water (Barnstead, USA) was used throughout chromatography. Other chemicals were of analytical grade and procured locally.
2.2.Animal collection and acclimatization
The experiments were conducted in accordance with local/national guidelines for experimentation in animals and approved by the animal ethics committee of Banaras Hindu University. All care was taken to prevent cruelty of any kind.Heteropneustes fossilisis a freshwater, airbreathing cat fish whose reproductive cycle can be divided into resting(November–January), preparatory (February–April), prespawning(May–June), spawning (July–August) and postspawning (September–October) phases (Chourasia & Joy, 2012a; Sundararaj, 1981, pp.1–82).
2.3.Seasonal study
AdultH. fossilis(30–50 g) were collected from local fish markets in the resting (December), preparatory ( first week of April), prespawning(June), spawning (July), and postspawning (September) phases. About 40–50 female fish were selected and maintained in flow-through cement tanks (76 × 48 × 40 cm) of 100-L capacity under natural photothermal conditions. They were fed daily minced goat liverad libitum.The acclimatized fish were anesthetized with MS222, weighed and sacrificed by decapitation. Ovaries were removed carefully on ice. The ovaries were weighed and gonadosomatic index (GSI) was calculated as gonad weight/body weight ×100 and expressed in percentage of the body weight. The ovaries were stored at -80 ℃ for a period up to 48 h, prior to microsome preparation.
2.3.1.Mitochondrial and microsomal preparation
Ovaries from five fish in each phase/experiment were pooled to form a sample and 5 such pooled samples constituted a group. The subcellular fraction was prepared by differential centrifugation of the homogenized tissues with slight modifications in buffers for the ovarian tissue (Shimada, Miura, & Imamura, 2006). All procedures were carried out at 4 ℃. The ovaries were minced and homogenized in a homogenization medium (100 mM potassium phosphate buffer pH 7.4 containing 100 mM KCl and 1 mM EDTA; 3 ml homogenization medium/g tissue) with an ultrasonicator (XL-2000, Microson, Misonix, USA) on ice bath. The homogenate suspension was centrifuged at 500×gfor 10 min to remove intact cells, macroscopic debris and nuclei, and the supernatant was removed and centrifuged at 15,000×gfor 20 min. The pellet containing mitochondria was stored as the mitochondrial fraction and the supernatant was removed and centrifuged at 105,000×gfor 60 min to obtain the microsomal pellet. The isolated mitochondrial and microsomal protein pellets were washed once with 100 mM potassium phosphate buffer pH 7.4 and suspended in a small volume of 100 mM potassium phosphate buffer pH 7.4. Total protein content in the pellet was assayed by the method of Lowry et al. (1951) using BSA as a standard.
2.4.Assay of Hsd3b activity
The mitochondrial and microsomal preparations obtained from the homogenate by differential centrifugation were pooled to make the enzyme source. Hsd3b activity was determined by measuring the conversion of P5to P4in mitochondrial plus microsomal fraction, according to Suzuki et al. (1980) with modifications. The enzyme preparation (1 mg/tube) was incubated in 1 mL incubation medium consisting of 0.25 mM NADP, 6.25 mM glucose-6-phosphate, 0.25 U glucose-6-phosphate dehydrogenase, 6.25 mM MgCl2, 0.25 mM NADPH, 100 μM pregnenolone (substrate), and 100 mM of sodium potassium phosphate buffer pH 7.4 in a 10 mL reaction tube. Reactions were carried out at 30 ℃ in a shaking bath for 30 min. The incubations were terminated by the addition of an equal volume of ethyl acetate. Steroids were extracted three times with 10 vol of cold diethyl ether. The extracts were evaporated to dryness under mild flow of nitrogen stream and reconstituted with ethanol: water (1:10 v/v), and the assay product P4was quantified by a commercially available competitive ELISA kit.
2.5.Assay of Cyp17a activity
Cyp17a activity was determined by measuring the conversion of P4to 17-OHP4in the microsomal fraction, as described by Matsunaga et al.(2001) with modifications. The microsomal preparation (1 mg/tube)was incubated in 1 mL incubation medium containing 0.25 mM NADP,6.25 mM glucose-6-phosphate, 0.25 U glucose-6-phosphate dehydrogenase, 6.25 mM MgCl2, 0.25 mM NADPH, 100 μM P4(substrate), and 100 mM of sodium potassium phosphate buffer pH 7.4 in a 10 mL reaction tube. Reactions were carried out at 30 ℃ in a shaking bath for 30 min. The incubations were terminated by the addition of an equal volume of ethyl acetate. Steroids were extracted as described above. The assay product 17-OHP4was quantified by a commercially available competitive ELISA kit.
2.6.Assay of Hsd20a and Hsd20b activity
Hsd20a and Hsd20b activities were determined in the microsomal fraction by measuring the conversion of 17-OHP4to 17, 20α-DP or 17,20β-DP according to the method of Shimada et al. (2006) and Thibaut and Porte (2004), respectively, with slight modifications. Brie fly,the microsomal preparation (1 mg/tube) was incubated with 1 ml incubation medium consisting of 0.25 mM NADP, 6.25 mM glucose-6-phosphate, 0.25 U glucose-6-phosphate dehydrogenase, 6.25 mM MgCl2, 0.25 mM NADPH, 100 μM 17-OHP4(substrate), and 100 mM of sodium potassium phosphate buffer pH 7.4 in a 10 ml reaction tube.Reactions were carried out at 30 ℃ in a shaking bath for 30 min. The incubations were terminated by the addition of an equal volume of ethyl acetate. Steroids were extracted as described above. The extracts were evaporated to dryness under a mild flow of nitrogen stream and reconstituted with acetonitrile: water (1:1v/v) and injected onto the reverse phase HPLC column. The reduction products 17,20α-DP and 17,20β-DP were determined by HPLC-UV detection (see below).
2.7.Assay of cytochrome P450 aromatase (Cyp19a1) activity
Cyp19a1 activity was assayed by the competitive ELISA method according to Matsui et al. (2005) with modifications, as described in our earlier study (Chourasia & Joy, 2008a). Brie fly, the microsomal preparation (1 mg/tube) was incubated with 1 ml incubation medium consisting of 1.7 mM NADP, 2.8 mM glucose-6-phosphate, 1.0 IU glucose-6-phosphate dehydrogenase, 3.3 mM magnesium chloride, 0.1 mM NADPH, 50 nM testosterone (substrate) in 100 mM potassium phosphate buffer (pH 7.4) in a 10 ml reaction tube and incubated for 30 min at 28 ℃ under continuous shaking in a water bath. The incubations were terminated by the addition of an equal volume of ethyl acetate.Steroids were extracted as described above. The assay product E2was quantified by a commercially available competitive ELISA kit.
For all enzyme assays, 30 min boiled microsome preparations (denatured enzyme source) were used as the blank for the non-enzymatic conversion and the values were subtracted from the sample values.Enzyme activity was expressed as pmol P4formed/mg protein/30 min.
2.8.Enzyme kinetic study for determination of Km and Vmax
Adult female cat fish were collected in April (late preparatory phase;vitellogenic (VT) phase, GSI =5.35 ±0.56%) and June (prespawning phase; post-vitellogenic (PV) phase, GSI =9.06 ±0.11%). Michaelis-Menten constant (Km) for the substrate and maximum velocity (Vmax)was determined by incubating the individual enzyme reaction mixture,as mentioned above, in triplicate with 0, 25, 75, 100, 150, 200, 300, 400 and 600 nM of the respective enzyme substrates for 5 min according to the methods, described above. The apparent Kmand Vmaxwere calculated from the double reciprocal Lineweaver-Burk (LB) plots (Price &Stevens, 1999, pp. 118–153). The Kmand Vmaxwere calculated from the intercepts (-1 /Kmand 1/Vmax, respectively) on the X and Y-axis of the LB plots.
2.9.In vitro effects of 2-hydroxyE2 (2-OHE2) on steroidogenic enzymes and steroid secretion
In both preparatory (VT) and prespawning (PV) phases, acclimatized female cat fish were sacrificed by decapitation and ovaries were transferred to a sterile petri dish containing a fresh cooled incubation medium. The incubation medium consisted of 3.74 g NaCl, 0.32 g KCl, 0.16 g CaCl2, 0.1 g NaH2PO4·H2O, 0.16 g MgSO4·7H2O and 0.8 g glucose,dissolved in 1 L of triple distilled water and sterilized. The pH was adjusted to 7.5 with 1 N sterilized sodium bicarbonate. Penicillin benzoate (200,000 U) and streptomycin sulfate (200 mg) were added and stored at 4 ℃. Two sets of experiments were performed using 300 mg ovarian pieces in triplicate per group (n =5 fish). The tissues were incubated with 10 ml of incubation medium with each of 1, 100, and 1000 nM of 2-OHE2up to 24 h at 25 ℃ in sterile, 6-well polystyrene culture plates. The concentrations were selected from the previous study(Chourasia & Joy, 2012a). 2-OHE2was first dissolved in absolute ethanol (1 mg/ml) and then diluted in the incubation medium to make the above-mentioned concentrations. At every 4 h, the medium was collected and replenished with a fresh medium containing the required concentrations of the steroid. As a control, the ovarian pieces were incubated with a plain medium containing the vehicle (ethanol). After the incubation, one set of the sampled ovarian pieces were stored at-80 ℃ for the determination of enzyme activity. Microsomal and mitochondrial fractions were prepared and used for the assay of enzyme activity (Hsd3b, Cyp17a, Cyp19a, Hsd20a and Hsd20b), as described above. The ovary slices and the incubation medium from the second set were collected (group-wise) for steroid assays. Steroid was extracted, as described in Chourasia and Joy (2012a). The tissues were homogenized separately, group-wise in 4 vol of cold PBS (0.02 M, phosphate-buffered saline, pH 7.4) with an ultrasonic homogenizer at 0 ℃ for 5–10 s. The homogenate was centrifuged at 5000 g for 20 min at 4 ℃ and extracted with 3 vol of diethyl ether, three times. The ether phase was collected and pooled, evaporated and dried under N2and stored at -20 ℃ till quantification. The incubation medium was directly extracted with diethyl ether, as described above. The ether extracts of the tissue and corresponding medium were pooled to constitute each sample.
2.10.In vitro effects of hCG on steroidogenic enzyme activity
In the second in vitro study, the experiments were performed in both VT and PV phases. Three hundred milligrams of ovary pieces were separated (n =5 fish) and incubated in triplicate with 10 mL of incubation medium each with or without 1 μM 2-OHE2, 5 IU/mL hCG, alone or in combination for 24 h. The 5 IU/mL hCG dose was effective to stimulate significantly estrogen-2/4- hydroxylase activity and GVBD response in the cat fish (Chourasia & Joy, 2008a), The test medium was changed at 4 h interval. Control incubations were also run parallel with the incubation medium only. After the incubation, ovarian fragments were sampled and stored at -80 ℃ for the assay of steroidogenic enzyme activity, as described above.
2.11.Steroid assays
Progesterone, 17-OHP4, testosterone (T), E2and cortisol (F) levels were assayed using respective competitive ELISA kits (Dia Metra, Italy),following the manufacturer’s instructions. For the ELISA, the ether extract was reconstituted with 50 μL of ethanol: water (1:10 v/v) and 20 μL was used for the reaction. Standards in different concentrations, tissue samples (20 μL) and blank (20 μL) were assayed in triplicate on the same ELISA plate and processed, as described previously (Chourasia &Joy, 2012a). Blank zero or system blank was prepared by adding 20 μL of the vehicle ethanol: water (1:10 v/v) in the ELISA reaction mixture. The data were validated for sensitivity, cross reactivity, recovery, and intraand inter-assay variations (supplementary file). 17,20α-DP and 17,20β-DP levels were measured by the HPLC-UV detection method(Chourasia & Joy, 2012a). The steroids were quantified using a Shimadzu HPLC system with ultraviolet (UV) detector (SPD-10 AVP)(Kyoto, Japan) at 254 nm using a reversed phase C18column (150 ×45 mm. i.d. 5 μm, Luna, Phenomenex), protected by guard column (5 μm).The extract was reconstituted with 50 μL acetonitrile: water (1:1 v/v)and 20 μL was injected onto the reverse phase HPLC column. The samples were run for 30 min at a flow rate of 1 mL/min by applying a linear gradient from 100% solvent A (71% water and 29% acetonitrile)to 60% solvent B (100% acetonitrile) and then a 30–35 min linear gradient from 60% solvent B to 100% solvent. Under the HPLC conditions, 17,20α-DP was eluted at 13.61 min and 17,20β-DP at 15. 36 min.Peak area vs. concentration of the standards were plotted to prepare standard curves and the concentration of the steroids in the samples were plotted from the standard curves. The details of the validation study were described previously (Chourasia & Joy, 2012a) and given in the supplementary file.
2.12.Statistical analysis
Data were expressed as mean ±SEM and were analyzed by One-way analysis of variance (ANOVA), followed by Newman-Keuls’ test (P <0.05) for multiple group comparisons. The double-reciprocal Lineweaver–Burk (LB) plots were used to determine the apparentKmandVmax(Price & Stevens, 1999, pp. 118–153). TheKmandVmaxwere calculated from the intercept on the X-axis (1 /Km) and Y-axis (1/Vmax)of the LB plots. Data were analyzed by One-way ANOVA (P <0.001).
3.Results
3.1.Seasonal variation in ovarian steroidogenic enzyme activity
Ovarian Hsd3b, Cyp17a, Hsd20a and Hsd20b showed significant seasonal variations in their activities (Fig. 1). The enzymes showed the lowest activity in the resting phase when the GSI was the lowest. The activities increased differentially with the rise in the GSI in a seasondependent manner. The Hsd3b increased significantly in the preparatory phase with peak activity in the prespawning phase and declined significantly in the spawning and postspawning phases (F =139.98,One-way ANOVA,P <0.001; Newman-Keuls’ analysis,P <0.05). The Cyp17a activity was recorded a steep increase in the preparatory phase and then declined significantly through the prespawning, spawning and postspawning phases (F =108.35, One-way ANOVA,P <0.001; Newman-Keuls’ analysis,P <0.05). The Hsd20a and Hsd20b activities increased significantly through the preparatory and prespawning phases to the peak values in the spawning phase and declined in the postspawning phase (Hsd20a: F =54.17; Hsd20b: F =390.01, One-way ANOVA,P <0.001; Newman-Keuls’ analysis,P <0.05).
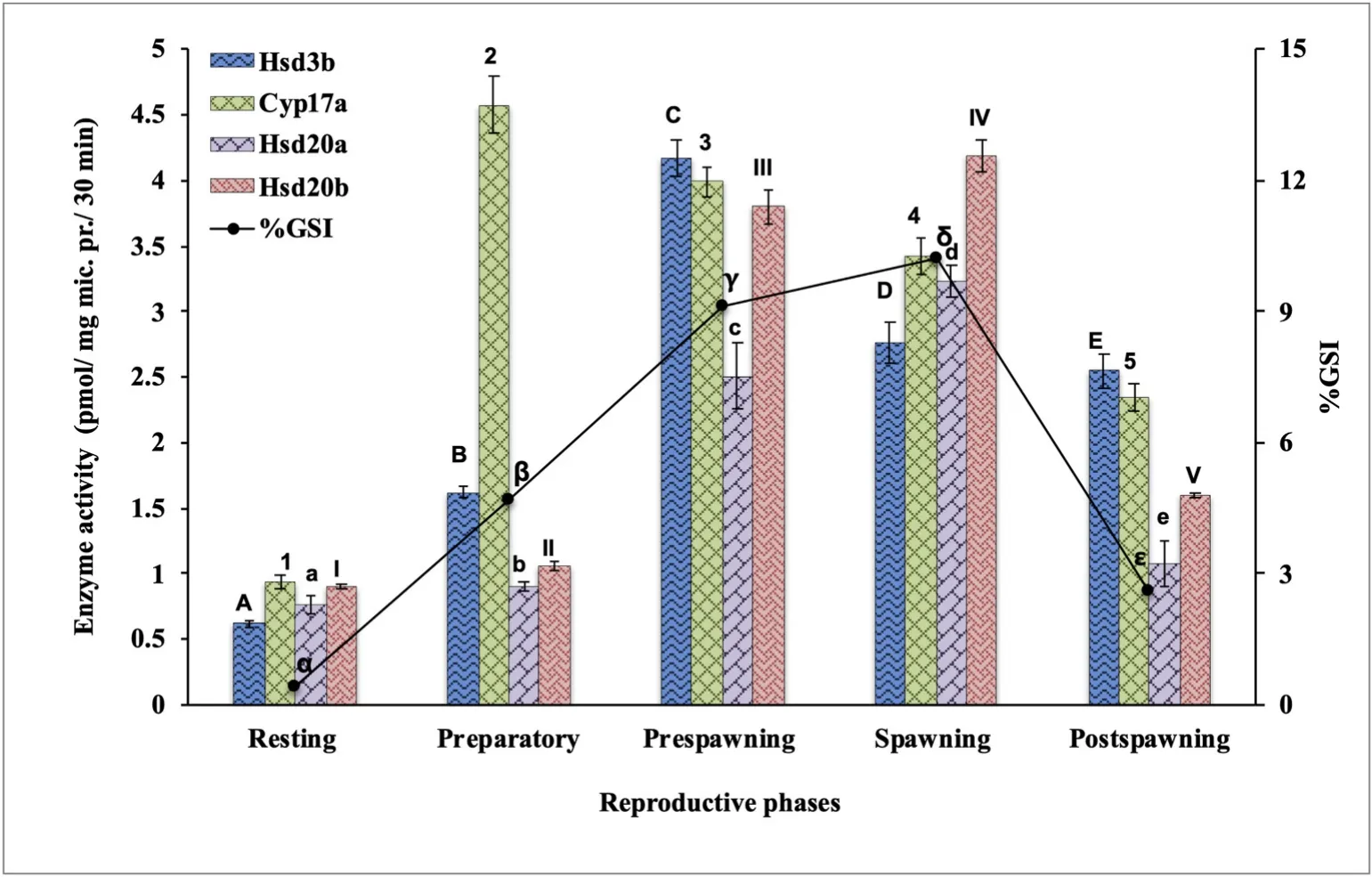
Fig. 1.Seasonal changes in steroidogenic enzyme activity in ovarian mitochondrial/microsomal fractions in the cat fish Heteropneustes fossilis. Values are mean ±standard error of mean (n =5). The data were analyzed by One-way ANOVA (P <0.001),followed by Newman-Keuls’ test (P <0.05).The enzyme activity in different seasons was compared and represented by different sets of symbols (A-E, a-e, α-ε, 1–5, I–V). The groups bearing the same symbol are not significantly different and those bearing different symbols are significant.
3.2.Enzyme kinetics and activity in preparatory and prespawning phases(VT vs. PV phase)
3.2.1.Hsd3b
As in the seasonal study, the ovarian Hsd3b activity peaked in the VT phase and decreased in the PV phase (Fig. 2A, Table 1; F =11.24, Oneway ANOVA,P <0.001; Newman-Keuls’ analysisP <0.05).). The apparentKmfor the substrate P5showed a significant variation the two phases, the value was higher in the VT phase (F =14.6). Similarly, the apparentVmaxvaried significantly in both phases with a significantly high value in the VT phase (F =11.4).
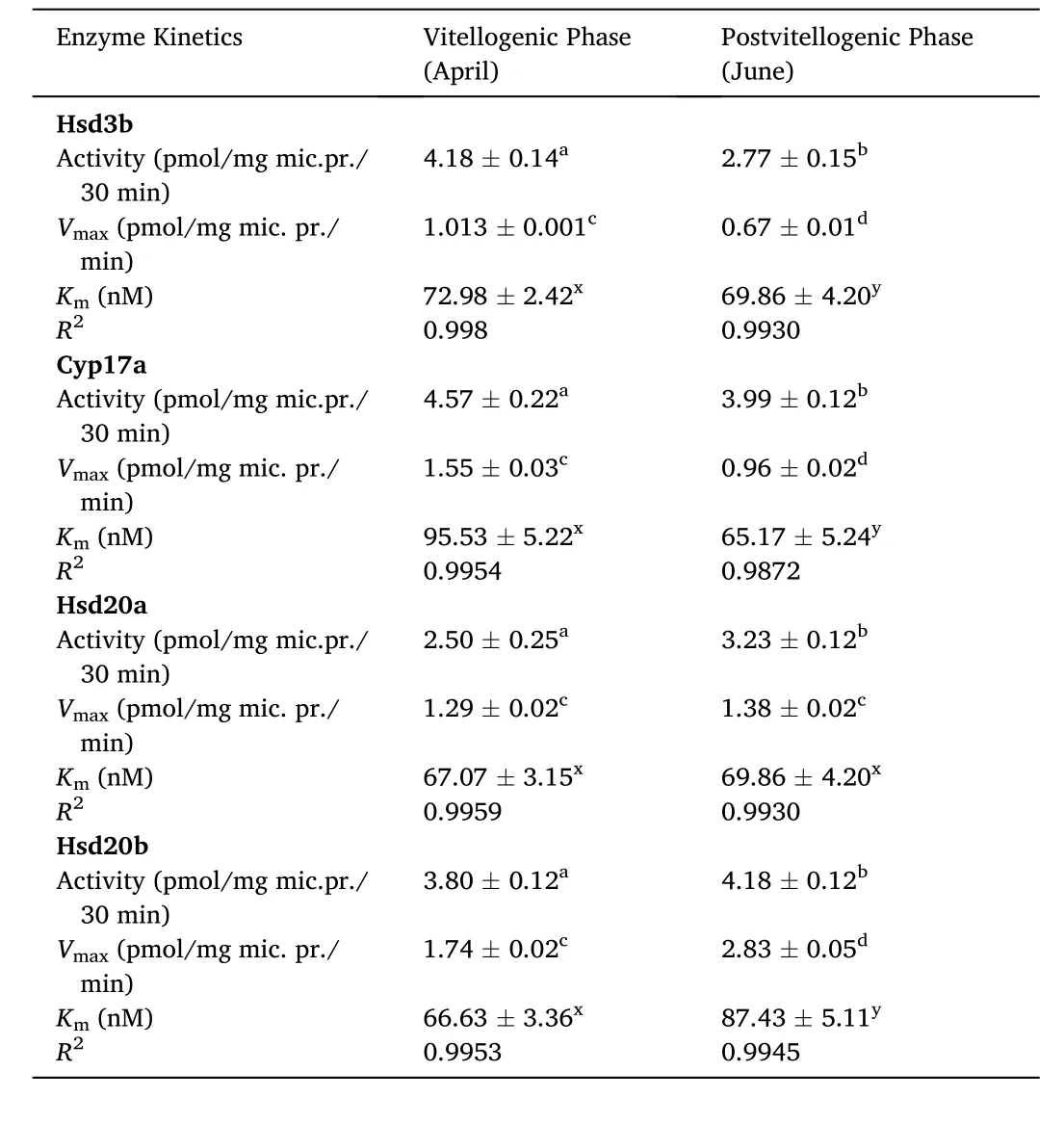
Table 1Enzyme activity and its kinetics in ovarian microsomes of cat fish Heteropneustes fossilis in vitellogenic and postvitellogenic phases. Values are mean ±SEM. Data were analyzed by one-way ANOVA (P <0.001), followed by Newman-Keuls’ test(P <0.05). Values are significantly different between the phases.
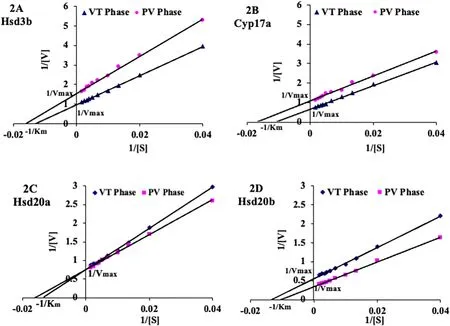
Fig. 2.Lineweaver–Burk plots of apparent Km and Vmax of Hsd3b (2A), Cyp17a (2B), Hsd20a (2C) and Hsd20b (2D) in vitellogenic (VT) and postvitellogenic (PV)ovarian microsomal/mitochondrial preparations. The intercepts at X-axis show the apparent Km (-1 /Km) and Y- axis show the apparent Vmax (1/Vmax). The R2 values are given in Table 1. Values are mean (n =3) ±SEM and analyzed by one-way ANOVA (P <0.001).
3.2.2.Cyp17a
The ovarian Cyp17a activity, apparentKmfor the substrate P4and apparentVmaxvaried significantly in the two phases with high values in the VT phase (Fig. 2B, Table 1; F (activity) =13.3, F (Km) =16.4, F(Vmax) =19.6, One-way ANOVAP <0.001; Newman-Keuls’ analysisP<0.05).
3.2.3.Hsd20a
The ovarian microsomal Hsd20a activity varied significantly in the two phases with a low value in the VT phase and a high value in the PV phase Fig. 2C, Table 1; F =14.6, One-way ANOVAP <0.001; Newman-Keuls’ analysisP <0.05). The apparentKmfor the substrate 17-OHP4and apparentVmaxdid not show any significant change between the two phases (F (Km) =9.34, F (Vmax) =5.32).
3.2.4.Hsd20b
The ovarian microsomal Hsd20b activity, apparentKmfor the substrate (17-OHP4) and apparentVmaxvaried significantly in the two phases with low values in the VT ovary and high values in the PV phase(Fig. 2D; Table 1; F (activity) =13.6, F (Km) =16.8, F (Vmax) =16.2,One-way ANOVAP <0.001; Newman-Keuls’ analysisP <0.05).
3.3.In vitro effects of 2-OHE2 on steroidogenic enzymes and steroid production
3.3.1.Hsd3b activity and progesterone level
The incubation of the ovarian slices with 2-OHE2in both phases elicited significant increases in Hsd3b with higher activity in the VT phase (Fig. 3A; F =92.55, One-way ANOVA,P <0.001). The Newman-Keuls’ analysis showed that the increases were significant in comparison to the control and among the different concentrations of 2-OHE2(P <0.05), except for the groups 100 nM vs. 1000 nM in the VT phase. The P4level showed a overall significant increase in both phases (Fig. 3B: F =77.68). The Newman-Keuls’ analysis showed that the increases were significant when compared with the control group and in the different steroid groups except between the 1 nM and 100 nM groups in the VT phase and 100 nM and 1000 nM groups in the PV phase.
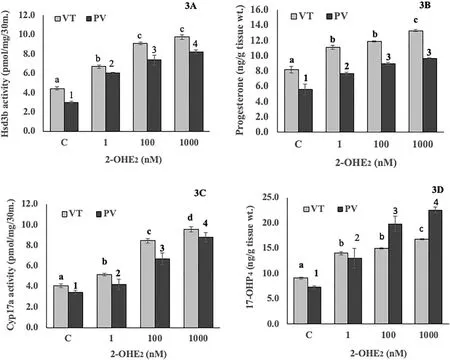
Fig. 3.Concentration effects of 2-hydroxyE2 on ovarian mitochondrial and microsomal Hsd3b activity (3A) and progesterone level (3B) and ovarian microsomal Cyp17a activity (3C) and 17-OHP4 level(3D) in vitellogenic (VT) and postvitellogenic (PV) phases. Values are mean ±SEM (n =5). Data were analyzed by oneway ANOVA (P <0.001), followed by Newman-Keuls’ test (P <0.05). The enzyme activity or steroid levels in different groups within each phase were compared (VT phase, a – d; PV phase, 1–4). The groups bearing different letters or numbers are significantly different.
3.3.2.Cyp17a activity and 17-OHP4 level
The incubation of the ovarian pieces with 2-OHE2elicited significant increases in Cyp17a activity (Fig. 3C; F =45.69, One-way ANOVA,P <0.001). The Newman-Keuls’ analysis showed a concentration-dependent increase in the enzyme activity in both phases as compared with the control groups (P <0.05). The 17-OHP4level showed an overall significant increase (Fig. 3D; F =44.62; One-way ANOVA,P <0.001). The increase was significantly high compared to the control group and varied significantly in the steroid groups except between 1 nM and 100 nM groups in the VT phase (Newman-Keuls’ analysis,P <0.05).
3.3.3.Hsd20a activity and 17,20α-DP level
The incubation of the ovarian slices with 2-OHE2elicited a signi ficant increase in Hsd20a in both phases (Fig. 4A; F =81.11, One-way ANOVA,P <0.001). The increase was significantly high compared to the control groups and among the steroid treatment groups (Newman-Keuls’ analysis,P <0.05) except between the 100 nM and 1000 nM groups in the VT phase. The 17, 20α-DP level elicited an overall significant increase (Fig. 4B; F =45.57, One-way ANOVA,P <0.001). The increase was significantly high in comparison to the control groups and among the different 2-OHE2groups (Newman-Keuls’ analysis,P <0.05)except between the 100 nM and 1000 nM groups in the VT phase.
3.3.4.Hsd20b activity and 17,20β-DP level
The incubation of the ovarian pieces with 2-OHE2elicited a signi ficant concentration-dependent increase in Hsd20b activity in both phases with higher activity in the PV phase (Fig. 4C; F =52.79, One-way ANOVA,P <0.001). The increase was significantly high in all steroidexposed groups compared to the control groups in both phases (Newman-Keuls’ analysis,P <0.05). The 17,20β-DP level (Fig. 4D; F =79.55)increased concentration-dependently with a higher increase in the PV phase in comparison to the control groups (Newman-Keuls’ analysis,P<0.05).
3.3.5.Cyp19a activity and E2 level
The incubation of the ovarian slices with 2-OHE2elicited a signi ficant concentration-dependent significant inhibition of Cyp19a activity in both phases (Fig. 5A; F =43.19, One-way ANOVA,P <0.001). The Newman-Keuls’ analysis showed that all the tested concentrations elicited a significant inhibition compared with the control groups (P <0.05). The enzyme inhibition was not significant between the 100 nM and 1000 nM groups. Similarly, the ovarian E2level decreased signi ficantly in a concentration-dependent (Fig. 5B; F =5303.60, one-way ANOVA,P <0.001). Multiple group comparisons showed a significant compared to the control groups and within the steroid-treated groups.
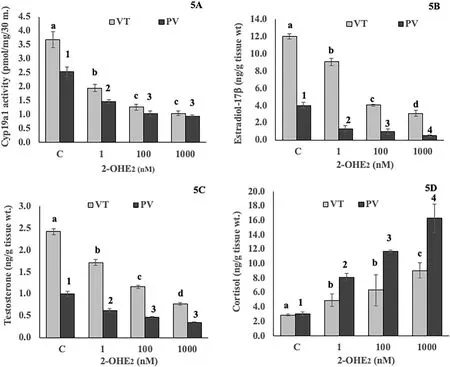
Fig. 5.Concentration effects of 2-hydroxyE2 on ovarian microsomal Cyp191a activity(5A), E2 level (5B), testosterone (5C) and cortisol (5D) levels in vitellogenic (VT) and postvitellogenic (PV) phases. Values are mean ±SEM (n =5). Data were analyzed by One-way ANOVA (P <0.001), followed by Newman-Keuls’ test (P <0.05). The enzyme activity or steroid levels in different groups within each phase were compared (VT phase, a – d; PV phase, 1–4). The groups bearing different letters or numbers are significantly different.
3.3.6.Testosterone and cortisol levels
In order to test whether 2-OHE2in fluenced androgen and corticosteroid secretion in the ovary, T and F levels were measured in vitro. The T level decreased in a concentration-dependent manner in both phases(Fig. 5C; F =20.27; Newman-Keuls’ analysis,P <0.05). On the other hand, the cortisol level was stimulated in a concentration-dependent manner with a higher increase in the PV phase (Fig. 5D; F =15.04;Newman-Keuls’ analysis,P <0.05).
3.4.In vitro effects of hCG, 2-OHE2, and the combination on enzyme activity
The incubation of the ovarian slices with hCG (5 IU/mL) and 2-OHE2(1 μM) alone or in combination elicited significant effects on Hsd3b,Cyp17a, Hsd20a, Hsd20b and Cyp19a activity in both VT and PV phases(Fig. 6; FVT=275.02; FPV=69.67, One-way ANOVA,P <0.001). The combination of hCG and 2-OHE2elicited a higher stimulatory effect on the enzyme activity than that of the individual incubations. The incubation with hCG stimulated Cyp19a activity while 2-OHE2inhibited the enzyme activity. The Newman-Keuls’ analysis (P <0.05) showed that all the steroid groups elicited significant differences as compared to the control groups in both phases (P <0.05). The co-incubation of hCG with 2-OHE2reversed the inhibitory effect of 2-OHE2and restored the enzyme activity compared to that of the control groups.
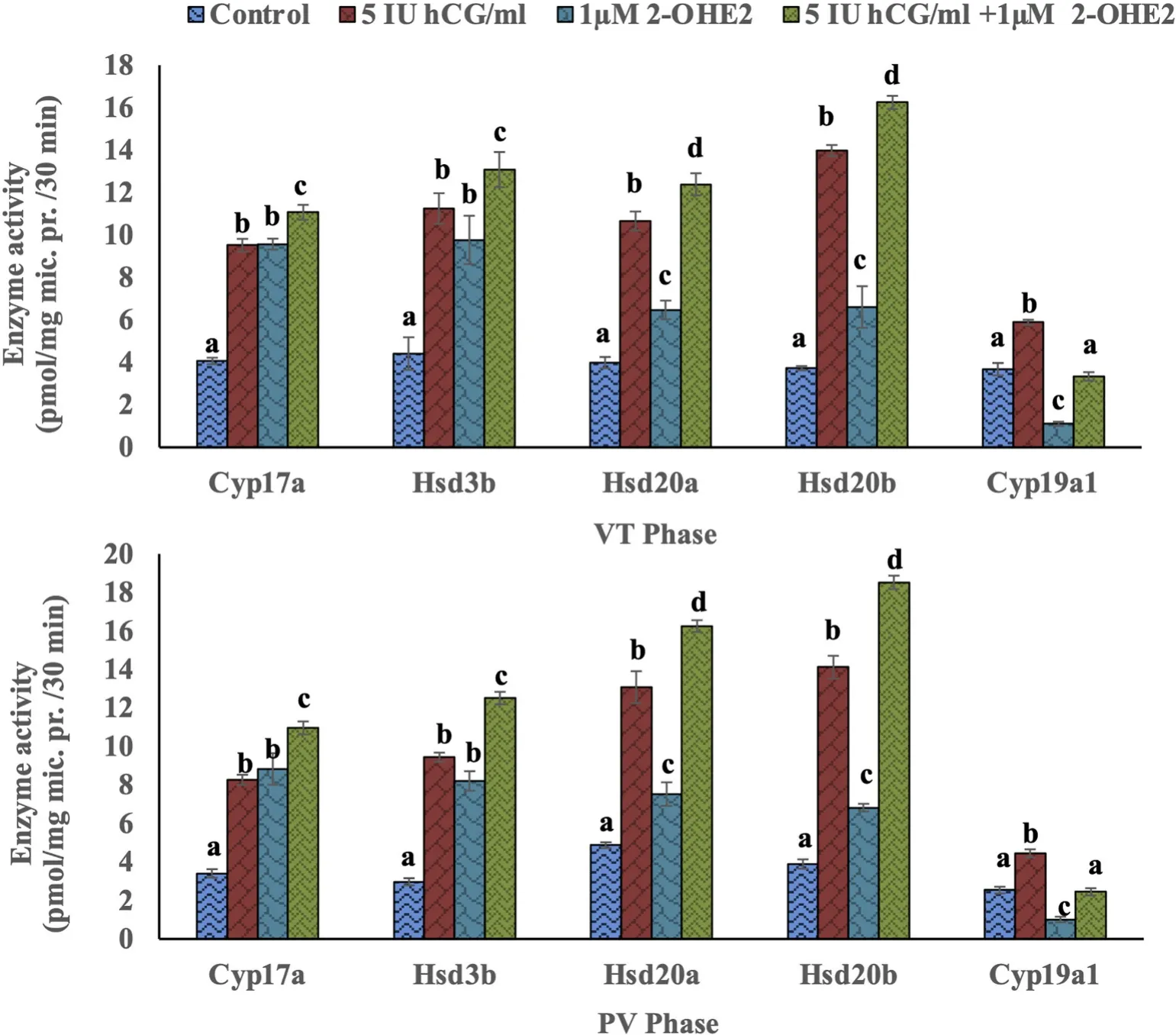
Fig. 6.In vitro effects of 5 IU/ml hCG and 1 μM/ml 2-hydroxyE2, alone or the combination, on Cyp17a, Hsd3b, Hsd20a, Hsd20b and Cyp19a1 activity in the ovary of the cat fish H. fossilis in the vitellogenic (VT)(upper panel) and postvitellogenic (PV)(lower panel) phases. Values are mean ±SEM (n =5). Data were analyzed by Oneway ANOVA (P <0.001) and Newman-Keuls’ test (P <0.05). The enzyme activity in different groups within each phase were compared (VT phase, a – d; PV phase, a-d).The groups bearing different letters are significantly different.
4.Discussion
4.1.Seasonal changes in enzyme activity
In the present study, ovarian microsomal/mitochondrial fractions were prepared to measure Hsd3b, Cyp17a, Hsd20a and Hsd20b activity during the different reproductive stages of the cat fish. Hsd3b is critical for the formation of Δ4-ketosteroids (P4and 17-OH P4) from Δ5-hydroxysteroids (P5and 17-OHP5) and is a major regulator of the Δ4–pathway (Kime, 1987; Tenugu et al., 2021; Tokarz et al., 2015). In the past, Hsd3b has been identified by various methods in different species(Bhat et al., 2018; Kazeto et al., 2003; Lai et al., 1998; Mishra & Chaube,2017; Raghuveer & Senthilkumaran, 2012; Sakai et al., 1994; Senthilkumaran et al., 2009). In the cat fish, Hsd3b activity showed significant variations with the activity recorded the lowest in the resting phase, steadily increasing in the preparatory phase to reach the peak in the prespawning phase and declined significantly in the spawning and postspawning phases. The peak activity in the prespawning phase preceded the rise in the GSI and vitellogenesis. The high enzyme activity during ovarian recrudescence also relate to the high turnover ofhsd3btranscripts reported in this and other cat fishes. In the African cat fishClarias gariepinus,Raghuveer and Senthilkumaran (2012) reported significantly higher abundance ofhsd3btranscripts in isolated stage II previtellogenic, and stage III vitellogenic follicles in comparison to the stage I perinuclear/primary follicles and stage IV post-vitellogenic follicles. InH. fossilis, brainhsd3bexpression was high in the preparatory and prespawning phases (Mishra & Chaube, 2017). In the Asian cat fishC. batrachus,ovarian and brainhsd3bmRNA expressions were higher in the preparatory and prespawning phases than in the spawning and post spawning phases (Bhat et al., 2018).
Cyp17a is an important enzyme in the Δ5-pathway catalyzing the formation of 17-OHP5, dehydroepiandrostone (DHEA) and androstenedione. Primarily, gonadal and adrenal tissues are the major sites of Cyp17a expression (Halm et al., 2003; Kazeto et al., 2000; Sakai et al.,1992; Trant, 1995; Wang & Ge, 2004). In the gonads, Cyp17a controls the production of T and 17-OHP4, the precursors for E2and 17, 20β-DP,respectively. The enzyme has been implicated in the induction of steroidogenic shift, prior to the onset of FOM (Kagawa et al., 1983;Nagahama, 1997; Nagahama et al., 1995; Planas et al., 2000; Sreenivasulu & Senthilkumaran, 2009a). Unlike Hsd3b activity, Cyp17a activity reached an early peak in the preparatory phase and declined significantly through the prespawning, spawning and postspawning phases, giving the lowest value in the resting phase. Several researchers have reported significant changes in thecyp17amRNA level during ovarian development in several teleost fish (Kazeto et al., 2000; Kumar et al., 2000; Sakai et al., 1992). In the channel cat fishIctalurus punctata,thecyp17atranscript level was the highest during recrudescence and mid-vitellogenesis (Kumar et al., 2000). InC. gariepinus,Cyp17a mRNA expression, protein content and activity were high during preparatory -prespawning stages (Sreenivasulu & Senthilkumaran, 2009a). In the zebra fish ovary, a high expression ofcyp17awas registered at all major developmental stages of follicles, including the previtellogenc, vitellogenic, and postvitellogenic full-grown follicles, and suggested that its regulation may take place at the translational or post-translational level(Wang & Ge, 2004). In female fathead minnow (Pimephales promelas),the expression ofcyp17awas negatively related with the development of the ovary with its expression level being high at early stages but declining with the growth of the gonads (Halm et al., 2003). In contrast,in female rainbow trout and Japanese eel, the peak Cyp17a mRNA level was registered during FOM and ovulation, the elevated expression was linked to the increased production of the MIH, 17,20β-DP (Kazeto et al.,2000; Sakai et al., 1992).
Hsd20a and Hsd20b: The incubation of the cat fish ovarian microsomes with 17-OHP4resulted in the formation of 17,20α-DP and 17,20β-DP, as reported inCyprinus carpio(Thibaut & Porte, 2004). The Hsd20a and Hsd20b activities were low in the resting and preparatory phases,increased in the prespawning and spawning phases, and declined in the postspawning phase. Both enzyme activities peaked in the spawning phase and the Hsd20a activity was lower than the Hsd20b activity in all phases. In Nile tilapia, a high expression ofhsd20btranscripts was reported in post-vitellogenic immature follicles within 1–2 h of the hCG treatment during FOM (Senthilkumaran et al., 2002). In the murrelChanna striatus, a lower expression ofhsd20bin previtellogenic follicles,and a higher expression in full-grown follicles was reported after 2 h of the hCG treatment (Sreenivasulu et al., 2005). InC. gariepinus, an induction ofhsd20bexpression was reported during the hCG-induced oocyte maturation, in vitro and in vivo, and specific inhibitors of carbonyl reductase (CR) inhibited not only the recombinant protein catalytic activity but also the hCG-induced oocyte maturation (Sreenivasulu & Senthilkumaran, 2009b). In rainbow trout (Oncorhychus mykiss) ovarian tissue, the expression of two subunits ofhsd20baandhsd20bb was reported, and both tended to be more highly expressed during FOM (Vazirzadeh & Guiguen, 2017). However, recent studies in Nile tilapia (Aranyakanont et al., 2020) and masu salmon (Ijiri et al.,2017) could not linkcr/hsd20bexpression to the production of the MIH(17,20β-DP); instead, the MIH production was linked to a novel 17β-hydroxysteroid dehydrogenase, type 12-like(hsd17b12l). Thus, it is possible that species variation exists with regard to the enzyme responsible for the production of MIH (Nagahama, 1997; Guan et al.,1999; Tanaka et al., 2002; Sreenivasulu & Senthilkumaran, 2009b; Ijiri et al., 2017; Aranyakanont et al., 2020). 17,20β-DP is identified as the MIH in a wide range of teleost including the cat fish that induces the maturation of oocytes (Mishra & Joy, 2006a; Nagahama et al., 1985;Scott & Canario, 1987; Senthilkumaran, 2011). There are not many studies on the catalytic role of Hsd20a or its product 17,20α-DP in fish gonads. 17, 20α-DP has been identified in male teleosts (Schoonen et al.,1987; Canario & Scott, 1989; Kime et al., 1992). Based on these studies,the involvement of 17,20α-DP in FOM, spermiation, or in other physiological functions has been proposed in gold fish (Kime & Scott, 1993),European cat fishSilurus glanis(Kime et al., 1993), zebra fish (van den Hurk et al., 1987) andC. carpio(Thibaut & Porte, 2004). In the cat fish,we reported previously that 17,20α-DP stimulated FOM and inhibited estrogen hydroxylase activity, second to 17,20β-DP (Chourasia & Joy,2010). The presence of the two enzymes and their products in the cat fish ovary suggests that both steroids are involved in the regulation of FOM and E2metabolism.
The seasonality in the activities of all the studied enzymes follows the annual reproductive cycle of the cat fish, which is dependent on the cyclic changes in the environmental photoperiod and temperature.(Sundararaj, 1981, pp. 1–82; Acharjee et al., 2017). The low enzyme activity in the resting phase coincides with short photoperiod and low temperature when the BPG axis is dormant. The high activity during ovarian recrudescence is associated with long photoperiod and high temperature, which activate the BPG axis (Acharjee et al., 2017; Chaube et al., 2020). Human hCG stimulated enzyme activities in the cat fish(Fig. 6), and in other telosts like Nile tilapia,C. gariepinusandC. striatus(Raghuveer & Senthilkumaran, 2012; Senthilkumaran et al., 2002;Sreenivasulu et al., 2005; Sreenivasulu & Senthilkumaran, 2009b). The seasonal study shows that the peak enzyme activity is reached at different time points during the recrudescence, which is in fluenced by substrate (s) concentration and temperature and pH-optima that regulate the enzyme reaction.
4.2.Seasonal changes in enzyme kinetic parameters
The apparent Kmand Vmaxvalues were determined in the VT and PV phases to show their relationships to the seasonal changes in the enzyme activity. The Km and Vmax values of Hsd3b and Cyp17a showed significant differences between the VT and PV phases, the kinetic data of Hsd20a did not vary significantly between the two phases, and that for Hsd20b was higher in the PV phase. In the cat fish, Cyp19a elicited a similar pattern like Hsd3b and Cyp17a, with testosterone as the substrate (Chourasia & Joy, 2008b). A high Km value denotes low substrate affinity for the enzymes. A low substrate affinity may be due to its ability to catalyze multiple substrates. Hsd3b, Cyp17a and Cyp19a are catalyzed by more than one substrate. InC. batrachus,Singh and Singh(1985) reported different substrate affinities for Hsd3b. The authors showed that P5was utilized more efficiently as the substrate, followed by 17-OHP5and DHEA. Seasonal changes in the substrate abundance or in the lipid composition of microsomal/mitochondrial preparations may also affect enzyme affinity for a given substrate (Zhao et al., 2001). The apparent Vmax for Hsd3b and Cyp17a is higher in the VT phase. However, the apparent Vmax for Hsd20a did not vary significantly between the two phases, though the mean values were higher in the PV phase.TheVmaxwas higher for Hsd20b in the PV phase. In the present study,TheVmaxis positively associated with the enzyme activities.
4.3.Effects of 2-OHE2 on enzyme activity and steroid secretion
This is the first study reporting broad actions of 2-OHE2on ovarian steroidogenesis. We have reported in the cat fish ovary that estrogens are catabolized to catecholestrogens, hydroxy-and methoxy-estrogens. The hydroxyestrogens are biologically active and are involved in the induction of 17,20β-DP (MIH) and prostaglandin synthesis causing FOM and ovulation, simultaneously inhibiting Cyp19a activity and E2secretion (Senthilkumaran & Joy, 2001; Mishra & Joy, 2006a, b; Chourasia &Joy, 2008a, b; Chourasia & Joy, 2012a, b; Joy & Chaube, 2013; Chaube et al., 2021). In view of these reports, we investigated whether 2-OHE2modulate the expression of other steroidogenic enzymes. The data show that the expression of Hsd3b, Cyp17a, Hsd20a and Hsd20b activity and production of the corresponding steroids (P4, 17-OHP4, 17,20α-DP and 17,20β-DP) were stimulated by 2-OHE2in a concentration-dependent manner in both VT and PV phases. On the other hand, as reported previously (Chourasia & Joy, 2008b), 2-OHE2inhibited Cyp19a and E2level in a concentration-dependent manner. Furthermore, 2-OHE2is a strong inhibitor of GPER/GPR30 E2membrane receptor in zebra fish follicles (Chourasia et al., 2015), By blocking E2actions, the high cAMP level that maintains the meiotic arrest is lifted, sensitizing the follicles to respond to the MIH. This study also showed that the T level is inhibited by 2-OHE2, as reported previously (Chourasia & Joy, 2012a). In the cat fish and other teleosts, cortisol and other corticosteroids exhibit oocyte maturational activity to varying degrees (Sundararaj, 1981, pp.1–82). Corticosteroids like cortisol and corticosterone are synthesized by the cat fish ovary (Mishra & Joy, 2006b). In the present study, ovarian cortisol levels were stimulated, similar to progestins. Though we did not measure the concerned enzymes involved in T or cortisol synthesis, it is very likely that the hydroxyestrogen may be targets for these enzymes.Future studies are required to focus on these aspects.
The LH-like placental gonadotropin hCG is used extensively to investigate gonadotropic role in teleosts. It stimulates steroidogenesis,gametogenesis, and FOM and ovulation or spermiation, and therefore is employed as a spawning agent in artificial reproduction of fishes (Fostier et al., 1983; Mishra & Joy, 2006b; Raghuveer & Senthilkumaran, 2012;Sundararaj, 1981, pp. 1–82; Van Der Kraak, 2009). In the present study,hCG stimulated the activities of the studied enzymes in both VT and PV phases. Interestingly, a synergistic effect was noticed when 2-OHE2was co-incubated with hCG on the activities of all the enzymes. In the case of Cyp19a, the co-incubation reversed the inhibitory effect of 2-OHE2and restored the Cyp19a activity. Summing up, 2-OHE2exerted dual effects on the steroidogenic enzyme activities; stimulating on Hsd3b, Cyp17a,Hsd20a and Hsd20b activity and inhibiting Cyp19a activity. In this manner, the hydroxyestrogen promoted progestin and cortisol (C21)pathways and inhibited the androgen-estrogen (C19–C18) pathway.Hydroxyestrogens like 2-OHE2seems to the triggering factor causing steroidogenic shift for the resumption of meiotic maturation (Joy &Chaube, 2013).
4.4.Conclusions
The enzymes under investigation showed significant seasonal variations during the annual reproductive of female cat fish with low activity in the gonad resting phase and high activity during ovarian recrudescence. Hsd3b and Cyp17a showed high activity during the early part of oogenesis while Hsd20a and Hsd20b activity remained high towards late oogenesis (spawning phase). The kinetic parametersKmand Vmax showed significant variations between the VT and PV phases with high apparentKmvalues (low substrate affinity) and high apparentVmaxin the VT phase for Hsd3b and Cyp17a. On the other hand, Hsd20a did not elicit any significant difference in the kinetic parameters between the two phases. Hsd20b showed high apparentKmvalues (low substrate affinity) and highVmaxin the PV phase. 2-OHE2exerted a stimulatory effect on Hsd3b, Cyp17a, Hsd20a and Hsd20b activities, the corresponding steroid products, and cortisol, and an inhibitory effect on Cyp19a, E2and testosterone. Estrogen hydroxylation seems to be the triggering factor underlying the steroidogenic shift at the onset of FOM and ovulation. This study highlights the importance of the estrogen metabolites and their roles in natural and induced reproduction of fish.
Funding
The research work was funded by grant No. SP/SO/C-13/2001) of Department of Science & Technology, New Delhi to KPJ.
CRediT authorship contribution statement
Tapan Kumar Chourasia: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Radha Chaube: Resources, Data curation, Writing – review &editing. Keerikkattil Paily Joy: Conceptualization, Resources, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no con flict of interest.
Acknowledgments
The research work was funded by grant No. SP/SO/C-13/2001) of Department of Science & Technology, New Delhi to KPJ, which is gratefully acknowledged. We are thankful to the Head for facilities at the Department of Zoology, Banaras Hindu University, Varanasi, India. KPJ is currently an INSA Senior Scientist at the Department of Biotechnology, CUSAT, Kochi, India.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aaf.2022.03.010.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Understanding the impact of stress on teleostean reproduction
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
