Efficacy of neoadjuvant chemotherapy for initially resectable colorectal liver metastases:A retrospective cohort study
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide. Approximately 20% of patients with CRC present with synchronous distant metastases, and another 20% develop metachronous metastases[1].
2. The youngest of whom was called Dummling: Sometimes the translations of the tale call the youngest son Simpleton . Bruno Bettelheim sees the number three as representing the ego14, super-ego, and id (102). Bettelheim sees the simpleton character as the fairy tale s rendering15 of the original debilitated16 state of the ego as it begins its struggle to cope with inner world of drives and with the difficult problems which the outer world presents (75).Return to place in story.
The liver is the most common metastatic site of CRC[2]. Hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). The 5-year overall survival (OS) rate after curative hepatectomy has been reported to range from 45 to 61%. However, the postoperative recurrence rate is high (approximately 75%), especially in the remnant liver[3]. To improve surgical outcomes, neoadjuvant chemotherapy (NAC) has been used to treat initially resectable CRLMs. In the EORTC 40983 trial[4], 364 patients with resectable CRLMs were randomly assigned to a perioperative 5-fluorouracil/folinic acid/oxaliplatin (FOLFOX4) group and a surgery alone group. Better recurrence-free survival, but no OS benefit, was observed in patients in the chemotherapy group. Therefore, upfront hepatectomy is recommended for patients with resectable CRLMs[3,5].
Several studies[6-9] have identified risk factors for recurrence and prognosis after hepatectomy for CRLMs, including positive lymph node status of the primary colorectal lesion, appearance time, largest tumor diameter, number and distribution of CRLMs, and preoperative carcinoembryonic antigen(CEA)/carbohydrate antigen 19-9 Levels. A greater number of risk factors were associated with early recurrence or poor prognosis. Hence, there are cases of early recurrence after upfront hepatectomy alone in the resectable CRLMs, and in selected high-risk patients, NAC may improve long-term survival. We investigated the effectiveness of NAC for initially resectable CRLMs, based on risk stratification according to prognostic factors.
MATERIALS AND METHODS
Study design
A total of 644 patients underwent their first hepatectomy for CRLMs at our institution between January 1992 and December 2019. Among them, 297 resectable cases were included in this study. Among these cases, patients with synchronous liver metastases who received liver-first surgery or simultaneous resection of CRLM and the primary lesion were excluded. Patients were stratified into an upfront hepatectomy group (238 patients) and a NAC group (59 patients) (Figure 1). No patient received preoperative chemotherapy before resection of the primary lesion. Poor prognostic factors for upfront hepatectomy were identified using multivariate logistic regression analysis. Propensity score matching was performed using baseline characteristics, and clinical outcomes were compared between the groups, according to the number of poor prognostic factors.
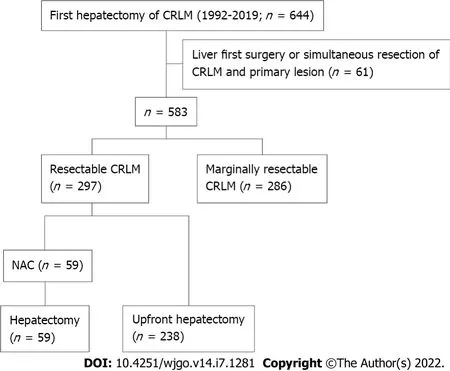
Clinicopathological characteristics
As the years went by, I left Visakhapatnam and travelled around the world, met many beautiful girls at the various exotic places I visited. But I never forgot her! A man s first love would always have an enduring place in his heart.
Propensity score matching was performed to minimize the differences in baseline characteristics between the upfront hepatectomy and NAC groups. The propensity score for each patient was estimated by logistic regression analysis using the primary tumor- and liver metastasis-related variables.
To determine the efficacy of NAC for initially resectable CRLMs.
Indications for NAC
The criteria for resectable CRLMs were:(1) No extrahepatic metastases; (2) Liver tumor in one lobe only,or no more than three tumors in both lobes; (3) Favorable tumor location, without invasion of major vascular structures; (4) Maximum tumor diameter ≤ 80 mm; and (5) Sufficient planned residual liver volume[10]. The criteria for unresectable CRLMs were uncontrollable extrahepatic metastases and insufficient residual liver capacity. Originally, NAC was administered to those with marginally resectable CRLMs who did not satisfy either of these criteria[10]. However, there were patients who underwent upfront hepatectomy (at their own request) although they met the criteria for NAC initially.Conversely, there were patients who received NAC although they met the criteria for resectable CRLM initially. Therefore, patients who met the criteria for resectable CRLM included those who underwent upfront hepatectomy or received NAC.
NAC
Patients received NAC according to the abovementioned criteria. Some patients in the NAC group were treated with chemotherapy by another physician, who considered the CRLMs to be unresectable.However, when the patients were referred to our hospital, the CRLMs were judged to have met the criteria for resection prior to the start of chemotherapy. Regarding NAC regimens, fluoracyl and folinic acid had been used. After oxaliplatin- and irinotecan-based regimens became available, these were widely used as NAC. The combined use of molecularly-targeted agents was also considered, based on
status. Hepatic arterial infusion was considered for elderly patients, or those who could not continue systemic chemotherapy due to side effects. The response to NAC was evaluated by contrastenhanced computed tomography (CT)/magnetic resonance imaging, according to the Response Evaluation Criteria in Solid Tumors (version 1.1)[11]. The number of treatment cycles varied because of the retrospective nature of the study. Hepatectomy was performed ≥ 4 wk after the last administration of chemotherapy. When bevacizumab was used, an interval of ≥ 6 wk was maintained.
Adjuvant chemotherapy after hepatectomy
Adjuvant chemotherapy (hepatic arterial or intravenous infusion or systemic or oral administration of fluoracyl and folinic acid, oxaliplatin, or irinotecan) was considered for all patients who underwent hepatectomy[10]. However, it has not been administered actively since 2019, as few studies have shown a survival benefit[12,13].
Hepatectomy
Hepatectomy with negative surgical margins was performed in principle with non-anatomical procedures. Anatomical hepatectomy was performed, if it was advantageous, in terms of complete resection (R0), operative time, blood loss, or invasiveness. Portal vein embolization or two-stage hepatectomy was planned when the remnant prognostic score was low, based on volumetry, the indocyanine green retention rate, and patients’ age[14]. Intraoperative ultrasonography was performed in all cases to detect occult tumors undetected by preoperative imaging, and to confirm the anatomical relationships between tumors and vasculobiliary structures, and the absence of residual tumors in the remnant liver. Parenchymal dissection was performed mainly using ultrasonic dissectors[14]. R0 resection was considered complete when the pathologist assessed free resection margins.
Outcomes
OS was defined as the time from hepatectomy until death from any cause. Disease-free survival (DFS)was defined as the time from hepatectomy until the first recurrence. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1)[11]. Synchronous CRLMs were defined as metastases to the liver at the time of resection of the primary CRC.
Unable to stifle23 the song, Goebbels vented24 his anger on the singer. He ordered lLale Andersen put under surveillance and had rumors25 spread that she was a friend of Jews. Andersen did have close Jewish friends. To one in Switzerland, she wrote letters saying how much she wanted to get out of Germany.
Follow-up
Patients were examined for recurrence after hepatectomy using contrast-enhanced CT (every 4–6 mo),blood tests, and tumor markers (every 2–3 mo). When recurrence in the remnant liver was suspected,magnetic resonance imaging was performed, and the appearance of new lesions was investigated.Extrahepatic recurrence in the chest and pelvis was detected on CT. Fluorodeoxyglucose positron emission tomography was sometimes performed to detect other distant metastases. Recurrence was diagnosed when imaging studies confirmed new lesions showing typical features of CRC/CRLMs,compared with previous images. Recurrent CRLMs were treated with repeat resection, if applicable.When there was no indication for resection, chemotherapy, radiotherapy, or palliative care was chosen.
Statistical analyses
Quantitative variables were expressed as medians (interquartile ranges), and categorical variables as numbers and percentages. Continuous data were compared using the Mann–Whitney
test, and categorical data using the chi-square test. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using stepwise logistic regression. Statistically significant variables in the univariate analysis were included in the multivariate analysis. All statistical analyses were conducted using SPSS Base 11.0 J (Chicago, IL, United States). A
value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics before propensity score matching
Before propensity score matching, there were 238 patients in the upfront hepatectomy group and 59 patients in the NAC group (Table 1). Variables that were significantly different between the upfront hepatectomy and NAC groups included age (≥ 60 years) (
< 0.001), primary tumor location (right) (
=0.03), lymph node metastases (≥ 1) (
< 0.001), depth of tumor invasion [adjacent organ invasion (T4b)] (
= 0.01), number of liver metastases (≥ 4) (
< 0.001), appearance time (synchronous) (
< 0.001), tumor distribution (bilobar) (
< 0.001), and staged hepatectomy (performed) (
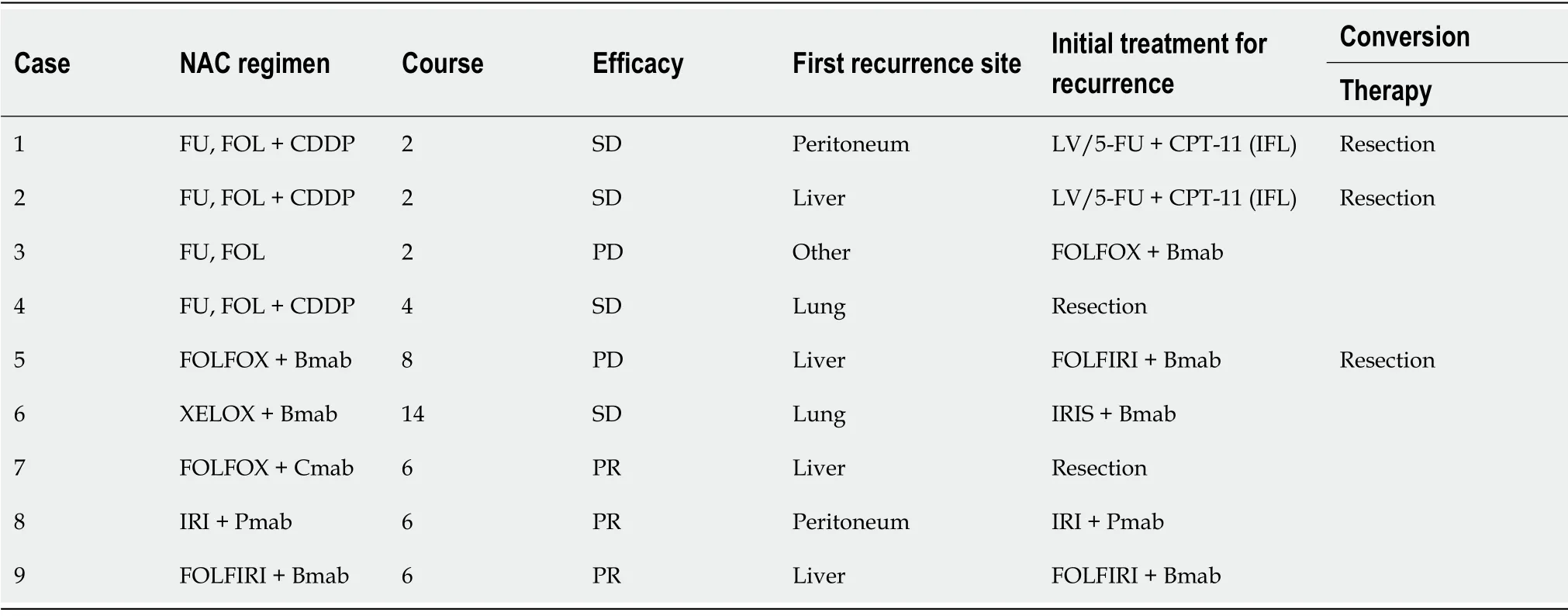
= 0.04). The NAC regimens were as follows:Oxaliplatin-based chemotherapy (35 patients), with molecularly-targeted agents[bevacizumab (14 patients), cetuximab (three patients), and panitumumab (seven patients)]; irinotecanbased chemotherapy (four patients), with molecularly-targeted agents [bevacizumab (two patients) and panitumumab (two patients)]; oxaliplatin- and irinotecan-based chemotherapy (nine patients), withmolecularly-targeted agents [bevacizumab (six patients) and cetuximab (one patient)]; fluorouracil and folinic acid (nine patients), with cisplatin (seven patients); and chemotherapy, including hepatic arterial infusion (two patients). Responses to NAC were defined as follows:Complete response (no patient),partial response (34 patients), stable disease (22 patients), or progressive disease (three patients). The median number of treatment cycles was 6 (range from 2 to 25).

Prognostic factors for upfront hepatectomy
In univariate analysis, preoperative CEA levels (≥ 10 ng/mL) (
= 0.01), primary histological type (other than well/moderately differentiated) (
= 0.01), primary lymph node metastases (≥ 1) (
= 0.001),lymphatic invasion (≥ 1) (
= 0.02), and adjuvant chemotherapy (performed) (
= 0.02) were associated with poor OS in the upfront hepatectomy group (238 patients). Preoperative CEA levels [hazard ratio(HR), 1.948; 95% confidence interval (CI):1.252–3.031;
= 0.003], primary histological type (HR, 2.971;95%CI:1.038–8.503;
= 0.04), and primary lymph node metastases (HR, 1.623; 95%CI:1.020–2.583;
=0.04) were independent prognostic factors in multivariate analysis (Table 2).
Holding the Prince by the arms, not so much to do him honour as to restrain his impatience, they proceeded by slow degrees up the steps of the Temple, and when they at last reached the top he thought his long waiting must be at an end
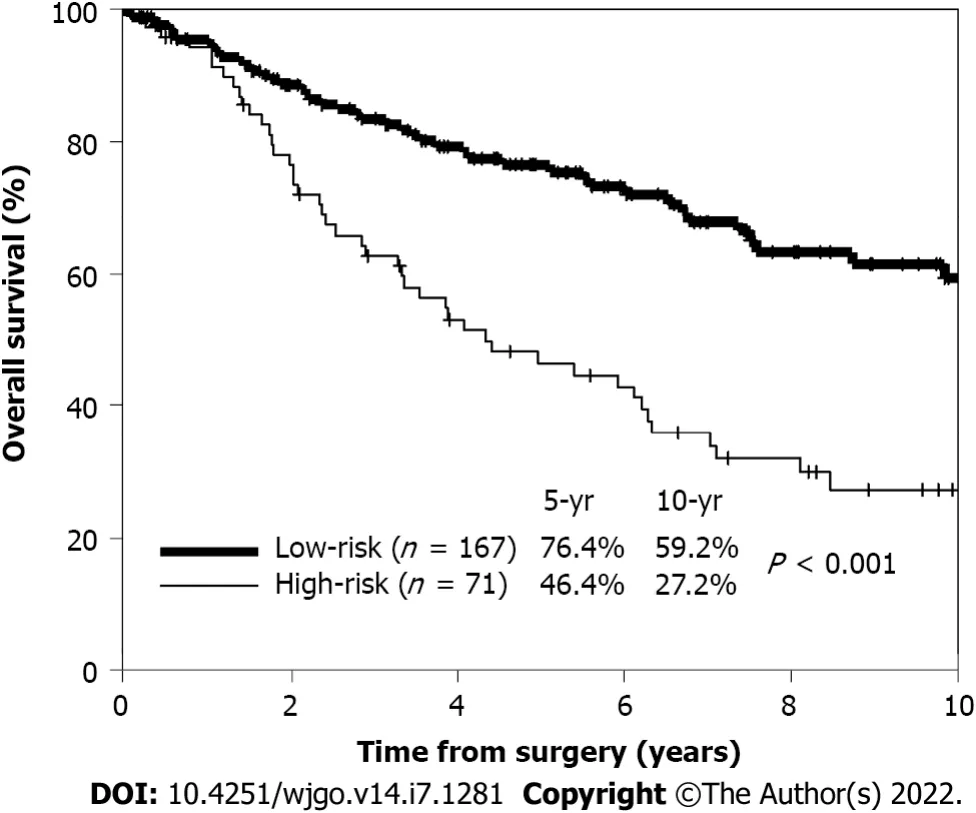
The 5-year OS rates of patients with zero (59 patients), one (108 patients), and two (71 patients) risk factors were 83%, 73%, and 46%, respectively. No patient had three risk factors. High-risk patients were defined as those with two or more risk factors, while low-risk patients were defined as those with zero or one risk factor. The 5-year OS rate of high-risk patients (71 patients) was significantly worse than that of low-risk patients (167 patients) (46.4%
76.4%;
< 0.001) (Figure 2).
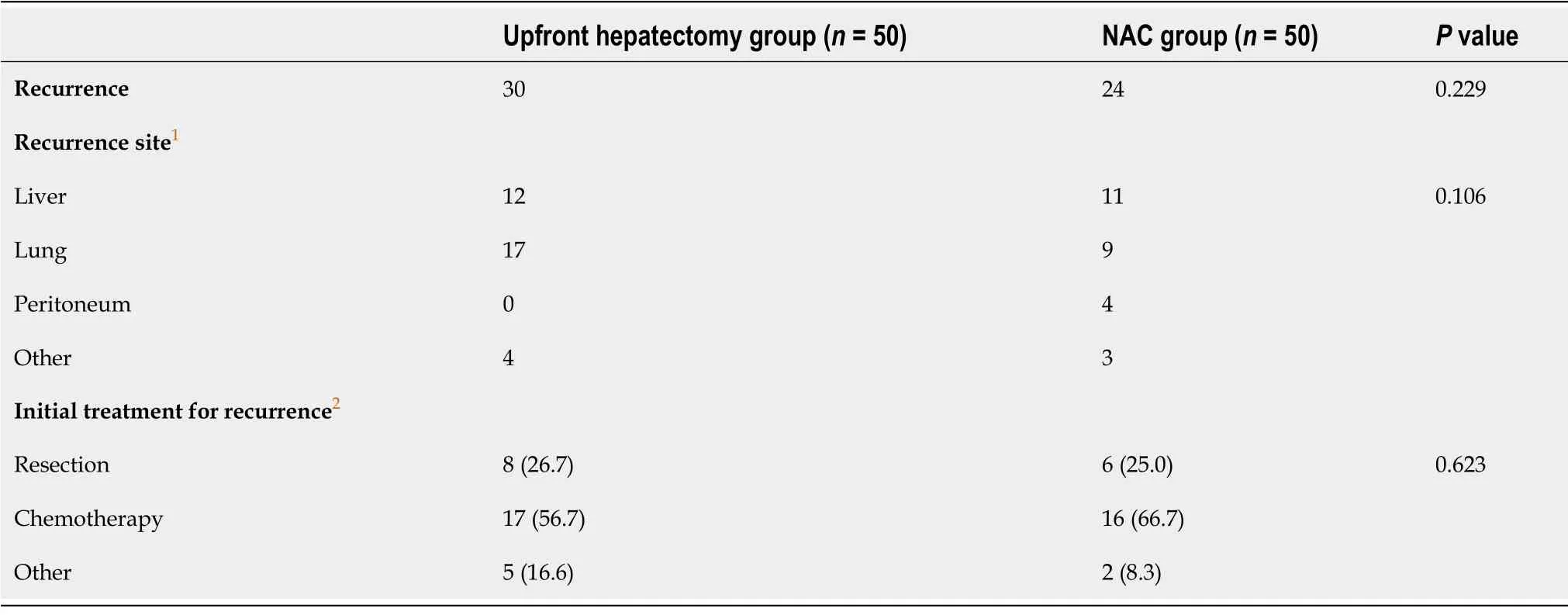
We demonstrated that the OS rate, but not the DFS rate, of high-risk patients was significantly higher in the NAC group than in the upfront hepatectomy group. The post-recurrence clinical course after the first hepatectomy differed between the two groups. The treatment strategy for recurrence showed that chemotherapy was initially selected most frequently in both the upfront hepatectomy and NAC groups,although resection of not only the intrahepatic, but also the extrahepatic, recurrence site is crucial for prolonging the survival of patients with CRLMs[17]. However, in the NAC group, there were conversion cases from chemotherapy to resection, and consequently, there were more resection cases in the NAC group than in the upfront hepatectomy group (56%
27%), although this was not significant.Based on these results, the reason for a better OS rate among high-risk patients in the NAC group may be that the most effective and tolerable chemotherapy regimen has already been established in patients receiving NAC before their first hepatectomy, and appropriate regimens may be available from the start of treatment for recurrence. In fact, the OS rate of the NAC group after recurrence was significantly higher than that of the upfront hepatectomy group (
= 0.04).
Baseline characteristics after propensity score matching
Fifty patients in the upfront hepatectomy group were matched with 50 patients in the NAC group.Patients with insufficient preoperative data or without a suitable match were excluded. After matching preoperative baseline characteristics, treatment-related factors (staged hepatectomy, surgical margins,and adjuvant chemotherapy) were comparable between the two groups (Table 3). The NAC regimens after propensity score matching were as follows:Oxaliplatin-based chemotherapy (30 patients), with molecularly-targeted agents [bevacizumab (11 patients), cetuximab (three patients), and panitumumab(seven patients)]; irinotecan-based chemotherapy (four patients), with molecularly-targeted agents[bevacizumab (two patients) and panitumumab (two patients)]; oxaliplatin- and irinotecan-based chemotherapy (eight patients), with molecularly-targeted agents [bevacizumab (five patients) and cetuximab (one patient)]; fluorouracil and folinic acid (six patients), with cisplatin (four patients); and chemotherapy, including hepatic arterial infusion (two patients). Responses to NAC were defined as follows:Partial response (30 patients), stable disease (17 patients), or progressive disease (three patients). In total, there were 30 responders and 20 non-responders. The median number of treatment cycles was 6 (range from 2 to 25). The upfront hepatectomy group comprised 18 high-risk patients and 32 low-risk patients. The NAC group comprised 13 high-risk patients and 37 low-risk patients (Table 3).The background characteristics were comparable when stratified by high- and low-risk, respectively.
BROTHER1 took sister2 by the hand and said: Look here; we haven1 t had one single happy hour since our mother died. That stepmother3 of ours beats us regularly every day,4 and if we dare go near her she kicks us away. We never get anything but hard dry crusts to eat -- why, the dog under the table is better off than we are. She does throw him a good morsel2 or two now and then. Oh dear! if our own dear mother5 only knew all about it! Come along, and let us go forth3 into the wide world together. 6
Clinical outcomes after propensity score matching
Short-term outcomes, including the amount of intraoperative bleeding, frequency of red blood cell transfusions, postoperative complications, and length of postoperative hospital stay, were not significantly different between the two groups. In the NAC group, there was one complication of Clavien–Dindo grade IV. In this patient, five cycles of irinotecan-based chemotherapy were administered as NAC. Partial resection of segments 7 and 8, with right hepatic vein reconstruction, was performed 4 wk after the last cycle of NAC. Laparotomy hemostasis was performed on postoperative day 5, due to bleeding from the surface of the hepatic dissection.
The following clinicopathological variables were analyzed:Patient-related:Age (< 60
≥ 60 years), sex(male
female), and initial CEA level (< 10
≥ 10 ng/mL); primary tumor-related:Site of the primary lesion (right
left), primary histological type (well/moderately differentiated
others), lymph node metastases (0
≥ 1), depth of tumor invasion [adjacent organ invasion (T4b)
others], lymphatic invasion (0
≥ 1), and venous invasion (0
≥ 1); liver metastasis-related:Number (1–3
≥ 4),maximum diameter (< 40
≥ 40 mm), appearance time (synchronous
metachronous), and tumor distribution (unilobar
bilobar); and treatment-related:Staged hepatectomy (performed
not performed), surgical margins (exposed
not exposed), and adjuvant chemotherapy after primary resection and after hepatectomy (administered
not administered). In addition, left-sided tumors included carcinomas in the descending colon, sigmoid colon, and rectum; and right-sided tumors included carcinomas in the cecum, ascending colon, and transverse colon.
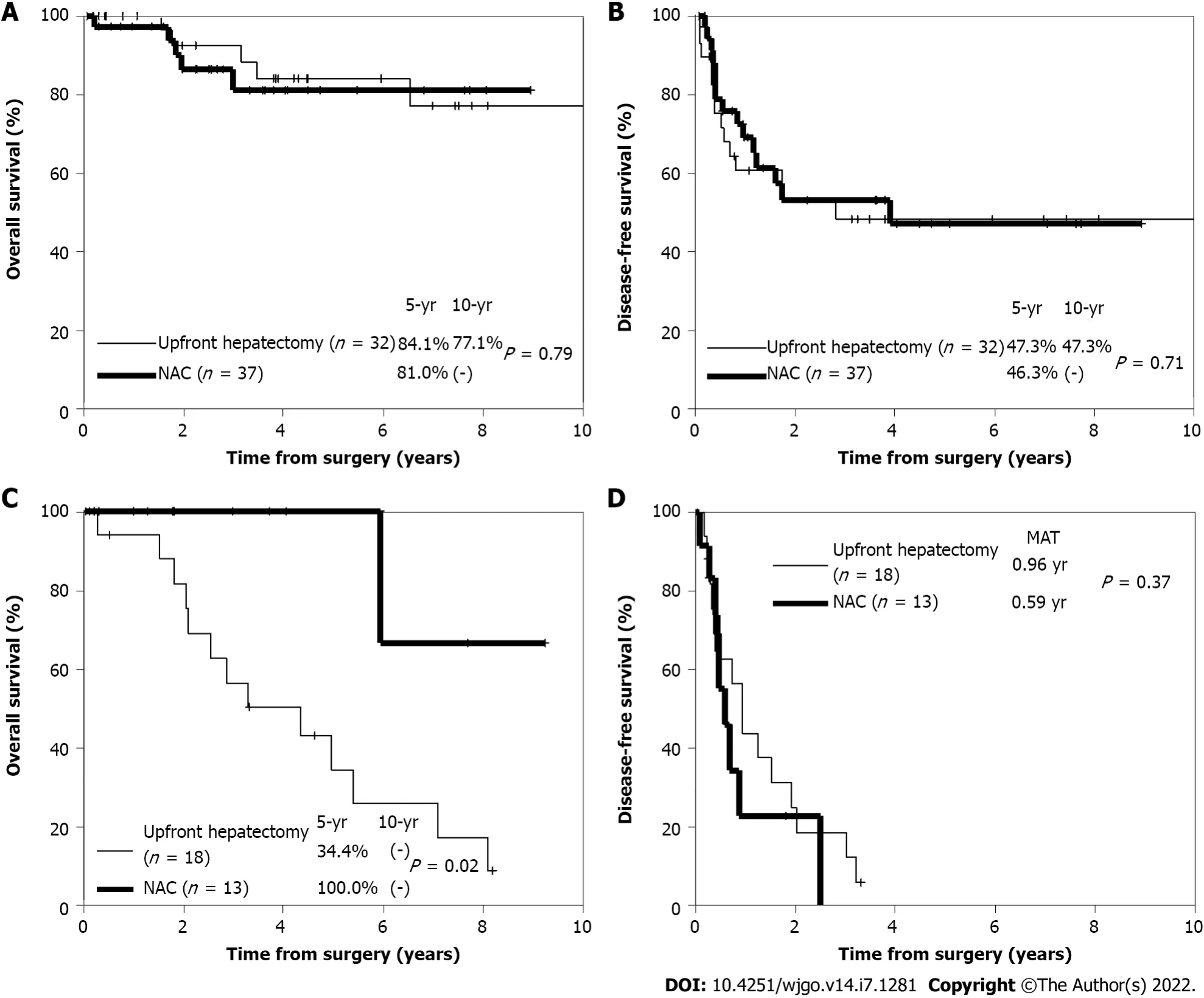
Regarding long-term outcomes, there was no significant difference in the 5-year OS rate between the upfront hepatectomy and NAC groups (63%
83%;
= 0.13) after propensity score matching. Among low-risk patients, there was also no significant difference in the 5-year OS rate (84.1%
81.0%;
= 0.79)(Figure 3A) or 5-year DFS rate (47.3%
46.3%;
= 0.71) (Figure 3B) between the two groups.Conversely, among high-risk patients, the 5-year OS rate was significantly higher in the NAC group than in the upfront hepatectomy group (100%
34.4%;
= 0.02) (Figure 3C). However, there was no significant difference in the 5-year DFS rate between the two groups (
= 0.37) (Figure 3D).
The study was reviewed and approved for publication by our Institutional Reviewer.
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide. The liver is the most common metastatic site of CRC, and hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). Upfront hepatectomy is recommended for patients with resectable CRLMs. However, there are cases of early recurrence after upfront hepatectomy alone in the resectable CRLMs. In selected patients, neoadjuvant chemotherapy (NAC) may improve long-term survival.


DISCUSSION
This study revealed a significantly worse OS rate of patients in resectable CRLMs with two or more risk factors [primary histological type (other than well/moderately differentiated), lymph node metastases(≥ 1), and preoperative CEA levels (≥ 10 g/mL)] who met the high-risk criteria compared to those who met the low-risk criteria. Among high-risk patients, the OS rate of those who received NAC wassignificantly higher than that of those who underwent upfront hepatectomy after propensity score matching. It is a novel finding that the efficacy of NAC for resectable CRLMs was demonstrated after risk stratification and propensity score matching.
The definition of resectable CRLM varies in the literature[3,4,6,15]. In studies that examined the effectiveness of NAC for resectable CRLMs, resectable CRLM was defined as a maximum of four tumors[4]; four or fewer tumors with a maximum diameter of < 5 cm[3]; or (1) A ≥ 30% residual liver volume(regardless of tumor number and size); (2) Resectable or already resected primary tumor; and (3) No unresectable extrahepatic metastases[16]. Some studies did not show a benefit of NAC for resectable CRLMs[3,4]. This may be because the criteria for resectable CRLMs were not specific enough to restrict the patient group to those for whom NAC is truly effective. Even when NAC was shown to be effective,it was considered without propensity score matching[16]. The definition of resectable CRLM in our database is more detailed and the efficacy of NAC was assessed by risk stratification.
Colorful plastic sleds were shoved in the back of the Bronco and stashed9 in the little available trunk space of warm cars idling in the sub-zero Christmas chill. The moon was out and the trees were covered with frost, glittering like a snow globe in a happy child s hand.
Conversely, disadvantages of NAC include the risk that hepatectomy may not be performed in patients who do not respond to NAC. We showed that the effect of chemotherapy was progressive in 6% of NAC cases. To avoid missing the timing of hepatectomy, it is important to evaluate the efficacy of chemotherapy every 2–3 cycles. Other disadvantages of NAC include liver damage and perioperative complications induced by the NAC drugs. Sinusoidal dilation caused by oxaliplatin and steatohepatitis caused by irinotecan have been reported[18]. Furthermore, prolonged systemic NAC alters the liver parenchyma and increases morbidity after major resection[19]. Although many centers specializing in hepatobiliary procedures have reported mortality rates of < 5% after major liver surgery, the morbidity of hepatectomy may have increased with the advent of NAC, due to the hepatic parenchymal damage caused by chemotherapy[5]. The short-term outcomes of the NAC group in this study were comparable to those of a previous study[5]. However, one case of postoperative bleeding was observed after irinotecan-based chemotherapy. As hepatectomy was performed after a sufficient drug interval, no sinusoidal dilation or steatohepatitis was observed in the resected specimen. Postoperative bleeding in this case may have resulted from a complicated hepatic dissection surface. Therefore, careful surgical procedures are required, even after a sufficient drug interval.
Limits of the study
This study has several limitations. The first is its single-center design with limited sample size. Second,its retrospective nature introduces the inevitable risk of selection bias, which could not be completely eradicated, despite using propensity score matching to reduce confounding by indication. Lastly, it has been reported that molecular biological factors, such as
status and microsatellite instability, are prognostic[20,21]. However, this information was unavailable in the present study.
CONCLUSION
Our findings suggest that NAC may improve the prognosis of patients with resectable CRLMs who have at least two of the following risk factors:Preoperative CEA levels (≥ 10 ng/mL), primary histological type (other than well/moderately differentiated), and lymph node metastases (≥ 1). Future prospective, multicenter studies with larger sample sizes are needed to validate these findings
ARTICLE HIGHLIGHTS
Research background
Somehow I managed to lug4 the wet clothes to the laundromat. I spent most of the day washing and drying clothes and thinking how love had disappeared from my life. Staring at the graffiti() on the walls, I felt as wrung-out as the clothes left in the washers.
51. Perish miserably: Burning occurs often in fairy tales. It is symbolic120 of purification (Matthews 1986). The witch being burnt can also represent evil destroying itself (Luthi 1976).
Research motivation
Identifying the poor prognostic factors for upfront hepatectomy in resectable CRLMs and investigating the effectiveness of NAC are urgently needed to improve long-term survival of patients with resectable CRLMs.
Research objectives
Sometimes, if I go up into the attic and listen very carefully, I can almost hear his voice rising and falling, telling me stories I don t understand. I can almost see him in the corner, hunched12() over, listening to his radio and fanning himself. I can see him swishing his brush over the rice paper, and then pointing to me, telling me my own name.
Research methods
Among 644 patients who underwent their first hepatectomy for CRLMs at our institution, 297 resectable cases were stratified into an upfront hepatectomy group (238 patients) and NAC group (59 patients).Poor prognostic factors for upfront hepatectomy were identified using multivariate logistic regression analysis. Propensity score matching was used, and clinical outcomes between the upfront hepatectomy and NAC groups were compared according to the number of poor prognostic factors. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test.
Research results
As independent poor prognostic factors for overall survival (OS) in the upfront hepatectomy group,preoperative carcinoembryonic antigen (CEA) levels (≥ 10 ng/mL) (
= 0.003), primary histological type(other than well/moderately differentiated) (
= 0.04), and primary lymph node metastases (≥ 1) (
=0.04) were identified. High-risk status was defined as the presence of two or more risk factors. Fifty patients were matched in upfront hepatectomy and NAC groups respectively, after propensity score matching. Among high-risk patients, the 5-year OS rate was significantly higher in the NAC group (13 patients) than in the upfront hepatectomy group (18 patients) (100%
34%;
= 0.02).
Research conclusions
NAC was effective in patients with resectable CRLMs who had at least two of the following risk factors:Preoperative CEA levels (≥ 10 ng/mL), primary histological type (other than well/moderately differentiated), and lymph node metastases (≥ 1).
Research perspectives
NAC therapy may improve the prognosis of high-risk patients with resectable CRLMs.
I immediately informed the dive supervisor10 of my dilemma over the communicator. His instructions were unclear due to the fact that he, along with five other divers, were all laughing hysterically11.
Takeda K, Sawada Y, Yabushita Y, Honma Y, Kumamoto T and Watanabe J performed the research; Takeda K drafted the manuscript; Kunisaki C, Misumi T and Endo I revised the manuscript critically; all authors read and approved the final manuscript.
Recurrence after hepatectomy was observed in 30 (60%) patients in the upfront hepatectomy group and 24 (48%) patients in the NAC group. The difference between them was not statistically significant.The lung and remnant liver were the most frequent sites of recurrence in the upfront hepatectomy and NAC groups, respectively, and there was no significant difference in the distribution of initial recurrence sites. Regarding the initial treatment strategy for recurrence, resection and chemotherapy were adopted in 26.7% and 57.7% of patients in the upfront hepatectomy group and 25.0% and 66.7% of patients in the NAC group, respectively. The differences between them were not statistically significant(Table 4). Especially among high-risk patients, recurrence was observed in 15 (83%) of the 18 patients in the upfront hepatectomy group. Resection was adopted as the initial treatment strategy for recurrence in four patients, chemotherapy in six patients, and other therapies in five patients. None of the patients who received chemotherapy were converted to resection, and resection could only be performed in 27%of patients with recurrence. Conversely, recurrence was observed in nine (69%) of the 13 high-riskpatients in the NAC group. Resection was adopted as the initial treatment strategy for recurrence in two patients. Chemotherapy was adopted as the initial treatment strategy for recurrence in seven patients(the same regimen was used in all responders; a different regimen was used in non-responders), three ofwhom were converted to resection (Table 5). Consequently, resection was performed in 56% of patients with recurrence in the NAC group, which was higher than the proportion of high-risk patients in the upfront hepatectomy group (27%). The 5-year OS rate after the first recurrence in the NAC group was significantly higher than that in the upfront hepatectomy group (66.7%
17.9%;
= 0.04).
The requirement for written informed consent was waived owing to the retrospective nature of the study.
All the authors report no relevant conflicts of interest for this article.
The original anonymous dataset is available on request from the corresponding author at kazu1968@yokohama-cu.ac.jp.
The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
Japan
She was flushed and breathing hard when I entered her room. I slipped the tape into the recorder and held the microphone to her lips. Ruthie, Hannah, Molly?this is the most important tape. She held my hand and closed her eyes. Someday your daddy will bring home a new mommy. Please make her feel special. Show her how to take care of you. Ruthie, honey, help her get your Brownie uniform ready each Tuesday. Hannah, tell her you don t want meat sauce on your spaghetti(). She won t know you like it separate. Molly, don t get mad if there s no apple juice. Drink something else. It s okay to be sad, sweeties. Jesus cried too. He knows about sadness and will help you to be happy again. Remember, I ll always love you.
Then she cracked her second nut, and all the forest behind her seemed to be in fire and flames, and the evil spirits howled even worse than on the previous day; but the contract they would not give up
Kazuhisa Takeda 0000-0001-8092-6206; Yu Sawada 0000-0002-5641-1103; Yasuhiro Yabushita 0000-0003-1090-1614; Yuki Honma 0000-0001-6560-2895; Takafumi Kumamoto 0000-0002-9928-4386; Jun Watanabe 0000-0002-7187-3664; Ryusei Matsuyama 0000-0001-5131-0023; Chikara Kunisaki 0000-0002-0843-8766; Toshihiro Misumi 0000-0001-8339-2895; Itaru Endo 0000-0001-5520-8114.
Fan JR
A
Fan JR
1 de Ridder JAM, van der Stok EP, Mekenkamp LJ, Wiering B, Koopman M, Punt CJA, Verhoef C, de Wilt JH.Management of liver metastases in colorectal cancer patients:A retrospective case-control study of systemic therapy
liver resection.
2016; 59:13-21 [PMID:26994469 DOI:10.1016/j.ejca.2016.02.003]
2 Ishida K, Uesugi N, Hasegawa Y, Sugimoto R, Takahara T, Otsuka K, Nitta H, Kawasaki T, Wakabayashi G, Sugai T.Proposal for novel histological findings of colorectal liver metastases with preoperative chemotherapy.
2015; 65:367-373 [PMID:25940915 DOI:10.1111/pin.12300]
3 Hirokawa F, Asakuma M, Komeda K, Shimizu T, Inoue Y, Kagota S, Tomioka A, Uchiyama K. Is neoadjuvant chemotherapy appropriate for patients with resectable liver metastases from colorectal cancer?
2019; 49:82-89[PMID:30255329 DOI:10.1007/s00595-018-1716-x]
4 Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery
surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983):long-term results of a randomised, controlled, phase 3 trial.
2013; 14:1208-1215 [PMID:24120480 DOI:10.1016/S1470-2045(13)70447-9]
5 Boostrom SY, Nagorney DM, Donohue JH, Harmsen S, Thomsen K, Que F, Kendrick M, Reid-Lombardo KM. Impact of neoadjuvant chemotherapy with FOLFOX/FOLFIRI on disease-free and overall survival of patients with colorectal metastases.
2009; 13:2003-9; discussion 2009 [PMID:19760306 DOI:10.1007/s11605-009-1007-3]
6 Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer:analysis of 1001 consecutive cases.
1999; 230:309-18; discussion 318 [PMID:10493478 DOI:10.1097/00000658-199909000-00004]
7 Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer.
2008; 32:1097-1107 [PMID:18200429 DOI:10.1007/s00268-007-9348-0]
8 Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE.Hepatic resection for metastatic colorectal adenocarcinoma:a proposal of a prognostic scoring system.
1999; 189:291-299 [PMID:10472930 DOI:10.1016/s1072-7515(99)00089-7]
9 Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score:emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases.
2004; 139:1168-1172 [PMID:15545561 DOI:10.1001/archsurg.139.11.1168]
10 Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I. R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy.
2011; 37:336-343 [PMID:21277151 DOI:10.1016/j.ejso.2011.01.007]
11 Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M,Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours:revised RECIST guideline (version 1.1).
2009; 45:228-247 [PMID:19097774 DOI:10.1016/j.ejca.2008.10.026]
12 Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R.Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases:FFCD ACHBTH AURC 9002 trial.
2006; 24:4976-4982 [DOI:10.1200/jco.2006.06.8353]
13 Hasegawa K, Saiura A, Takayama T, Miyagawa S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H,Oba M, Takahashi M, Mizunuma N, Matsuyama Y, Watanabe T, Makuuchi M, Kokudo N. Adjuvant Oral Uracil-Tegafur with Leucovorin for Colorectal Cancer Liver Metastases:A Randomized Controlled Trial.
2016; 11:e0162400[PMID:27588959 DOI:10.1371/journal.pone.0162400]
14 Tanaka K, Shimada H, Kubota K, Ueda M, Endo I, Sekido H, Togo S. Effectiveness of prehepatectomy intra-arterial chemotherapy for multiple bilobar colorectal cancer metastases to the liver:a clinicopathologic study of peritumoral vasculobiliary invasion.
2005; 137:156-164 [PMID:15674195 DOI:10.1016/j.surg.2004.07.007]
15 Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, Starzl TE. Experience in hepatic resection for metastatic colorectal cancer:analysis of clinical and pathologic risk factors.
1994; 116:703-10; discussion 710[PMID:7940169]
16 Ninomiya M, Emi Y, Motomura T, Tomino T, Iguchi T, Kayashima H, Harada N, Uchiyama H, Nishizaki T, Higashi H,Kuwano H. Efficacy of neoadjuvant chemotherapy in patients with high-risk resectable colorectal liver metastases.
2021; 26:2255-2264 [PMID:34519930 DOI:10.1007/s10147-021-02024-5]
17 Imai K, Yamashita YI, Miyamoto Y, Nakagawa S, Okabe H, Hashimoto D, Chikamoto A, Baba H. The predictors and oncological outcomes of repeat surgery for recurrence after hepatectomy for colorectal liver metastases.
2018; 23:908-916 [PMID:29619592 DOI:10.1007/s10147-018-1273-8]
18 Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases.
2006; 24:2065-2072 [PMID:16648507 DOI:10.1200/JCO.2005.05.3074]
19 Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases.
2006; 243:1-7 [PMID:16371728 DOI:10.1097/01.sla.0000193603.26265.c3]
20 Jácome AA, Vreeland TJ, Johnson B, Kawaguchi Y, Wei SH, Nancy You Y, Vilar E, Vauthey JN, Eng C. The prognostic impact of RAS on overall survival following liver resection in early
late-onset colorectal cancer patients.
2021; 124:797-804 [PMID:33208919 DOI:10.1038/s41416-020-01169-w]
21 Fujiyoshi K, Yamamoto G, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, Sakamoto H, Akagi K. High concordance rate of KRAS/BRAF mutations and MSI-H between primary colorectal cancer and corresponding metastases.
2017; 37:785-792 [PMID:28000889 DOI:10.3892/or.2016.5323]
 World Journal of Gastrointestinal Oncology2022年7期
World Journal of Gastrointestinal Oncology2022年7期
- World Journal of Gastrointestinal Oncology的其它文章
- Gut microbiome and pancreatic cancer cachexia:An evolving relationship
- Prospects and applications of enucleation in solid pseudopapillary neoplasms of the pancreas
- Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma
- KIFC3 promotes proliferation, migration and invasion of esophageal squamous cell carcinoma cells by activating EMT and β-catenin signaling
- Differences of core genes in liver fibrosis and hepatocellular carcinoma:Evidence from integrated bioinformatics and immunohistochemical analysis
- Increased 5-hydroxymethylcytosine is a favorable prognostic factor of Helicobacter pylori-negative gastric cancer patients
