Prospects and applications of enucleation in solid pseudopapillary neoplasms of the pancreas
INTRODUCTION
Solid pseudopapillary neoplasms (SPNs) of the pancreas account for approximately 1%-3% of all pancreatic neoplasms[1,2]. To date, surgery remains the only curative treatment for SPN patients[3-5].Conventional pancreatectomy, such as pancreaticoduodenectomy and distal pancreatectomy, as the mainstream surgical options, has achieved good results and prognoses.
In recent years, enucleation, as an organ-sparing surgical method, has been increasingly widely used in the treatment of some benign and low-grade malignant tumors, including SPNs[1,4,6,7]. Compared to conventional pancreatectomy, enucleation can preserve the physiological function of the pancreas to the maximum extent while treating tumors, lengthen the life of patients, and improve their quality of life[1,7].
20.Two pearls: Pearls often symbolize innocence53, purity, faith, wealth, health, salvation54, and self-sacrifice (Olderr 1986).Return to place in story.
Enucleation has undoubtedly come to stay as an alternative surgical procedure for SPNs. However, to improve and widen the application of enucleation in SPNs, some problems must be solved in the future.This review article summarizes findings published in recent years on the enucleation of SPNs as well as potential future developments and directions.
FEASIBILITY AND ADVANTAGES OF ENUCLEATION APPLICATION IN SPNS
The low-grade malignancy of SPNs has been widely accepted, and reports of SPNs have become more extensive and in depth in recent years. To date, surgical treatment of SPNs, which has a 5-year survival rate of more than 95%, is still the only treatment that can achieve curative effects[2,3,8]. All patients who are eligible for surgical treatment should be encouraged to undergo relevant management, as surgery is indicated even if R1 resection is performed[8,9]. Surgery, if possible, is also a good option for patients with local progression and metastasis at the time of diagnosis, and distant metastasis is not an absolute contraindication to surgical treatment[3,9-12]. The specific surgical method is determined by the location, size, intraoperative pathology, and surrounding tissue invasion and distant metastasis of the tumor.
Clinical manifestations associated with SPNs are often nonspecific[5]. The most common symptom is abdominal pain[13]. For instance, patients with SPNs at the head of the pancreas do not experience obstructive jaundice and pancreatitis like those with other malignant pancreatic tumors[3]. In addition,some patients have no symptoms and are first discovered accidentally by epigastric imaging[4,10]. On the one hand, the awareness of the public about health management has gradually improved, and the state and individuals are paying increasing attention to timely physical examinations. On the other hand, with the expansion and improvement of imaging techniques worldwide, there has been an increase in the incidence of SPNs, and tumors are being increasingly detected at an early stage in asymptomatic patients. The earlier the tumor is detected, the smaller the tumor is likely to be, the more opportunities there are for surgical treatment, the more surgical options that are available, and the better the outcome.
Enucleation is expected to preserve normal pancreatic function and improve postoperative quality of life by preserving normal pancreatic tissue to a large extent. However, several cases of positive surgical margins in SPN patients undergoing enucleation have been reported[1,17]. To treat tumors while preserving organ function, we need to pay attention to the following points:First, further research and investigation are needed to determine the appropriate distance from the tumor to the surgical margin.Second, tumor characteristics, such as the composition, size, and shape of the tumor as well as its relationship with surrounding blood vessels, should be carefully evaluated by intraoperative ultrasound and other equipment, and then the resection scope can be determined. Finally, the determination of negative margins by intraoperative frozen section is of great significance to the prognosis of patients.We recommend multipoint biopsies on the tissue margins of the three-dimensional structure of the tumor to confirm the status of the margins, especially the dorsal side of the tumor in the visual blind area during surgery.
In recent decades, the concepts of minimally invasive surgery and enhanced recovery after surgery have had a great influence on the surgical treatment of relevant diseases. In addition, technological innovation and research achievements provide support for and guarantee for the development of surgical strategies toward more minimally invasive and accurate directions. In the stage of rapid development of medicine, people pursue not only survival but also quality of life. For younger patients,especially pediatric patients, it is extremely critical to be able to treat the tumor and preserve normal function to the greatest extent to improve postoperative quality of life.
We sang all the way home from church. At lunch, Mom had a surprise for us. She had bought a dozen eggs, and we had boiled Easter eggs with our fried8 potatoes! Late that afternoon, the minister drove9 up in his car. Mom went to the door, talked with him for a moment, and then came back with an envelope in her hand. We asked what it was, but she didn’t say a word. She opened the envelope and out fell a bunch10 of money. There were three crisp11 twenty-dollar bills, one ten-dollar bill and seventeen one-dollar bills.
But one day, as the brothers were as usual doing the honours to their guests, an old man turned to them and said, Yes, it is all most beautiful, but there is still something it needs! And what may that be? A pitcher3 of the water of life, a branch of the tree the smell of whose flowers gives eternal beauty, and the talking bird
Furthermore, older age is an independent risk factor for recurrence[21] and is significantly associated with tumor recurrence[22]. In addition, as there are no significant differences in margin status,peripheral tissue invasion, postoperative complications, disease-free survival, or overall survival between male and female patients, the prognosis of SPNs has been reported to be similar between male and female patients[21]. Therefore, for elderly male and female patients with SPNs, surgery should be more radical, and postoperative follow-up should be more frequent[11].
Compared with conventional surgical methods, enucleation removes the tumor while preserving as much of the normal pancreatic parenchyma as possible, which is closely related to the postoperative quality of life of patients, especially young patients. Importantly, according to recent studies[1,7,15],compared with conventional surgical methods, enucleation does not increase the risk of tumor recurrence or metastasis in SPN patients. Previous studies[1,2,7], including one of our studies, have reported the safety and efficacy of enucleation as an organ preservation method in the surgical management of SPNs, and it has some advantages over conventional pancreatectomy in some cases(Figure 1). Even if the tumor is located in the head of the pancreas, enucleation is safe and can ensure adequate margins[4,7,16]. Our previous study revealed that enucleation had a shorter duration of surgery, less blood loss, lower rate of exocrine insufficiency, and comparable morbidity compared with conventional pancreatectomy[7]. Compared with conventional pancreatectomy, enucleation does not require digestive tract reconstruction, reducing surgical complexity and the risk of associated postoperative complications.
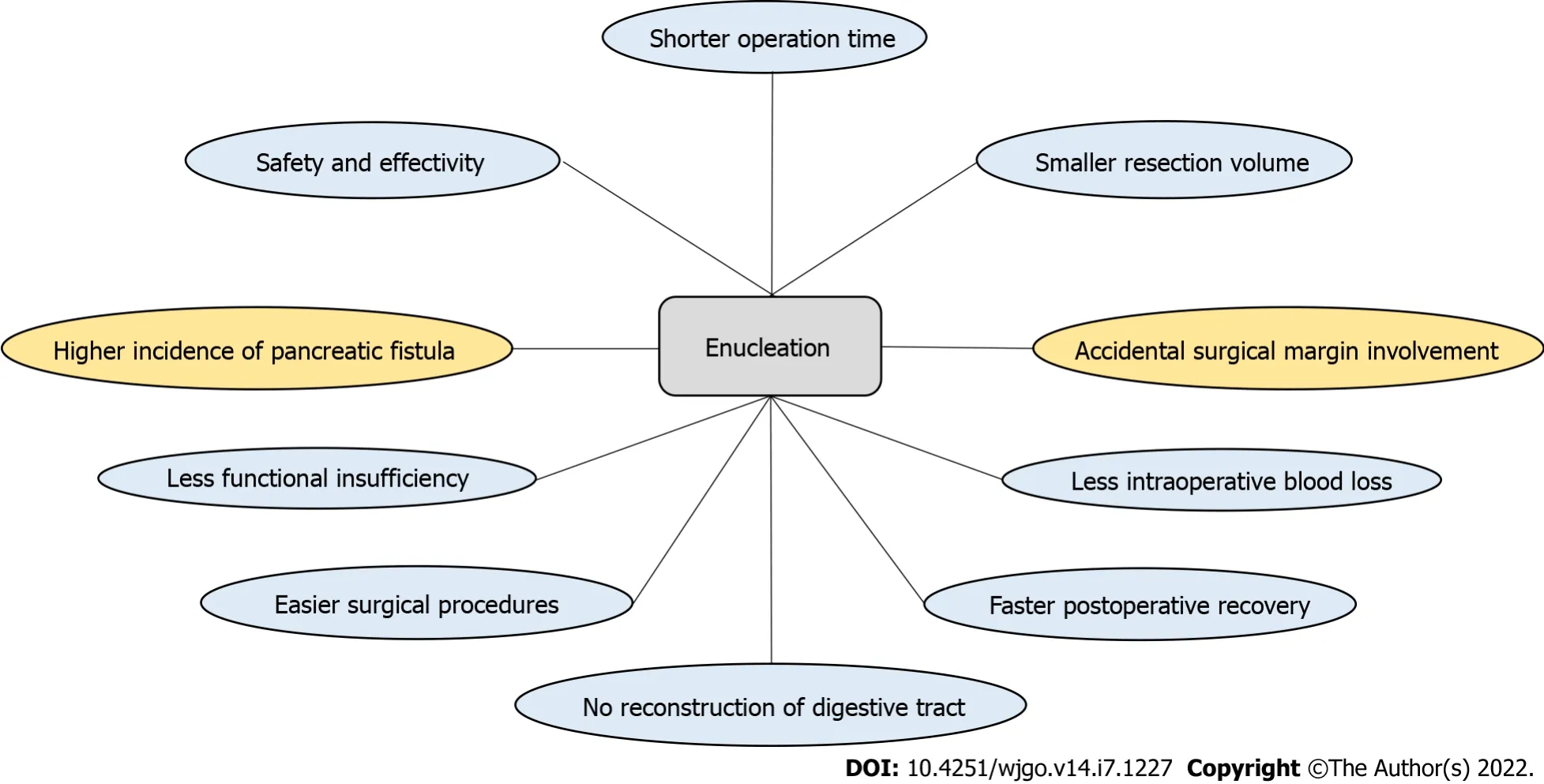
In summary, enucleation, as a safe and effective surgical procedure, should be more widely used in appropriate patients. An increasing number of studies have reported that enucleation can be achieved successfully by laparotomy, laparoscopy, or even robotic techniques (Table 1).

PRECAUTIONS OF AND RELEVANT SUGGESTIONS FOR ENUCLEATION APPLICATION IN SPNS
For pediatric patients
There are differences in some clinical characteristics between children and adults, such as sex composition, mean diameter of the tumor, and common tumor sites[17]. Cho
[1] showed that compared with conventional surgery in children, enucleation is safe and effective and reported some indications for enucleation. In that study, enucleation had a similar rate of morbidity and mortality as conventional pancreatectomy, prevented tumor recurrence, and reduced the incidence of postoperative pancreatic fistula. Even in pediatric patients who must undergo conventional pancreatectomy, the spleen should be preserved to prevent potentially dangerous infections associated with splenectomy. As a special population, the monitoring and management of pediatric patients should be strengthened to reduce other complications caused by prolonged hospitalization.
For pregnant patients
A diagnosis of SPN during pregnancy is rare and poses a threat to both the mother and the fetus.Sometimes, large cystic-solid masses of the pancreas found in pregnant women should be considered SPNs[18]. A 26-year-old woman who was diagnosed with SPN at 21 wk of gestation underwent tumor enucleation for SPN at 22 wk of gestation, and a healthy female infant was delivered vaginally at 39 wk and 5 d of gestation[10]. Similarly, another woman who was 26 years old underwent enucleation for SPN at 14 wk of gestation and gave birth to a mature female baby at 38 wk[18]. Surgery during pregnancy should be performed in cooperation with the surgeon and obstetrician to remove the tumor while ensuring the safety of the mother and fetus. Generally, the second trimester is the most favorable time window for surgical intervention for SPNs because fetal organogenesis is complete and the size of the fetus is adequate, which can reduce the influence of spontaneous abortion in early pregnancy and the influence of the large size of the fetus in late pregnancy on the difficulty of the operation[10].
Sex differences
To date, there are still no good tumor markers of suggesting the occurrence, development, recurrence,and metastasis of SPNs. Laboratory tests, including tumor markers, are nonspecific[3,4]. With regard to current imaging techniques and the development of tumor markers associated with SPNs, imaging is of higher value during follow-up. It is expected that more studies will be conducted to find relevant markers that can detect abnormalities early and provide help for the early diagnosis and treatment of this tumor. In addition, surgery-related complications, such as pancreatectomy-related diabetes mellitus, need to be monitored because most patients have no obvious symptoms early on.
Overall, the tumors are significantly larger in females with SPNs than in males[21], but the tumors are more aggressive and develop at a later age in men[11]. In terms of composition, the mean solid component is significantly higher in male patients than in female patients[21]. For immunohistochemical staining, the expression of β-catenin is significantly decreased in male patients, but vimentin expression is significantly increased in male patients[21]. More research is expected to explain the underlying causes of these differences.
Imaging examination is widely used in the diagnosis and differential diagnosis of various diseases. In a study of SPNs, the accuracies of imaging diagnoses for SPNs in male and female patients were 54.0%and 70.5%, respectively[21]. However, in imaging diagnoses, SPNs in male patients were more likely to be misdiagnosed as malignant tumors than those in female patients, with misdiagnosis rates of 37.7%and 10.7%, respectively[21]. These results suggest that when imaging alone is insufficient to determine a diagnosis or differential diagnosis, other examinations, such as preoperative pathological examination,may be necessary to supplement the deficiency in imaging and improve the overall diagnostic accuracy for SPNs.
In fact, conventional pancreatectomy achieves the primary goal of negative margins while extensively removing the normal pancreatic parenchyma. Extensive excision of normal pancreatic tissue at the same time as tumor excision increases the risk of postoperative endocrine and exocrine pancreatic insufficiency[6]. Falconi
[14] showed that the incidences of endocrine insufficiency in pancreatic parenchymal-preserving resection, distal pancreatectomy, and pancreaticoduodenectomy were 3%,14%, and 18%, respectively. For wide surgical resection-induced pancreatic dysfunction, the lifetime psychological and physical effects of replacement therapy are enormous and unacceptable.
Histopathological features
A definite pathological diagnosis can guarantee the application of enucleation in SPN. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) can achieve pathological tissue acquisition, and SPNs can be diagnosed preoperatively and differentiated from other diseases. Lubezky
[3] reported that the sensitivity and specificity of EUS-FNA were 90.9% and 100%, respectively. In addition, intraoperative frozen sections are important for determining the presence of an involved margin. If intraoperative frozen sectioning reveals invasive features (such as adenocarcinoma or carcinoma), conventional surgery should be performed[7].
In fact, there is still no consensus on the malignant characteristics of SPNs[1,8]. In 2010, the World Health Organization (WHO) classified SPNs as a low-grade malignant neoplasm. Prior to this, the malignant components of solid pseudopapillary carcinoma (SPC) of the pancreas were defined by the WHO as vascular invasion, perineural invasion, or deep invasion into the surrounding pancreatic parenchyma. Importantly, recurrence and metastasis of SPNs may occur even in the absence of microscopic features similar to SPC of the pancreas, and these features may not cause malignant behavior[1,8]. For example, in a study, 98 of 351 patients with SPNs presented with malignant features,but recurrence occurred in 9 of the 317 patients who underwent surgery for SPNs and had a follow-up of more than 6 mo[8]. Among these nine patients who relapsed after surgery, eight had R0 resection and six did not meet the WHO definition of SPC[8].
In summary, there is no consensus on the malignant characteristics of SPNs of the pancreas, and the malignant components of SPC of the pancreas may not be absolute contraindications for enucleation with negative surgical margin. However, it should be noted that during enucleation of SPNs with peripheral tissue invasion, more peritumor pancreatic tissue should be resected than that resected during enucleation of SPNs without peripheral tissue invasion.
Relationship between the tumor and surrounding tissue
The main procedures for enucleation are summarized in Figure 2, and some keys to enucleation are described below. Some preoperative and intraoperative auxiliary examinations are closely related to the correct diagnosis and evaluation of the relationship between the tumor and the surrounding tissues,such as the MPD, common bile duct (CBD), and mesenteric vessels. Although the measurement is less precise, in patients with postoperative complications, computed tomography can be used to detect tumors close to the MPD, and a distance between the tumor and the MPD of less than 2-3 mm is a risk factor for postoperative pancreatic leakage[15]. Intraoperative ultrasound can be further used to evaluate the tumor and provide guidance for surgical resection. Importantly, multiple lesions may occur in patients with SPNs[7].
Postoperative pancreatic fistula
Enucleation has been reported to be associated with a higher risk of postoperative pancreatic leakage,and pancreatic leakage is more serious than conventional pancreatectomy, especially in patients with tumors larger than 3 cm and tumors close to the MPD[1,7,14,25]. Cho
[1] reported that the most common postoperative complication in pediatric patients was postoperative pancreatic fistula (POPF).Although the overall incidence of pancreatic leakage was similar in the enucleation and conventional pancreatectomy groups, mild grade A symptoms mainly occurred in the conventional pancreatectomy group, and the incidence of grades B and C symptoms was more common in the enucleation group,which consequently prolonged the duration of maintaining drainage with POPF in the enucleation group[1]. Patients with tumors at the head and neck of the pancreas had a higher incidence of complications than those with tumors at other sites after enucleation for pancreatic benign tumors[6]. However,it should be noted that postoperative pancreatic fistula was not associated with further progression to pancreatic insufficiency after pancreatectomy[14]. Because of the higher incidence of pancreatic fistula as a short-term complication after enucleation, it is not advised to choose a conventional surgical approach imprudently that will increase the risk of postoperative pancreatic dysfunction.
Although the results of current conservative treatments for pancreatic leakage are good, more methods for reducing postoperative pancreatic leakage are expected.
Pancreatic excision and onset of diabetes mellitus
Kwon
[26] found that the pancreatic resection volume (in milliliters) and resected volume ratio (in percentage) were associated with the onset of diabetes mellitus after distal pancreatectomy, especially in patients with a high pancreatic resected volume ratio (> 35.6%) in distal pancreatectomy. It is suggested to preserve as much normal pancreatic tissue as possible under the condition of ensuring a positive margin to reduce the risk of postoperative dysfunction. For patients diagnosed with severe impairment of pancreatic function before surgery, conventional pancreatectomy, which enables negative margins to be achieved more easily, should be considered.
I sincerely petitioned that your classroom would be a safe haven for my child to grow and learn, lending itself to the crazy, yet somehow perfect, mixture of self-discipline and controlled instruction
Different types of enucleation
Enucleation has been gradually completed
laparotomy, laparoscopy, and robotic approaches, each of which has certain characteristics. For example, open enucleation is more suitable for tumors that are deep or posterior lesions and located to the right of the superior mesenteric vein[6]. Laparoscopic tumor enucleation is also feasible for the treatment of some SPN patients and has certain advantages[2,27].Laparoscopic enucleation has a clear and magnifying optical field, which can make the resection more detailed and may be beneficial to the protection of the MPD[24]. Compared with open surgery, the robotic approach provides an alternative for SPNs in the head of the pancreas without increasing the incidence of clinically relevant pancreatic fistula or other major complications, and patients can obtain a favorable prognosis[28]. There are few reports on surgical procedures for enucleation in patients with SPNs, and some surgical details can be seen in these articles[6,24,28,29], including our previous one[7].
Surgical key points of enucleation
SPNs can occur anywhere in the pancreas[2], even outside the pancreas[23], and they can be solid, solidcystic, or cystic in composition. For enucleation, it is important to carefully evaluate the tumor size,location, depth of implantation into the pancreas, and distance between the pancreatic duct and tumor margin because it may be difficult to distinguish between the tumor and normal tissue and the relationship between the surrounding organs during surgery. While laparoscopic resection of tumors deeply embedded in the pancreas is technically feasible and safe compared to that of superficial tumors,it is more challenging[24]. Accidental damage to important surrounding structures may result in serious complications. If the main pancreatic duct (MPD) is damaged during enucleation, there is an increased risk of forced conversion of enucleation to conventional pancreatectomy, postoperative pancreatic leakage, and iatrogenic pancreatic duct stenosis.
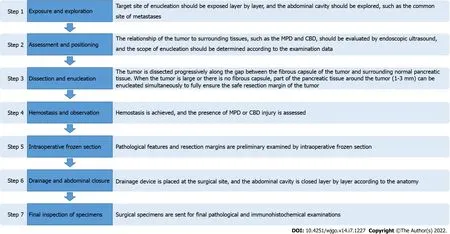
Correctly exposing the location of the tumor is critical for surgery because it can appear anywhere in the pancreas. In the process of enucleation, the tumor can be taped to expose the boundary between the tumor and normal tissue more clearly, which is conducive to the complete enucleation of the tumor and the preservation of normal pancreatic parenchyma[7]. Similarly, traction sutures are beneficial for enucleation of solid pancreatic tumors[6]. In the dissection of tumors from the surrounding normal pancreatic parenchyma, the use of monopolar cautery is more efficient than the use of an ultrasonic scalpel for fine dissection[6]. Parenchymal sutures and a tissue sealant can be used in patients whose hemostasis and pancreatostasis cannot be satisfactorily managed with bipolar cautery and ultrasound scalpel[7].
Pancreatic duct stents can be used in patients with a small distance between the tumor and the pancreatic duct to facilitate intraoperative identification of the location of the pancreatic duct and reduce the risk of accidental intraoperative injury to the pancreatic duct. If the mass is near the confluence region, a Foley catheter can be inserted into the CBD to avoid accidental injury during surgery[25]. The possibility of damaging the MPD can be reduced by preoperative endoscopic implantation of pancreatic duct stents as intraoperative guidance[6].
Drainage tubes and some measures to the enucleated sites are applied to reduce postoperative complications, unnecessary invasive procedures, and even reoperation. Some surgeons who apply fibrin glue and absorbable fibrin sealant patches to the enucleated sites in most patients[6]. In a case report of SPN, a drainage stent was placed in the MPD of the patient before surgery, and the surgeon used only interrupted sutures to close the pancreatic parenchymal defect after enucleation[29]. Even without the use of drainage tubes, the patient was discharged 2 wk after surgery without postoperative complications[29]. In the future, more research findings and inventions are expected to reduce the incidence of pancreatic leakage after enucleation.
The integrity of the MPD and CBD can be confirmed by intraoperative cholangiopancreatography with methylene blue[25]. For patients with proven MPD or CBD damage, polyprolene sutures can be used to repair or rebuild the tube[25]. The Roux-Y loop can be used to treat patients with suspected MPD injury or a wide wounded area (diameter > 3 cm) of the pancreatic parenchyma[7]. For patients with severe MPD damage, a fine silicon tube can be inserted into the MPD as a stent, and the other side of the silicon tube can be inserted through the papilla into the duodenal cavity, which is fixed with soluble sutures[23].
For benign pancreatic tumors
For benign pancreatic tumors, Falconi
[14] revealed that atypical resection has an acceptable risk of postoperative complications and significantly reduces the risk of long-term complications. Lu
[25]reported that enucleation is recommended for benign or low-grade tumors of the proximal pancreas,and large tumors and proximity to the MPD are not absolute contraindications, although the postoperative fistula rate would be high. Laparoscopic enucleation is safe and effective for benign and low-grade malignancies and is associated with favorable perioperative outcomes[6]. Although these findings relate to benign or low-grade pancreatic tumors, they may also apply to SPNs, which are a member of the group.
A wrong order given in front of the reef- the slightest hesitation- and the boat would be lost, Then it would be all over with me and Martin too! This thought passed through Jurgen s mind one day while theywere out at sea, where his foster-father had been taken suddenlyill
UNANSWERED QUESTIONS REGARDING ENUCLEATION IN SPNS AND FUTURE PROSPECTS
What are the best surgical indications?
According to the above findings, it can be concluded that the lower the degree of malignancy of the tumor, the farther the distance from the MPD to the tumor margin, and the smaller the volume of the removed pancreatic tissue, the more suitable enucleation is. However, the specific critical value still lacks relevant data, so there is no unified view. Higher-level evidence is needed to further explore the following questions:How do the location and size of the tumor affect the indications for enucleation due to anatomic factors? What is the effect of tumor components on indications, and are solid or cystic component tumors more suitable for enucleation? Can surgical indications for enucleation be relaxed for people seeking a higher quality of life, and what are the indications for this group?
Then the doll s eyes began to shine like two stars and it became alive. It ate a little and said: Do not fear, little Vasilissa. Go where thou hast been sent. While I am with thee no harm shall come to thee from the old witch. So Vasilissa put the doll back into her pocket, crossed herself and started out into the dark, wild forest.
How can complications, especially pancreatic fistula, be prevented and intervened?
Common postoperative complications, such as pancreatic leakage and emerging diabetes, are related to the exocrine and endocrine functions of the pancreas. The problems related to pancreatic leakage have been presented in the section about postoperative pancreatic fistula and surgical procedures of enucleation mentioned in this paper. New-onset diabetes mellitus (NODM) should be monitored for a long time to prevent multisystem harm caused by the loss of glucose homeostasis. Due to the uneven distribution of islets in the pancreas, the resection volume of patients with NODM caused by resection at different sites needs to be further studied to guide the control and prediction of postoperative NODM.
How can the surgical margin be guaranteed?
The king s ability to give dresses with these celestial qualities implies that even the heavens and gods are conspiring69 against Donkeyskin s wishes at this point in the tale.Return to place in story.
What is the prognosis of long-term oncology?
Although the majority of patients have a good prognosis, approximately 15% of patients present with malignant signs of peripheral organ invasion and metastatic disease[14]. The absence of malignant histological appearance cannot completely exclude the risk of postoperative metastasis and recurrence,so regular oncological follow-up and long-term surveillance are important for the early detection and further treatment of metastasis and recurrence.
He dug in his heels, held on even tighter, nobody could say he wasn t a fighter. The water seething7 and boiling, turned bright red then dark as that grisly catfish became a shark.
In a small number of patients, distant metastasis can occur in the peritoneum, perirenal lymph nodes,colon, small intestine, and other sites[30]. Usually, the most common site of postoperative metastasis is the liver[2]. When a suspicious liver mass is detected during postoperative follow-up, it should be differentiated from primary hepatocellular carcinoma and nonpancreatic metastatic tumors.Percutaneous liver biopsy with immunohistochemistry can be used to confirm the diagnosis. Even if liver metastases occur, patients with SPNs can achieve long-term survival with timely surgical treatment.
Through observations and studies, there are certain differences between male and female patients that deserve attention. Approximately 90% of SPNs occur in adolescents and young adult women[19]. The male–female ratio is approximately 1:10, and SPNs are a common diagnosis in females under 40 years old undergoing pancreatectomy[2,3,16]. In our previous study, male patients, with an average age of 43.1 years, were older than female patients, and there were more asymptomatic male patients[20].
CONCLUSION
Enucleation has undoubtedly come to stay as an alternative surgical procedure for SPNs. However,many questions remain unresolved, and future directions toward the best surgical indication, the prevention and intervention of complications, especially pancreatic fistula, intraoperative resection margin safety assessment, and long-term oncology prognosis remain to be evaluated and should be explored in future clinical trials.
Wang R and Li J contributed equally to this work; Chen YH and Liu XB conceptualized and designed the manuscript; Wang R, Li J, and Tan CL drafted the manuscript; all authors revised the manuscript for important intellectual content; Liu XB and Chen YH supervised the work of the manuscript; Liu XB obtained research funding; all authors have read and approved the final manuscript.
the Key Research and Development Projects in Sichuan Province, No. 2019YFS0043; and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, No. ZY2017302-1.3.5.
A miller symbolizes greed, habitual and uncreative thinking as well as logic as a feeble protection against passion (Olderr 1986).Return to place in story.
The authors have no conflicts of interest to declare. No benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article.
All day long, I had been very busy. Picking up trash, cleaning bathrooms and scrubbing floors were all on my agenda for the day. My grown children were coming home for the weekend. I went grocery shopping and prepared for a barbeque supper, complete with ribs1 and chicken. I wanted everything to be perfect.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
China
By this time the Three Bears thought their porridge would be cool enough, so they came home to breakfast. Now Goldilocks had left the spoon of the Great, Huge Bear, standing5 in his porridge.
Rui Wang 0000-0003-0829-152X; Yong-Hua Chen 0000-0001-8485-0755.
He spread carefully out on a fine white cloth his Mirabelle plums, which looked for all the world as if they had been freshly gathered, and when he saw the Princess coming out of church he began to call out in a feigned49 voice: Fine plums! lovely plums! How much are they? said the Princess
Yan JP
Wang TQ
Yan JP
1 Cho YJ, Namgoong JM, Kim DY, Kim SC, Kwon HH. Suggested Indications for Enucleation of Solid Pseudopapillary Neoplasms in Pediatric Patients.
2019; 7:125 [PMID:31001506 DOI:10.3389/fped.2019.00125]
2 Eric D, Milosavljevic V, Gonzalez-Urquijo M, Tadic B, Veselinovic M, Grubor N, Jelic D, Bjelovic M. Laparoscopic enucleation of Frantz's tumor of the pancreas:Case report and literature review.
2021; 64:102221[PMID:33796288 DOI:10.1016/j.amsu.2021.102221]
3 Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A,Nakache R, Klausner JM. Solid pseudopapillary neoplasm of the pancreas:Management and long-term outcome.
2017; 43:1056-1060 [PMID:28238521 DOI:10.1016/j.ejso.2017.02.001]
4 Namur GN, Ribeiro TC, Souto MM, Figueira ER, Bacchella T, Jureidini R. MINIMALLY INVASIVE SURGERY FOR PSEUDOPAPILLARY NEOPLASM OF THE PANCREAS.
2016; 29:97-101 [PMID:27438035 DOI:10.1590/0102-6720201600020008]
5 Silano F, de Melo Amaral RB, Santana RC, Neves VC, Ardengh JC, do Amaral PCG. Yield of surgery in solid pseudopapillary neoplasms of the pancreas:A case series and literature review.
2021; 13:589-599 [PMID:34163575 DOI:10.4251/wjgo.v13.i6.589]
6 Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Jun ES, Sin SH, Kim HE, Park KM, Lee YJ. Enucleation for benign or low-grade malignant lesions of the pancreas:Single-center experience with 65 consecutive patients.
2015; 158:1203-1210 [PMID:25633730 DOI:10.1016/j.surg.2014.10.008]
7 Wang X, Chen YH, Tan CL, Zhang H, Xiong JJ, Chen HY, Ke NW, Liu XB. Enucleation of pancreatic solid pseudopapillary neoplasm:Short-term and long-term outcomes from a 7-year large single-center experience.
2018; 44:644-650 [PMID:29525465 DOI:10.1016/j.ejso.2018.01.085]
8 Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW, Kim SW; Korean Pancreatic Surgery Club. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection:a multicenter analysis in Korea.
2014; 260:348-355 [PMID:24743622 DOI:10.1097/SLA.0000000000000583]
9 Hosokawa I, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Takayashiki T, Ishihara T, Yokosuka O,Miyazaki M. Preoperative diagnosis and surgical management for solid pseudopapillary neoplasm of the pancreas.
2014; 21:573-578 [PMID:24535774 DOI:10.1002/jhbp.96]
10 Huang TT, Zhu J, Zhou H, Zhao AM. Solid pseudopapillary neoplasm of pancreas in pregnancy treated with tumor enucleation:Case report and review of the literature.
2018; 21:1234-1237 [PMID:30156213 DOI:10.4103/njcp.njcp_39_18]
11 Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas:distinct patterns of onset, diagnosis, and prognosis for male versus female patients.
2008; 143:29-34[PMID:18154930 DOI:10.1016/j.surg.2007.07.030]
12 Wang P, Wei J, Wu J, Xu W, Chen Q, Gao W, Jiang K, Miao Y. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas:A single institution experience with 97 cases.
2018; 18:415-419 [PMID:29548800 DOI:10.1016/j.pan.2017.12.012]
13 Farhat W, Ammar H, Amine Said M, Mizouni A, Bouazzi A, Abdessaied N, Ben Mabrouk M, Ben Ali A. Solid pseudopapillary neoplasm of the pancreas:a report of 10 cases and literature review.
2020; 90:1683-1688[PMID:31989788 DOI:10.1111/ans.15701]
14 Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours.
2008; 95:85-91 [PMID:18041022 DOI:10.1002/bjs.5652]
15 Brient C, Regenet N, Sulpice L, Brunaud L, Mucci-Hennekine S, Carrère N, Milin J, Ayav A, Pradere B, Hamy A, Bresler L, Meunier B, Mirallié E. Risk factors for postoperative pancreatic fistulization subsequent to enucleation.
2012; 16:1883-1887 [PMID:22872510 DOI:10.1007/s11605-012-1971-x]
16 Afridi SA, Kazaryan AM, Marangos IP, Røsok BI, Fretland ÅA, Yaqub S, Edwin B. Laparoscopic surgery for solid pseudopapillary tumor of the pancreas.
2014; 18:236-242 [PMID:24960486 DOI:10.4293/108680813X13753907291837]
17 Yalçın B, Yağcı-Küpeli B, Ekinci S, Orhan D, Oğuz B, Varan A, Kutluk T, Akyüz C. Solid pseudopapillary neoplasm of the pancreas in children:Hacettepe experience.
2019; 89:E236-E240 [PMID:31033126 DOI:10.1111/ans.15111]
18 Feng JF, Chen W, Guo Y, Liu J. Solid pseudopapillary tumor of the pancreas in a pregnant woman.
2011; 74:560-563 [PMID:22319967]
19 Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas.
2005; 6:185-186 [PMID:15737835 DOI:10.1016/S1470-2045(05)01770-5]
20 Cai YQ, Xie SM, Ran X, Wang X, Mai G, Liu XB. Solid pseudopapillary tumor of the pancreas in male patients:report of 16 cases.
2014; 20:6939-6945 [PMID:24944486 DOI:10.3748/wjg.v20.i22.6939]
21 Wei G, Luo Q, Fang J, Li X, Shi Y, Li Y, Sun L. The Sex Features of Patients With Solid Pseudopapillary Neoplasms of the Pancreas:A Retrospective Study.
2022; 12:844182 [PMID:35252013 DOI:10.3389/fonc.2022.844182]
22 Karsenti D, Caillol F, Chaput U, Perrot B, Koch S, Vuitton L, Jacques J, Valats JC, Poincloux L, Subtil C, Chabrun E,Williet N, Vanbiervliet G, Belkhodja H, Charachon A, Wangermez M, Coron E, Cholet F, Privat J, Le Baleur Y, Bichard P,Ah Soune P, Lecleire S, Palazzo M; from the GRAPHE. Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Pancreatic Solid Pseudopapillary Neoplasm Before Surgical Resection:A European Multicenter Registry-Based Study on 149 Patients.
2020; 49:34-38 [PMID:31856077 DOI:10.1097/MPA.0000000000001460]
23 Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases:Case report.
2017; 9:497-501 [PMID:29290920 DOI:10.4251/wjgo.v9.i12.497]
24 Xu J, Li F, Zhan H, Liu H, Wu D, Hu S, Wang L. Laparoscopic enucleation of pancreatic tumours:a single-institution experience of 66 cases.
2021; 91:106-110 [PMID:33205607 DOI:10.1111/ans.16450]
25 Lu WJ, Cai HL, Ye MD, Wu YL, Xu B. Enucleation of non-invasive tumors in the proximal pancreas:indications and outcomes compared with standard resections.
2017; 18:906-916 [PMID:28990381 DOI:10.1631/jzus.B1600597]
26 Kwon JH, Kim SC, Shim IK, Song KB, Lee JH, Hwang DW, Park KM, Lee YJ. Factors Affecting the Development of Diabetes Mellitus After Pancreatic Resection.
2015; 44:1296-1303 [PMID:26390413 DOI:10.1097/MPA.0000000000000404]
27 Senthilnathan P, Dhaker KC, Kaje V, Naidu SB, Sarvani M, Sabnis SC, Srivatsan Gurumurthy S, Nalakilli VP, Anand Vijay N, Rajapandian S, Praveen Raj P, Parthasarathi R, Palanivelu C. Laparoscopic management of solid pseudo papillary neoplasm of pancreas in tertiary care center from south India.
2017; 17:927-930 [PMID:29054814 DOI:10.1016/j.pan.2017.09.003]
28 Jin JB, Qin K, Yang Y, Shi YS, Wu ZC, Deng XX, Chen H, Cheng DF, Shen BY, Peng CH. Robotic pancreatectomy for solid pseudopapillary tumors in the pancreatic head:A propensity score-matched comparison and analysis from a single center.
2020; 43:354-361 [PMID:31327550 DOI:10.1016/j.asjsur.2019.05.016]
29 Tanaka K, Misawa T, Haruki K, Saito R, Gocho T, Akiba T. Enucleation of solid pseudopapillary tumor with a preoperative nasopancreatic drainage stent in a child.
2017; 10:438-441 [PMID:28635016 DOI:10.1111/ases.12397]
30 Agaimy A, Haller F. CTNNB1 (β-Catenin)-altered Neoplasia:A Review Focusing on Soft Tissue Neoplasms and Parenchymal Lesions of Uncertain Histogenesis.
2016; 23:1-12 [PMID:26645457 DOI:10.1097/PAP.0000000000000104]
31 Li Z, Zhang Z, Liu X, Hu W, Mai G, Zhang Y, Lu H, Zeng Y, Tian B. Solid pseudopapillary tumor of the pancreas:the surgical procedures.
2011; 41:91-96 [PMID:21191697 DOI:10.1007/s00595-009-4225-0]
32 Yu P, Cheng X, Du Y, Yang L, Xu Z, Yin W, Zhong Z, Wang X, Xu H, Hu C. Solid Pseudopapillary Neoplasms of the Pancreas:a 19-Year Multicenter Experience in China.
2015; 19:1433-1440 [PMID:26001371 DOI:10.1007/s11605-015-2862-8]
33 Nakagohri T, Kinoshita T, Konishi M, Takahashi S, Gotohda N. Surgical outcome of solid pseudopapillary tumor of the pancreas.
2008; 15:318-321 [PMID:18535771 DOI:10.1007/s00534-007-1264-z]
34 Butte JM, Brennan MF, Gönen M, Tang LH, D'Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center.
2011; 15:350-357 [PMID:20824369 DOI:10.1007/s11605-010-1337-1]
35 Sugito K, Furuya T, Kaneda H, Masuko T, Ohashi K, Inoue M, Ikeda T, Koshinaga T, Tomita R, Maebayashi T. Longterm follow-up of nutritional status, pancreatic function, and morphological changes of the pancreatic remnant after pancreatic tumor resection in children.
2012; 41:554-559 [PMID:22158069 DOI:10.1097/MPA.0b013e318232a6e2]
36 Salvia R, Bassi C, Festa L, Falconi M, Crippa S, Butturini G, Brighenti A, Capelli P, Pederzoli P. Clinical and biological behavior of pancreatic solid pseudopapillary tumors:report on 31 consecutive patients.
2007; 95:304-310[PMID:17326131 DOI:10.1002/jso.20685]
37 Matos JM, Grützmann R, Agaram NP, Saeger HD, Kumar HR, Lillemoe KD, Schmidt CM. Solid pseudopapillary neoplasms of the pancreas:a multi-institutional study of 21 patients.
2009; 157:e137-e142 [PMID:19818965 DOI:10.1016/j.jss.2009.03.091]
38 Morikawa T, Onogawa T, Maeda S, Takadate T, Shirasaki K, Yoshida H, Ishida K, Motoi F, Naitoh T, Rikiyama T,Katayose Y, Egawa S, Unno M. Solid pseudopapillary neoplasms of the pancreas:an 18-year experience at a single Japanese Institution.
2013; 43:26-32 [PMID:23114787 DOI:10.1007/s00595-012-0345-z]
39 Takamatsu S, Nagano H, Ohtsukasa S, Kawachi Y, Maruyama H. A case of spontaneous ruptured solid pseudopapillary tumor of pancreas resected by laparoscopic surgery.
2013; 2013:953240 [PMID:23737801 DOI:10.1155/2013/953240]
40 Jurić I, Pogorelić Z, Stepan JG, Kuzmić IP. Extremely rare presentation of Frantz's tumour:synchronous localisation in the pancreatic head and tail.
2014; 59:e8-e12 [PMID:25035290 DOI:10.1177/0036933014543222]
41 Karakas S, Dirican A, Soyer V, Koç S, Ersan V, Ates M. A pancreatic pseudopapillary tumor enucleated curatively.
2015; 10:118-120 [PMID:25828476 DOI:10.1016/j.ijscr.2015.03.040]
42 Stewart CL, Meguid C, Chapman B, Schulick R, Edil BH. Evolving Trends Towards Minimally Invasive Surgery for Solid-Pseudopapillary Neoplasms.
2016; 23:4165-4168 [PMID:27510845 DOI:10.1245/s10434-016-5491-x]
43 Esposito C, De Lagausie P, Escolino M, Saxena A, Holcomb GW 3rd, Settimi A, Becmeur F, van der Zee D. Laparoscopic Resection of Pancreatic Tumors in Children:Results of a Multicentric Survey.
2017; 27:533-538 [PMID:28225638 DOI:10.1089/lap.2016.0630]
44 Scandavini C, Valente R, Rangelova E, Segersvärd R, Arnelo U, Permert J, Svensson PJ, Stenman J, Del Chiaro M.Pancreatectomies for pancreatic neoplasms in pediatric and adolescent age:A single institution experience.
2018; 18:204-207 [PMID:29277262 DOI:10.1016/j.pan.2017.12.009]
 World Journal of Gastrointestinal Oncology2022年7期
World Journal of Gastrointestinal Oncology2022年7期
- World Journal of Gastrointestinal Oncology的其它文章
- Gut microbiome and pancreatic cancer cachexia:An evolving relationship
- Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma
- KIFC3 promotes proliferation, migration and invasion of esophageal squamous cell carcinoma cells by activating EMT and β-catenin signaling
- Differences of core genes in liver fibrosis and hepatocellular carcinoma:Evidence from integrated bioinformatics and immunohistochemical analysis
- Efficacy of neoadjuvant chemotherapy for initially resectable colorectal liver metastases:A retrospective cohort study
- Increased 5-hydroxymethylcytosine is a favorable prognostic factor of Helicobacter pylori-negative gastric cancer patients
