Simultaneous interventional therapy for coarctation of the aorta combined with intracristal ventricular septal defect in older age adult
Jian-Ming WANG, Xian-Yang ZHU, Jia-Wang XIAO, Qi-Guang WANG✉
Department of Congenital Heart Disease, General Hospital of Northern Theater Command, Shenyang, China
Coarctation of the aorta (CoA) is an uncommon congenital cardiovascular malformation, which is rare associated with several other cardiac and vascular anomalies, such as bicuspid aortic valve, ventricular septal defect (VSD), patent ductus arteriosus and aortic arch hypoplasia.[1,2]Open surgical repair is the usual treatment for neonatal CoA, whereas balloon angioplasty and stent placement are the treatment of choice for older children and adults.[3]Simultaneous surgery repair for CoA and its accompanying other cardiac anomalies had been reported and intensive study.[4]However,few articles published in the literature involving percutaneous simultaneous interventional therapy for CoA and its accompanying cardiac anomalies. What’s more, previous studies showed early treatment is preferred, as older age at the time of treatment is associated with increased risk.[5]Here, we described a particular case of simultaneous percutaneous treatment of CoA combined with VSD at older age.
A 56-year-old man who complained of recurrent amaurosis after activity for ten months and progressive exacerbation with intermittent syncope for 20 days.On examination, there was a normal dynamic pulse at the rate of 86 beats/min and blood pressure measured with appropriate size cuff of 165/100 mmHg at upper limb, meanwhile 132/90 mmHg at lower limb. Cardiovascular physical examination revealed the heart boundary expands to the lower left with grade 3/6 systolic murmur along the 3rdto 4thribs at the left edge of sternum. There was no conduction block or any types of arrhythmias under 24-hour Holter monitoring analysis. Echocardiography revealed that VSD (0.5 cm, type of outflow) with right aortic sinus prolapse, prompting coarctation of the descending aorta. The left ventricular and ventricular septum hypertrophy were obvious with normal ejection fraction. A small fissure like defect was found in the subaortic ventricular septum with a size of about 0.17 cm × 0.30 cm under computed tomography angiography. The 3D volume rendering images were reconstructed, which revealed that aortic isthmus was folded and narrowed, and the size of the narrowest section was about 12.3 mm × 14.8 mm (Figure 1). Therefore, the diagnosis of CoA combined with intracristal VSD was made.

Figure 1 CTA preoperative evaluation. (A): A small fissure like defect (red arrow) was found in the subaortic ventricular septum with a size of about 1.7 mm × 3.0 mm was observed; (B): the aortic isthmus was folded and narrowed (red arrow) were found from 3D volume rendering images; (C): CTA revealed coarctation of the aorta at sagittal CTA view; and (D): the size of the narrowest section of aortic was 12.3 mm × 14.8 mm at coronal section view.CTA: computed tomography angiography.
The patient was subjected to cardiac catheterization and angiography for further confirmation of the accurate diagnosis. The 6F pigtail catheter was imported along the right radial artery to the ascending aorta (pressure: 182/80 mmHg) and the left ventricle (pressure: 182/0 mmHg). The pressure of the descending aorta was measured through the femoral artery by end-hole catheter (150/80 mmHg). The left ventricle angiography showed that two right outlets of VSD were measured to be 0.4 cm and 0.3 cm, respectively. Although the intracristal VSD was adjacent to aortic valve right aortic sinus prolapse, aortic regurgitation was not found on supravalvular angiography. A 6/4 the Amplatzer Duct Occluder II (ADO II, AGA Medical, Golden Valley, MN, USA) was selected according to the angiography and transthoracic echocardiogram. A cut pigtail catheter was carefully manipulated to pass through the defect into the right ventricle, and 0.035” noodle wire was advanced into the pulmonary artery. Subsequently, the delivery sheath was replaced into the right ventricle. An appropriate device was retrogradely introduced through the delivery sheath. There was no residual shunt or aortic regurgitation under left ventriculography or supravalvular angiography after successful implantation of the 6/4 ADO II occluder. The tortuous aortic isthmus and localized stenosis without patent ductus arteriosus were found in the positive and lateral angiography of the ascending aorta. The diameter of the positive narrowing was 1.46 cm, the diameter of the expansion after narrowing was 3.55 cm, and the diameter of the diaphragmatic segment was 1.5 cm. The diameter of the lateral narrowing was 1.37 cm, the diameter of the expansion after narrowing was 2.47 cm and the diameter of the diaphragmatic segment was 2.93 cm. After intravenous injection of 10 mg morphine, we preclosed with the Perclose A-T device (Abbott Vascular Device, Abbott Park,IL, USA) at the femoral artery. The 6F end-hole catheter was delivered along the femoral artery to the left subclavian artery, replacing exchange-length 260 cm super-stiff wire, introducing the 14F delivery sheath to the narrowed proximal end of CoA, and shaping the first Cheatham-Platinum (CP, NuMed Corp.,Hopkington, NY, USA) stent using the balloon-in-balloon (BIB) catheter from the delivery sheath. The size of the CP covered stent was 8 cm × 4.5 cm, the size of BIB balloon produced by NUMED Company was 11 cm in diameter of inner capsule and 22 cm in diameter of outer capsule. After manually pushing the contrast medium to determine the appropriate position of the stent, the internal capsule pressure of five atmospheres and the external capsule pressure of four atmospheres were used to effectively expand the narrowing part for three times until the stent was fully expanded and the concave sign disappeared. In consideration of narrowing the tortuous proximal end,another one 8 cm × 3.9 cm stent was implanted at proximal (Figure 2). The pressure curves at the proximal and distal ends of the CoA showed there was no pressure gradient, both 152/80 mmHg measured. The proximal end angiography showed that the stents adhered well to the arterial wall, and there was no contrast agent between the stents and the arterial wall. Access site was closed using Perclose A-T device without complication. Total 6000 U heparin calcium was administered during the operation.
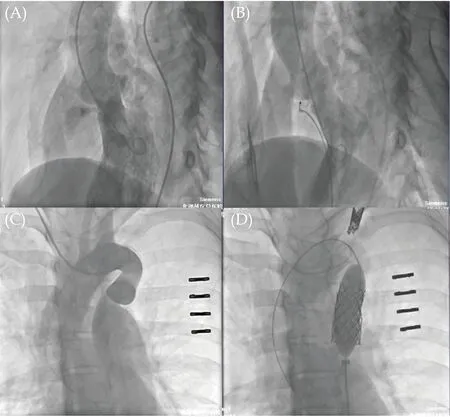
Figure 2 The angiography video review. (A): The intracristal ventricular septal defect was found in left ventricle angiography; (B):a 6/4 the Amplatzer Duct Occluder II occluder was implanted without residual shunt under left ventriculography; (C): the tortuous aortic isthums and localized stenosis without patent ductus arteriosus were found in the positive angiography of the ascending aorta; and (D): two Cheatham-Platinum covered-stents were successfully deployed using the balloon-in-balloon catheter.
The optimal position of the occluder and covered stents without dislodgement was confirmed by first postoperative 24 h X-ray examination. The patient was discharged in stable condition on the seventh day after closure uneventfully. There was no displacement of the occluder and covered stents or aortic wall complications by computed tomography angiography reexamined at 6-month follow-up (Figure 3). There was no significant systolic gradient and recurrent obstruction by transthoracic echocardiogram and Xray examination as 12-month follow-up completed.The patient had good cardiopulmonary condition without amaurosis symptoms, but he suffered continued hypertension and needed treatment during the follow-up period.
Yet up until now, there has been no universal consensus on an optimal way to manage CoA combined congenital heart diseases. CoA associated with other aortic abnormalities which need surgical management has traditionally been subjected to two staged surgery. A hybrid single-staged procedure has been explored, which the benefits include a significant reduction of risk of a complex surgical procedure to a simple surgical operation with associated stent intervention, without elongated cardio-pulmonary bypass time.[6]With the development of interventional technology, use of interventional procedures in either sequential or simultaneous sessions has been reported in previous studies.[7]However, there are few reports of the patients with concurrent CoA and intracardiac malformations treated via a simultaneous transcatheter route.
CoA with intracardiac malformations, such as VSD,leads to the reduction of forward blood flow in the ascending aorta and aortic arch. Therefore, it is often combined with obvious aortic arch dysplasia and tortuous narrow shape. In this case, two CP covered-stents were successfully implanted for CoA without migration and side vessel occlusion. The NUMED BIB catheters, which cooperated with two CP coveredstents, provided better control of stent position and coped with old adult’s tortuous CoA.
In conclusion, simultaneous interventional therapy for complex coarctation associated with VSD is a viable option for some old age patients, but there are technically difficult considerations to complex congenital cardiovascular malformations individually. In the case of this old age patient, it was suggested that carefully observing the condition of cardiovascular malformations, and proper interventional appliances selection might contribute to procedural success.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
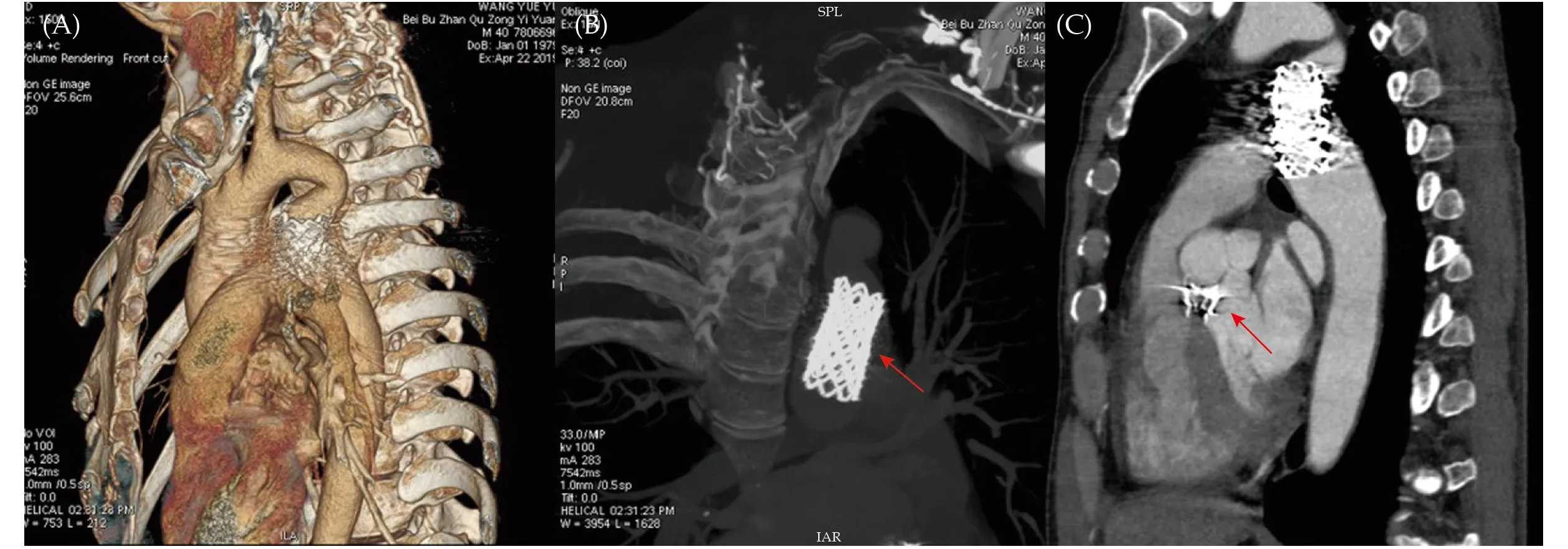
Figure 3 The CTA evaluation at 6-month follow-up. (A): Postoperative cardiovascular morphological observation from 3D volume rendering images; (B): a stable position and adaptable shape of covered-stents (red arrow) was confirmed under CTA examination; and(C): no displacement of the occluder (red arrow) and other complication was observed at 6-month follow-up. CTA: computed tomography angiography.
 Journal of Geriatric Cardiology2022年6期
Journal of Geriatric Cardiology2022年6期
- Journal of Geriatric Cardiology的其它文章
- Association between antiplatelet medication and cerebral microbleeds in stroke-free population
- Association between clustering of cardiovascular risk factors and resting heart rate in Chinese population: a cross-sectional study
- Effects of chronic obstructive pulmonary disease on longterm prognosis of patients with coronary heart disease postpercutaneous coronary intervention
- Efficacy of comprehensive remote ischemic conditioning in elderly patients with acute ST-segment elevation myocardial infarction underwent primary percutaneous coronary intervention
- Normalizing the dementia status in cardiovascular diseases:a perspective
- Acute myocardial infarction complicated with takotsubo syndrome in an elderly patient: case report and literature review
