Acute myocardial infarction complicated with takotsubo syndrome in an elderly patient: case report and literature review
Jing BAI, Wei XIANG, Ling-Yun KONG, Lan-Ting ZHAO, Fang LIU, Li-Feng LIU,Zhe TANG, Ping ZHANG,✉
1. Department of Cardiology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University,Beijing, China; 2. Department of Cardiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China; 3. Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Takotsubo syndrome (TTS), also known as stress-induced cardiomyopathy, which was first described in Japanese patients in 1990 by Sato,et al.,[1]is a syndrome that is being increasingly recognized worldwide and usually characterized by transient left ventricular (LV) systolic dysfunction after emotional or physical stress, with a good prognosis. TTS mimics acute coronary syndrome(ACS) because it is often presented with chest pain,ST-segment and T-wave changes on the electrocardiogram (ECG), and mildly elevated cardiac enzyme levels. Recent evidence shows that postmenopausal women are predominantly afflicted with this condition.[2,3]However, the exact pathophysiology of TTS remains to be elucidated. Relevant studies have found that TTS can occur simultaneously with acute myocardial infarction (AMI), and even culprit lesions can be found using intravascular imaging. The long- and short-term prognosis of TTS is the same as, or enen worse than that of AMI.[4,5]Thus,more clinical attention should be given rise on cases of AMI complicated with TTS.
An 82-year-old male presented to the fever clinic of our hospital with the chief complaint of having fever for one day. This was accompanied by lower abdominal discomfort for 12 h. Excluded from being suspected of having pneumonia caused by the novel coronavirus, the patient was referred to the emergency department. His fever was accompanied by generalized myalgia and cold intolerance, but with no chills, abdominal pain, or diarrhea. He also did not experience frequent, urgent, or painful urination. In addition, he did not have pharyngalgia, cough, expectoration of sputum, chest tightness, chest pain, or dyspnea. Physical examination after admission revealed a temperature of 39.1 °C, heart rate of 93 beats/min, blood pressure of 128/83 mmHg, oxygen saturation of 98%, respiratory rate of 28 breaths/min,and a body mass index of 24.1 kg/m2. Routine blood test analysis showed C-reactive protein levels of 124.95 mg/mL and a white blood cell count of 10.87 ×109/L. Chest computed tomography revealed no signs of infection. B-mode ultrasonography of the urinary tract revealed mild bilateral hydronephrosis,dilation of the right ureter, prostatic hyperplasia,and overfilling of the bladder. Therefore, bacterial infection of the urinary tract and acute urinary retention were considered. The patient underwent urethral catheterization and was administered intravenous levofloxacin 0.5 g once daily. Meanwhile, routine ECG examination was performed and found mildly elevated ST-segments in leads I, II, aVF, and V2-V6, with corrected QT interval (QTc) prolongation (Figure 1). Approximately 2 h later, ST-segments in leads I, III, aVF, and V2 were returned to baseline level, leads V3-V6 were depressed, and Twave in leads I, II, aVF, and V2-V6 was inverted on the ECG (Figure 1). The markers of myocardial injury were elevated: creatine kinase of 560.7 u/L, creatinine kinase-myocardial band of 9.76 ng/mL,myoglobin of 274.7 ng/mL, and hypersensitive cardiac troponin I (hs-cTnI) of 0.292 ng/mL. Although the patient did not report any chest pain or chest tightness, ACS was highly suspected by both the ECG and cardiac enzyme test results. After taking 300 mg aspirin and 600 mg clopidogrel bisulfate orally, he was admitted to the cardiac care unit. He didn’t have a history of hypertension, diabetes mellitus (DM), coronary heart disease, chronic kidney disease, or any infectious disease. He was not a smoker.

Figure 1 The recordings of ECG at the emergency department. (A): The ECG showed the mildly elevated ST-segments in leads I, II,aVF, and V2-V6, and QTc was 494 ms; and (B): the ECG showed ST-segments in leads I, II, aVF, and V2 were returned to baseline level,while in leads V3-V6 were depressed, and T-wave in leads I, II, aVF, and V2-V6 was inverted, and QTc was 483 ms. ECG: electrocardiogram.
After admission to the cardiac care unit, his temperature was 37.2 °C and blood pressure was 99/67 mmHg. In the semi-recumbent position, a few moist rales were heard in both his lungs and mild edema was noted in both the lower limbs. No heart murmur was heard. His abdomen sign was negtive. Hs-cTnI decreased after initial period of slight increase (the peak value of 0.310 ng/mL) (Figure 2), whereas the N-terminal-pro B-type natriuretic peptide (NT-pro-BNP) increased sharply (Figure 2). Ultrasonic cardiogram (UCG) showed LV wall was diffuse hypokinesia, with apical ballooning, and left ventricle ejection fraction (LVEF, 36%) was significantly decreased (Figures 3). The bedside chest X ray showed pulmonary edema (Figure 4). After intravenous furosemide and levofloxacin were used, rales in the lungs and edema of lower limbs gradually disappeared,and axillary temperature recovered to normal. Treatments such as the antiplatelet drugs, and improveheart failure therapies such as beta-blockers were used. The NT-proBNP of the patient peaked on the third day of admission, and effectively returned to baseline on the ninth day (Figure 2). Meanwhile,the T-wave inversion gradually improved and the QTc gradually returned to normal range on the ECG(Figure 5). The LV wall motion also gradually recovered and the balloon-like changes in the apical of the LV disappeared, also LVEF had returned to normal range on the UCG (Figures 2 & 3). Pulmonary edema showed alleviation in comparison with the results of the previous bedside X ray (Figure 4). The patient received the coronary angiography (CAG) on the eleventh day after admission and was found to have a lesion at the opening of the left anterior descending artery (LAD), with a stenosis of approximately 70%, and fuzzy shadows (Figure 6). Further intravascular ultrasound (IVUS) showed the hypoechoic and unstable thin-cap fibroatheroma with plaque burden of approximately 75%. Signs of plaque rupture were noted, but no thrombosis was observed (Figure 6). A stent was implanted from the proximal part to the opening of the LAD (Figure 6).Before discharged, speckle tracking UCG was performed to assess the myocardial motion, which was found to be nearly normal with globe longitudinal strain (GLS) of -21.3% (Figure 7). The patient was discharged from the hospital.

Figure 2 Changes in hs-cTnI, NT-proBNP and LVEF. (A): Trend of the hs-cTnI; (B): trend of the NT-proBNP; and (C): trend of the LVEF on echocardiography. hs-cTnI: hypersensitive cardiac troponin I; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminalpro B-type natriuretic peptide.

Figure 3 Changes in echocardiogram. (A & B): LV wall was diffuse hypokinesia, with apical ballooning, and LVEF 36% (August 6th);and (C & D): LV wall motion recovered and the balloon-like changes in the apical of the LV disappeared, also LVEF returned to normal range (68%) (August 16th). LV: left ventricular; LVEF: left ventricular ejection fraction.

Figure 4 Changes in bedside chest X ray. (A): The pulmonary edema was obvious (August 7th); and (B): the pulmonary edema was significantly reduced and the transparency of lung had increased after uretic therapeutic (August 11th).
The pathogenesis of TTS is not fully understood.It has been more widely indicated that catecholamine-induced cardiotoxicity and microvascular dysfunction as well as the complex action of neuroendocrine physiology contribute to the pathogenesis of TTS. Eventually, it was thought to involve the cognitive center of the brain and the pituitary-adrenal axis.[6,7]This theory is supported by the study of Wittstein,et al.,[8]who found that the serum catecholamine concentration was 2-3 times higher in patients with TTS than in patients with AMI.

Figure 5 Changes in electrocardiogram. Electrocardiogram showed the T-wave in leads I, II, aVF, and V2-V6 inversion gradually improved, and the QTc was 508 ms (A), 518 ms (B), 522 ms (C), and 387 ms (D) respectively, gradually returned to normal from August 9th to August 18th.
The diagnostic criteria of TTS was proposed by the Mayo Clinic, and it includes four parts: (1) transient dyskinesia or hypokinesia in the LV, with or without apical involvement; regional ventricular wall motion abnormalities extending beyond a single vascular distribution area; presence of stress-inducing factors; (2) absence of obstructive coronary artery disease; (3) new ECG abnormalities (ST-segment elevation and/or T-wave inversion), or significant increase in the serum cardiac troponin level; and (4)absence of pheochromocytoma and myocarditis.[9]However, in the past decade, TTS complicated with AMI has been reported in dozens of cases, thus challenging the diagnostic criteria suggested by the Mayo Clinic.[10-12]Chao,et al.[13]and Lindsay,et al.[14]reported that the incidence of takotsubo-like LV dysfunction induced by acute occlusion of LAD was approximately 26%, and its vulnerable population was similar to that of TTS, predominantly women.ACS, including spontaneous coronary artery dissection, complicated with TTS has been reported in a series of cases.[15-18]These findings suggest that TTS is triggered by ACS. Other researchers consider that TTS can trigger AMI in turn.[19]The third viewpoint is that the two diseases cannot be completely delinked,because they may be two different names for the same phenomenon.[14,16]
The clinical process of the current patient is more consistent with that asserted in the third viewpoint.On the one hand, although no vascular occlusion was noted on the coronary angiogram of the patient, fuzzy lesions at the opening of the LAD were noted.Moreover, signs of plaque rupture were found on IVUS imaging. These findings indicated that the patient had experienced an acute coronary event recently, it meant an AMI had occurred. No fresh thrombus was observed neither on the coronary angiogram nor on IVUS imaging, this is speculated that micro-thrombosis might occur after plaque rupture in the early stage and then dissolve rapidly and spontaneously, which didn’t occlude the LAD for very long time. This theory is supported by the atypical ischemic symptoms of the patient, with only a 2-hour ST-segment elevation on ECG, and mildly elevated hs-cTnI. Additionally, coronary angioplasty was performed on the eleventh day of admission rather than at the time of ST-segment elevated on ECG, so the opportunity may have been missed to detect the acute thrombus. On the other hand, the patient developed classical manifestations of heart failure, such as unable to lie supine, decreased oxygen saturation,with moist rales in both lungs, and pulmonary edema sign confirmed with chest X ray, and significantly increased NT-proBNP level, which did not match the less severe AMI, obviously. Furthermore, the contraction of LV wall was diffuse hypokinesia, which suggested the whole LV wall was affected, obviously, beyond the perfusion region of the LAD. This phenomenon cannot be explained by AMI, simply.Meanwhile, the LV structure and systolic function recovered quickly as transient of balloon-like changes in the apical wall and LVEF raised from 36%up to 68% in a few days. Almost normal myocardial motion was observed by speckle tracking echocardiography, conformed the reversible process of TTS, not the irreversible process of myocardial infarction. These supported the patient suffered from TTS. Based on the published literature, TTS occurs in patients of all ages. However, it occurs predominantly in postmenopausal women, with only 4%-13% of cases observed in men. Physical stress, instead of emotional stress, is more often associated with the onset of TTS in male.[2,3]It is worth noting that the presence of infective fever and urinary retention in the patient may have led to the activation of the sympathetic nervous system, and may have triggered the myocardial stress response, causing TTS and AMI. Therefore, TTS is not necessarily an independent disease. However, it is difficult to distinguish AMI from TTS at the time of onset, and it seems impossible to determine which condition is the precipitating factor.
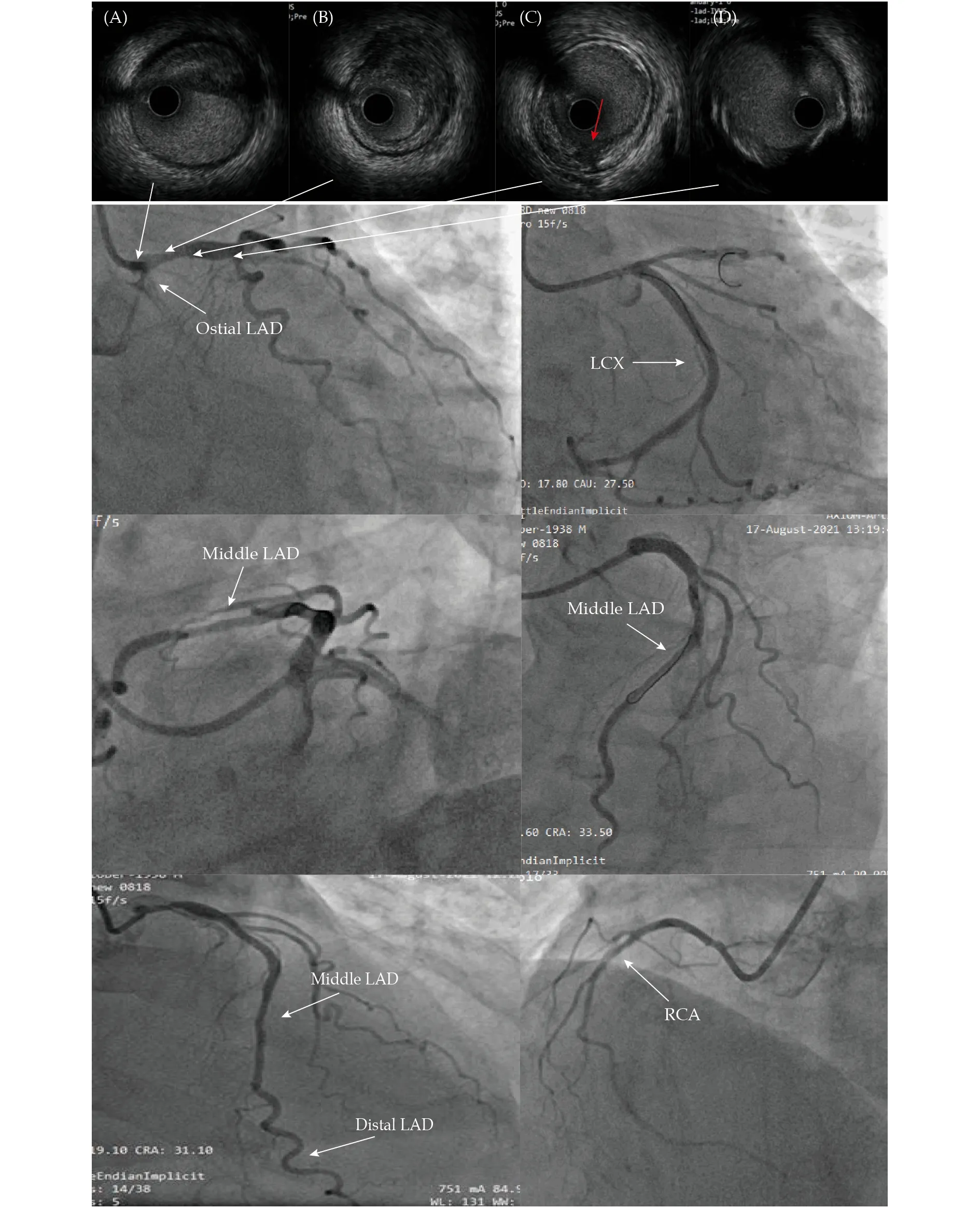
Figure 6 Coronary angiography and intravascular ultrasound images. The coronary angiogram showed there was a 70% stenosis lesion in the ostial of LAD, no significant stenosis lesion in LCX and RCA. Intravascular ultrasound showed low echo lipid plaque in the ostial and proximal LAD, with thin eccentric fibrous cap. Plaque rupture was seen (red arrow). LAD: left anterior descending artery;LCX: left circumflex coronary artery; RCA: right coronary artery.
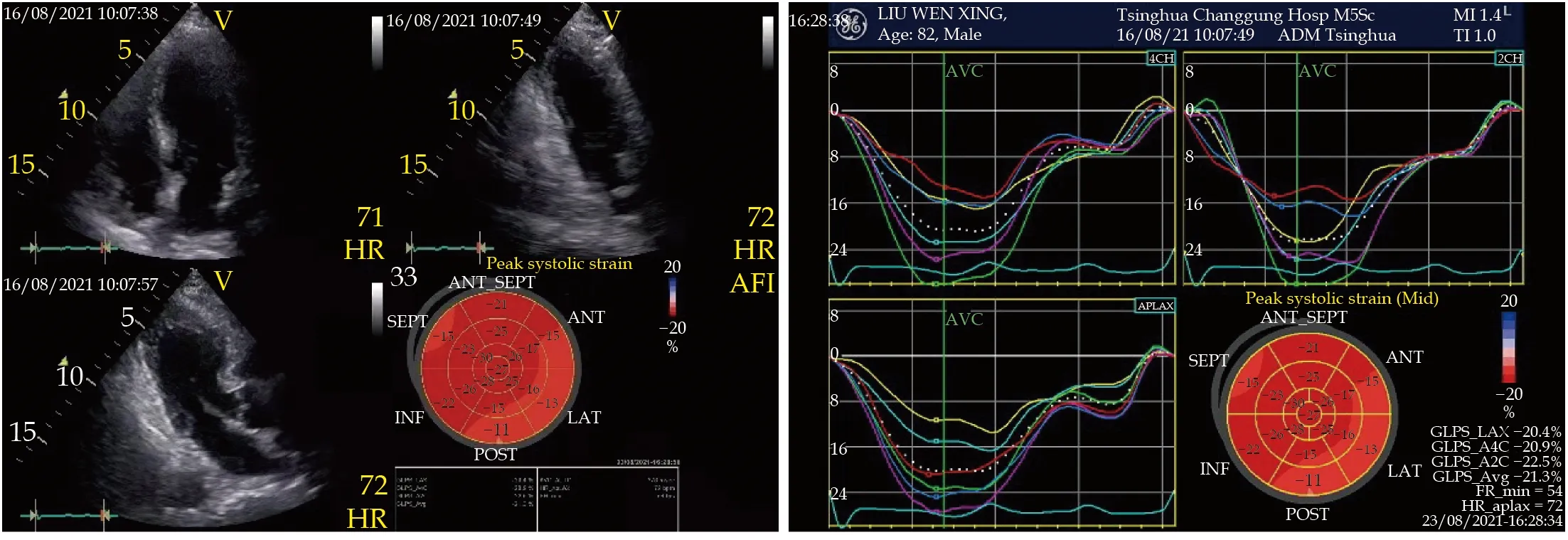
Figure 7 The speckle tracking echocardiography. The speckle tracking echocardiography showed the myocardial motion was nearly normal and globe longitudinal strain was -21.3%, but longitudinal strain slightly reduced in the basic segment and partial of the middle segment of the left ventricular anterior, lateral and post wall.
As reported in the literature, BNP and NT-proBNP levels increase significantly within 24 h after the onset of TTS and then subside slowly and completely in the following three months. In this patient, NTproBNP decreased quickly after peaking in a few days. The peak NT-proBNP level is associated with the severity of reversible periodic ventricular wall motion abnormality or systolic dysfunction assessed by UCG.[20]BNP or NT-proBNP levels can predict complications during hospitalization. In our patient, the peak NT-proBNP level was as high as 50,517 pg/mL, but fortunately, no severe cardiac complications occurred at hospital. The ECG of TTS is similar to that of ACS, but it lacks specificity. Common ECG findings in patients with TTS are a mild ST-segment elevation, widespread T-wave inversion, deeper T-wave, longer QTc, and less Q waves. The QTc shows an increasing trend in patients with TTS, but a decreasing trend in patients with ST-segment elevation myocardial infarction (STEMI). However,ECG alone cannot distinguish TTS from ACS. Wallström,et al.[21]reported a case-control study comparing the post-discharge symptoms reported by patients with TTS and AMI in the questionnaire, no difference was found in terms of the residual symptoms at the eighth week of discharge, which mainly manifested as fatigue, shortness of breath, and pain.
The prognosis of TTS was previously considered benign, but recent studies have reported its poor prognosis. Cardiogenic shock developed more frequently in patients with takotsubo than non-ST-segment elevation myocardial infarction (NSTEMI).[4]Cardiac rupture has also been reported in a very small cases (approximately 2.9%) with TTS.[22]TTS is with similar worse early and late mortality as STEMI and NSTEMI.[4]While, Stiermaier,et al.[5]found that the long-term mortality rate of patients with TTS was higher than that of patients with STEMI (hazard ratio = 1.58) by matching 286 patients with TTS and the same number of patients with AMI, after following up for mean 3.8 years, although no difference was noted between the two groups in the 28-day mortality and in the one-year mortality.[5]Male, Killip class III-IV, and DM are independent predictors for the risk of death in patients with TTS.[5]The cardiac function of this elderly man is Killip class III, but no traditional risk factors for coronary artery disease, such as hypertension, DM and so on. A good prognosis has been observed in our patient at present, but the long-term prognosis needs to be further observed.The successful treatment of this patient lies in the detection of plaque rupture at the opening of the LAD by CAG and IVUS, which were performed after restoring his cardiac function. Such lesions in LAD are at very high risk, which may lead to sudden death.This patient has strengthened our understanding of TTS complicated with AMI. Not all patients with TTS have a favorable prognosis, some may be accompanied with unstable lesions, even if their cardiac function is restored well.
TTS can occur simultaneously with AMI, and it may be a special manifestation of AMI. It needs to be treated with caution in clinical practice.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2022年6期
Journal of Geriatric Cardiology2022年6期
- Journal of Geriatric Cardiology的其它文章
- Association between antiplatelet medication and cerebral microbleeds in stroke-free population
- Association between clustering of cardiovascular risk factors and resting heart rate in Chinese population: a cross-sectional study
- Effects of chronic obstructive pulmonary disease on longterm prognosis of patients with coronary heart disease postpercutaneous coronary intervention
- Efficacy of comprehensive remote ischemic conditioning in elderly patients with acute ST-segment elevation myocardial infarction underwent primary percutaneous coronary intervention
- Normalizing the dementia status in cardiovascular diseases:a perspective
- Simultaneous interventional therapy for coarctation of the aorta combined with intracristal ventricular septal defect in older age adult
