Low Boiling-point Solvents Treatment of PEDOT∶PSS Film for Optimized Photovoltaic Cell Performance
LI Wei-guang,CHEN Jun-cong,ZHENG Yan-qiong,CHEN Yu-huan,CHEN Wei-an,LI Xi-feng
(1.School of Materials Science and Engineering,Shanghai University,Shanghai 200072,China;2.Key Laboratory of Advanced Display and System Applications of Ministry of Education,School of Mechatronic Engineering and Automation,Shanghai University,Shanghai 200072,China)
Abstract:As a popular hole transport material,poly(3,4-ethylenedioxythiophene)∶poly(styrene sulfonate)(PEDOT∶PSS)has been widely used in variety of optoelectronic devices.High boiling-point solvents were usually added into PEDOT∶PSS solutions to enhance its conductivity,but simultaneously resulted in evident leakage current due to solvent residue.However,low boiling-point solvents,which are more easily removed,have not been effectively utilized.In this study,we found that the performance of the corresponding poly(3-hexy-thiophene)(P3HT)∶[6,6]-phenyl-C61-butyric acid methyl ester(PC61BM)polymer solar cells(PSCs)was improved without increasing the leakage current after low boiling-point solvent treatment of PEDOT∶PSS films by dripping isopropanol,ethanol or acetone then spin-coating.Especially,after the isopropanol treatment,the short-circuit current density of PSC increased from 8.15 mA/cm2 to 9.17 mA/cm2,and the power conversion efficiency reached 3.5%,an 18.6% enhancement compared to the control device.The analysis revealed that the solvent-treated PEDOT∶PSS films demonstrated diffetent surface morphology,enhanced light transmission,and improved electrical conductivity due to the phase separation between PEDOT and PSS chains.Meanwhile,the hole transport capability of PEDOT∶PSS increased after treatment,especially after the isopropanol treatment.These results suggested that low boiling-point solvents could be effectively utilized by this method and improved the optoelectronic properties of the PEDOT∶PSS film.
Key words:poly(3,4-ethylenedioxythiophene)∶poly(styrene sulfonate)(PEDOT∶PSS);low boiling-point solvents;solvent treatment;polymer solar cells
1 Introduction
In recently years,organic photoelectric devices including organic light-emitting diodes(OLEDs),polymer solar cells(PSCs),touch panel displays,and organic photodetectors have become research hotspots[1-2].Through all these devices,poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate)(PEDOT∶PSS)as a popular hole transport material has been widely applied due to its excellent conductivity and film formation[3-4].Simultaneously,PEDOT∶PSS film shows ultra-high light transmittance and the potential to replace the traditional transparent electrode in optoelectronic devices[5-7].No matter as a candidate of transparent electrode or as a hole transport layer(HTL),the conductivity of PEDOT∶PSS needs to be further improved.Various strategies,including addition of small anions,methanesulfonic acid or alcohols,have been used to obtain comparable conductivity or bendable crystalline PEDOT∶PSS electrodes[7-13].In addition,high-molecular-weight soft substance doping or layer-by-layer doping efficiently suppressed the excessive agglomeration of PEDOT∶PSS[6,14],and removed a large number of the sulfonate component inside the film,finally significantly improved the phase separation morphology of the PEDOT∶PSS film.A frequently-used and effective method for improving the conductivity was to add relatively high boiling-point polar solvents,such as dimethyl sulfoxide(DMSO),N,N-dimethylformamide(DMF),ethylene glycol(EG)et al.to the PEDOT∶PSS solution[15].However,the common disadvantage brought about was an increased leakage current[16].In addition,when low boiling-point solvents,such as isopropanol,acetone,ethanol,et al.were directly added into the PEDOT∶PSS dispersion,the conductivity of the PEDOT∶PSS film didn't obviously improve,and the corresponding device efficiency improved only 1%-5%[17-18].It denotes that the addition of low boiling-point solvents was not an efficient strategy for conductivity enhancement of the PEDOT∶PSS film.Therefore,it is important to find a suitable method to improve the PEDOT∶PSS film using low boilingpoint solvents that can be more easily removed.
As an efficient upside-down technique,solvent treatment of organic solvent was frequently used for perovskite film preparation in perovskite solar cells or ultraviolet(UV)photodetectors[19-21].However,there is limited report for its application in organic films or organic solar cells,and furthermore there is no report on the usage of isopropanol,ethanol,and acetone in the solvent treatment process.
Here we found that the solvent treatment of PEDOT∶PSS filmviaisopropanol,ethanol,and acetone could effectively improve the polymer solar cells(PSCs)performance simultaneously without leakage current.Unlike the direct addition of high boilingpoint solvents,that tends to remain in the device,the insulating PSS chains can be dissolved in the solvent and dispelled.And the low boiling-point solvents can also be removed more easily under this method while ensuring that the PEDOT∶PSS films is not destroyed.After isopropanol treatment,the short-circuit current density of the poly(3-hexy-thiophene)(P3HT)based PSCs increased from 8.15 mA/cm2to 9.17 mA/cm2,and the power conversion efficiency reached 3.5%,an 18.6% improvement compared to the control device.Therefore,we investigated the detailed mechanism for the property improvement of PEDOT∶PSS filmsviathe solvent treatment with isopropanol,ethanol,and acetone,including the optical spectra(absorption and transmittance),surface morphology,solvent contact angle,sheet resistance and hole transport capability.These results suggest that low boiling-point solvents can be effectively utilized in this solvent treatment method to improve the optoelectronic properties of PEDOT∶PSS films.
2 Experiment
2.1 Materials
All chemicals and solvents were purchased commercially and used directly.PEDOT∶PSS dispersion(CleviosTMPVP Al4083)was purchased from Heraeus(Holding GmbH Hanau,Germany).P3HT,[6,6] -phenyl-C61-butyric acid methyl ester(PC61BM),and bathocuproine(BCP)were purchased from Luminescence Technology Corp.The organic solvents were purchased from Sigma-Aldrich.The molecular structures of the organic materials for the devices and organic solvents are shown in Fig.1(a).
2.2 Device Fabrication
The architecture of the PSC devices is ITO(150 nm)/PEDOT∶PSS(30 nm)/P3HT∶PC61BM(120 nm)/BCP(8 nm)/Al(100 nm),and its energy-level diagram is depicted in Fig.1(b).The indium tin oxide(ITO,15 Ω/□,150 nm)glass substrates were consecutively cleaned by ultrasonic bath in detergent,deionized water,acetone,and isopropanol,then dried in a drying cabinet.Prior to PEDOT∶PSS preparation,ITO substrates were treated in an UV/O3chamber for 15 min.

Fig.1 (a)Molecular structures of PEDOT∶PSS,BCP,P3HT,PC61BM,and organic solvents.(b)Schematic diagram of the energy levels of the PSC device.(c)Schematic diagram of solvent treatment process.
The pristine PEDOT∶PSS film was prepared by spin-coating of the PEDOT∶PSS dispersion onto ITO substrate at 3 500 r/min for 60 s,then annealed at 130 ℃for 15 min.For the solvent treatment process,80 μL of organic solvents was dropped onto the annealed PEDOT∶PSS film and held for 4 s,then spin coated at 4 500 r/min for 60 s,finally annealed at 130 ℃for 15 min,as illustrated in Fig.1(c).130 ℃is good for removing solvents used for treatment while maintaining the excellent properties of PEDOT∶PSS film.
The P3HT∶PC61BM solution was prepared by dissolving 20 mg∶20 mg in 1 mL of o-dichlorobenzene solvent in nitrogen glove box,then 80 μL solution was spin-coated at 800 r/min for 28 s onto the various PEDOT∶PSS film.Subsequently the BCP layer and Al cathode were vacuum deposited at 0.06 nm·s-1and 0.4 nm·s-1,respectively.The effective area of the PSC is 4 mm2decided by the overlap of anode and cathode.
The hole-only devices were prepared with the structure of ITO(150 nm)/PEDOT∶PSS(30 nm)/NPB(70 nm)/MoO3(3 nm)/Al(100 nm)by vacuum vapor deposition,and the evaporation rates for NPB,MoO3and Al were 0.04,0.03,0.40 nm·s-1respectively.
2.3 Characterization
All measurements of PSCs were carried out in ambient air immediately after manufacture without encapsulation.The current density-voltage(J-V)curves in dark and under illumination were measured using an AM1.5 G(100 mW·cm-2)solar simulator(Sun 3000,ABET Technologies)and Keithley 2400 source meter.The external quantum efficiency(EQE)spectra of PSCs were measuredvia7-SCSpec solar cell measurement system(7-STAR Co.,Ltd.,China).The atomic force microscope(AFM)images were performed by using a scanning probe microscope(Nanonavi SPA-400SPM,Japan).Transmission and absorption spectra were measured with a UV-Vis spectrophotometer(U-3900H,Hitachi,Japan).Hall measurement system(Accent HL5550LN2,Columbus,OH,United States)was used to test the sheet resistance of films.Solvent contact angle measurement in air was carried outviaKino optical contact angle and interfacial tensiometer(USA SL200KS).
3 Results and Discussion
To obtain a detailed insight into the treatment effect of low boiling-point solvents on PEDOT∶PSS film,we fabricated P3HT:PC61BM PSCs including PEDOT∶PSS film with and without(w/o)solvent treatment as HTL(Fig.2(a)).The corresponding current density-voltage(J-V)curves are depicted in Fig.2(b).The device parameters including the shortcircuit current density(Jsc),open-circuit voltage(Voc),fill factor(FF),power conversion efficiency(PCE),and PCE enhancement are summarized in Tab.1.

Tab.1 Device performance parameters of ITO/PEDOT∶PSS(30 nm)/P3HT∶PC61BM(120 nm)/BCP(8 nm)/Al(100 nm)under treatment with different low boiling-point solvents.Average photovoltaic parameters with standard deviations were obtained based on 12 cells for each set

Fig.2 (a)Schematic diagram of the PSC devices.J-V curves(b),EQE spectra(c)and dark state current curves(d)of the control device and the devices after solvent treatment with isopropanol,ethanol,and acetone.
By combining theJ-Vcurves and the parameters in Tab.1,viatreatment,the FF andJscshow significant improvement,andVocbasically keeps similar,therefore,the overall PCE has been significantly enhanced.TheJscimproves from 8.15 mA/cm2to 9.17,8.99,9.06 mA/cm2respectively.Isopropanol treatment also demonstrates an increased PCE from 2.95% to 3.50% with an enhancement of 18.6%.FF all shows different degrees of improvement.The series resistance(RS)decreases while the shunt resistance(RSH)increases after treatment by low boiling-point solvents.These results denote that the treatment of PEDOT∶PSS is appropriate to the three low boiling-point solvents.
We further compared the EQE spectra,as shown in Fig.2(c).It is obvious that in the range of 350-600 nm,the EQEs of treated PSCs are all improved,and it doesn't correspond to a particular wavelength,suggesting that the treated PEDOT∶PSS film would not influence the donor or acceptor layer,but just improved the interface between the PEDOT∶PSS and donor layer.
The equivalent circuit is a general method to describe the characteristics of PSCs,composed of an ideal current source,an ideal diode,a series resistanceRS,and a parallel resistanceRp.Iphis the generated current when the solar cell is exposed to light,andIDis the current through the diode.IRpis the current through the parallel resistorRp.If assuming the current flowing through the load resistanceRisI,then it satisfies the following relationship[22]:

whereI0is the diode reverse saturation current,nis the ideality factor,kis the Boltzmann constant,qis the electronic charge,andTis the temperature.In theoryVocis positively influenced byRp,and the increase ofRSwill greatly reduceIscand FF,which will make the device deviate from the ideal situation and greatly reduce PCE.In order to extract theRSof the PSC,dark current characteristics can be analyzed.The exponential part of the dark current characteristic under the positive bias can be described by a modified Shockley equation[22]:

wherej0is the reverse saturation current density,andAis the active area of PSCs.After low boiling-point solvents treatment,the darkJ-Vcurves of the device indicate no large difference together with the increase of FF and PCE of the device from Fig.2(d).However,after treatment with high boiling-point solvents,it is always possible to find that the dark current increases a lot followed by an increase ofRSand a decrease of FF[16].This is related to the rise in conductivity together with the leakage current increase in the treated PEDOT∶PSS film,which could result in the decrease of the FF.
The improvement of PSCs performance cannot be achieved without the change in the properties of PEDOT∶PSS film,and the PEDOT∶PSS films before and after treatment were further characterized.The absorption and transmission spectra of the pristine and treated PEDOT∶PSS films are indicated in Fig.3.The absorbance of the PEDOT∶PSS films after treatment decreases under the full band,the phenomenon is similar to the absorption change of some organic films treated with high boiling-point solvents[23].No any redshift is observed in the absorption peak in Fig.3(a),indicating that solvent treatment just affects the molecular stack but not change the molecular composition of PEDOT∶PSS.The transmittance of the PEDOT∶PSS film has been improved from 400 nm to 600 nm(Fig.3(b)).As for the multilayer film of PEDOT∶PSS/P3HT∶PC61BM,the absorption increases under the full band after treatment,implying that the molecular stack in the P3HT∶PC61BM layer is also influenced by the PEDOT∶PSS films.The changes in optical properties should be attributed to the changes in the surface morphology of PEDOT∶PSS[15].

Fig.3 Absorption(a)and transmission(b)spectra of the PEDOT∶PSS film with and without solvent treatment.Absorption(c)and transmission(d)spectra of the multilayer film of PEDOT∶PSS/P3HT∶PC61BM.
Subsequently,the PEDOT∶PSS films on quartz substrates before and after solvent treatment were characterized by AFM,as shown in Fig.4(a).The root mean square(RMS)roughness is 1.021,1.861,1.643,1.435 nm for pristine and treated PEDOT∶PSS films by isopropanol,ethanol,and acetone,respectively.Different from the large roughness change of high boiling-point solvent treatment[24],here the RMS roughness just changes slightly after treatment with low boiling-point solvent.Obvious differences in the surface morphology could be observed.The treated surface causes the film to become rougher,the height of the surface bumps to increase and more evenly distributed islands to appear.The highest protrusionviaisopropanol treatment reaches about 10 nm.The particle size of the pristine PEDOT∶PSS is around 30 nm and is prone to agglomeration,but this agglomeration is unevenly distributed.After the treatment,the overall morphology becomes more uniform,and the appearance of island-shaped particles becomes more regular,smaller and denser,as shown in Fig.4(b),which is a good improvement for enhancing the light absorption capacity of the active layer.
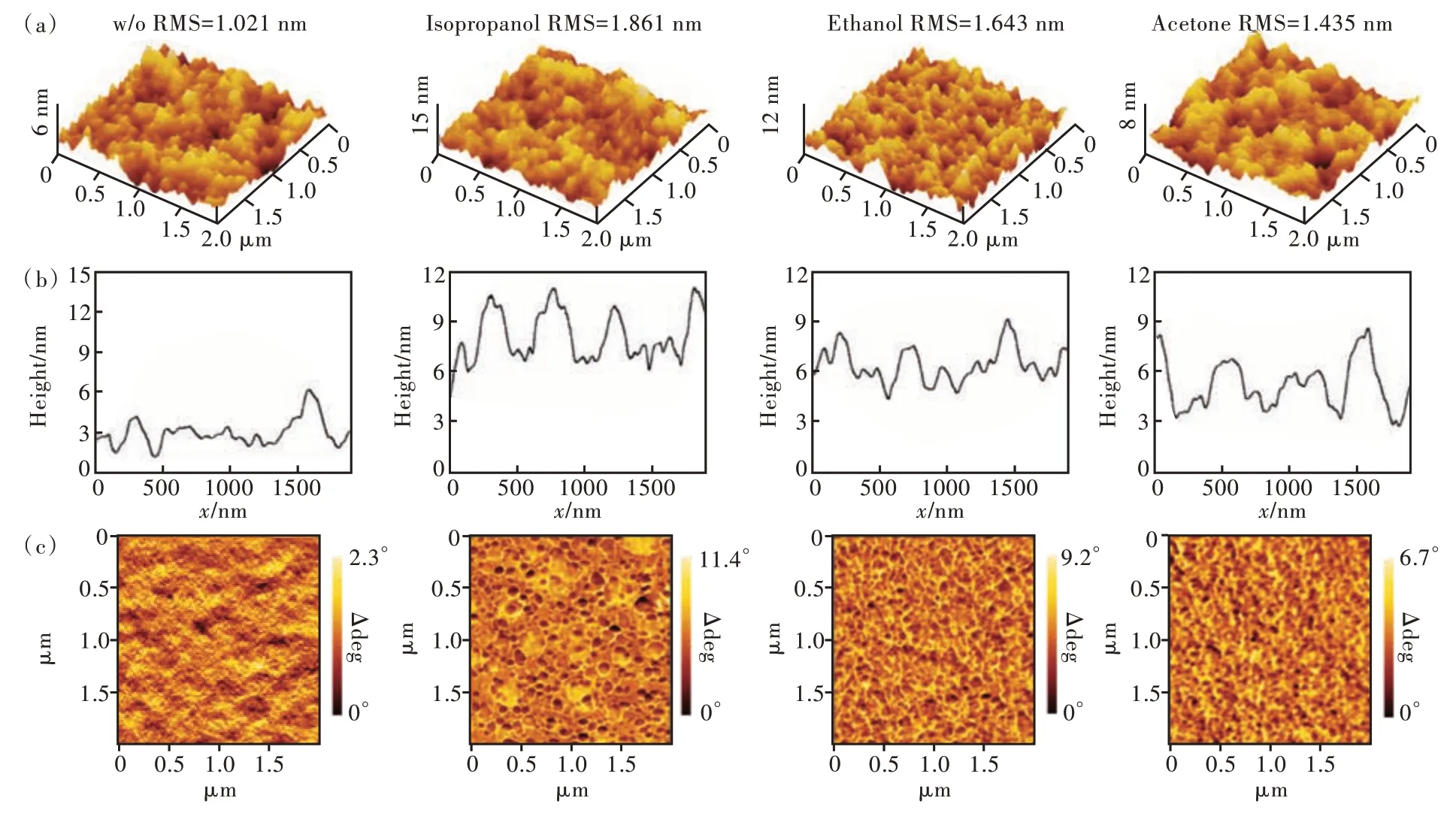
Fig.4 (a)Three-dimensional AFM images.Height profile at y=1 nm(b)and AFM phase images(c)of pristine and treated PEDOT∶PSS films by isopropanol,ethanol,and acetone,respectively.
The PEDOT∶PSS/ITO interface and its surface morphology have an extremely important effect on the performance of PSCs[23].The configuration change and surface islanding of the protrusions are supported by the AFM phase images(Fig.4(c)).The phase image of the pristine PEDOT∶PSS film clearly shows a large difference with other phase images.The bright areas in the AFM phase image correspond to PEDOT,while the dark areas correspond to PSS[25].It can be seen from Fig.4(c)that the phase value range of treated PEDOT∶PSS film all rises significantly relative to the pristine PEDOT∶PSS film,and the contrast between the light and dark area is more distinct.The phase separation between PEDOT and PSS becomes very explicit and continuous transport path appears[26].The isopropanol-treated PEDOT∶PSS film shows significant phase seperation different from the other two solvents treated films.This microstructure may result from the suitable boiling point of isopropanol,which is neither very low nor very high.It can ensure the enough depth of penetration to achieve the phase separation between PEDOT and PSS,and the solvent residual also can be completely removed by post-annealing,so it is the most suitable solvent among these three solvents.
Finite difference time domain(FDTD)optical simulations were used to depict the light field distribution in the device to get insight into the PSCs performance improvement mechanism.A plane wave in the visible range(from 300 nm to 700 nm)was chosen for the emission wavelength.The refractive index(n)and extinction coefficient(k)of each material were determined experimentally by ellipsometry and are shown in Fig.5(a)and 5(b),respectively.The boundary conditions in thexandydirections were set to periodic conditions to reduce the hardware requirements of the simulation.zdirection was set to perfect absorption boundary to minimize the effect caused by boundary reflections.The size of the island structure on PEDOT was set to a hemisphere with a radius of 5 nm,with a sparse island period of 100 nm and a dense island period of 50 nm.The period of the actual device island structure is much smaller.The optical effect of the island structure that appears on the PEDOT∶PSS film after treatment is investigated and the results are shown in Fig.5.Fig.5(c)shows the simulated absorption curves for the P3HT∶PC61BM.From the curve,it can be calculated that the sparse island structure enhances the light absorption of P3HT∶PC61BM by 7.1% and the dense island structure enhances its light absorption by 9.9%.Fig.5(d)-(f)show the simulated crosssectional light field distribution for the devices with pristine,sparse islanded and dense islanded PEDOT∶PSS.The black dashed line shows the P3HT∶PC61BM.From the simulation results,it can be seen that the field strength of the P3HT∶PC61BM without islanded structure of PEDOT∶PSS is about 3.6,and the field strength increases to 3.9 and 4.0 with the sparse and dense island structures in the PEDOT∶PSS layer,respectively.The light absorption capacity of P3HT∶PC61BM appears to be significantly enhanced,leading to an increase inJsc.And the light absorption capacity of P3HT∶PC61BM is enhanced more with increasing density of the island structures.
Fig.5 suggests that the island structure scatters the light passing through the hole transport layer,allowing more angular light to reach the active layer location,and the intensity shows a positive correlation with the density of the island structure.In the spectral results in Fig.3,the light absorption capacity of treated PEDOT∶PSS films in the visible range is degraded,while the results of multilayer films show enhanced absorption and reduced transmission,the transmission intensity of PEDOT∶PSS and absorption intensity of PEDOT∶PSS/P3HT∶PC61BM multilayer films increase in the order of w/o<ethanol<acetone<isopropanol,which is same as the deviceJscincreases and the more dense island structure of PEDOT∶PSS.This trend proves that the appearance of island structures throughout the visible range is a major factor in enhancing the absorption of the active layer and enhancing the cell performance,especially after isopropanol treatment.

Fig.5 Refractive index(n)(a)and extinction coefficient(k)(b)values of materials used for simulation.(c)Simulated absorption spectra of P3HT∶PC61BM films including pristine and island structured PEDOE∶PSS films.(d)-(f)Simulated crosssectional light field distribution for the devices with pristine,sparse islanded and dense islanded PEDOT∶PSS films.
In addition,the water surface contact angles of the pristine and treated PEDOT∶PSS films were measured.The PEDOT chains are hydrophobic and PSS chains are hydrophilic[27].The change of water contact angle before and after solvent treatment can indicate the hydrophobic ability of the film.As depicted in Fig.6,the contact angles of the pristine and treated films by isopropanol,ethanol,and acetone are 17.574°,23.024°,22.848° and 21.381°,respectively.The increase of the water contact angle indicates that the number of PSS chains decreases during solvent treatment,leaving more hydrophobic PEDOT on the film surface.This is because the solvent treatment shields the coulombic interaction between the PEDOT and PSS chains,and the disconnected PSS chains dissolve in the solvent and are carried out of the film during the spin coating process[28].This result well proves the occurrence of phase separation in the films.

Fig.6 Water surface contact angles of the pristine and treated PEDOT∶PSS films(a)by isopropanol(b),ethanol(c),and acetone(d).
The o-dichlorobenzene surface contact angles of the pristine and treated PEDOT∶PSS films were measured.As depicted in Fig.7,the contact angles of the pristine and treated films by isopropanol,ethanol,and acetone are 7.564°,5.602°,6.347°and 6.776°,respectively.Cassie theory points out that the contact angle is not only related to the hydrophilicity and hydrophobicity,but also related to the surface roughness of the solid surface[29].When the used solvents show high surface tension,for hydrophobic surfaces with contact angles over 90°,the rise in the surface roughness can increase the surface contact angle.And for hydrophilic surfaces with contact angles less than 90°,the rise in the surface roughness will decrease the surface contact angle[30].The declining contact angle here also proves that the surface roughness is further increased after treatment.The contact angle of o-dichlorobenzene for the pristine PEDOT∶PSS film is only 7.564°,indicating that o-dichlorobenzene has good spreading properties on the PEDOT∶PSS film for the subsequent spreading of the active layer.And after solvent treatment,all the o-dichlorobenzene contact angles show a decreasing trend,implying that the solvent treatment make odichlorobenzene spread more easily on the PEDOT∶PSS film,and the interfacial contact between PEDOT∶PSS and the active layer becomes better.

Fig.7 O-dichlorobenzene surface contact angles of the pristine and treated PEDOT∶PSS films(a)by isopropanol(b),ethanol(c)and acetone(d).
There has been extensive research on the conformation of PEDOT∶PSS as a polyelectrolyte in solvents[31-32].The PSS chains exhibit no conductivity,and the PEDOT chains are the fundamental component of conduction[24].Therefore,the proportion and the linear conformation of the PEDOT chains have a significant impact on the conductivity of the film.In order to clarify whether low boiling-point solvents treatment improved the conductivity of the PEDOT∶PSS films,the sheet resistance were measured and the results were summarized in Fig.8(a)and Tab.2.As shown in Fig.8(a),the sheet resistance of the pristine film is about 7.24×108Ω/□.After treatment,the sheet resistance of all the films decline significantly.Especially the isopropanol treatment decreases the sheet resistance to 6.39×106Ω/□.The decrease in sheet resistance would not originate from the possible residual of solvent,as the chosen temperature of 130 ℃is well above the boiling-point of the low boiling-point solvents used.Here it is speculated that the decrease in the resistance of the square is derived from the change of film morphology and the conductive path[33].

Fig.8 (a)Sheet resistance of pristine and treated PEDOT∶PSS films by isopropanol,ethanol,and acetone,respectively.(b)JV curves for the corresponding hole-only devices.

Tab.2 Sheet resistance of the PEDOT∶PSS films with and without solvent treatment
In this regard,we believe that the enhancement of PSC performance after isopropanol treatment needs to consider in addition the enhanced effect of light absorption in the active layer due to the island structure,as well as the conductivity of PEDOT∶PSS film.The hole-only devices with the architecture of ITO(150 nm)/PEDOT∶PSS(with and without solvent treatment)(30 nm)/NPB(70 nm)/MoO3(3 nm)/Al(100 nm)were prepared.The correspondingJ-Vcurves are shown in Fig.8(b).The current density of the solvent-treated hole-only devices is slightly higher at the same voltage compared to the control device,indicating that the hole transport capacity is enhanced after treatment.Together with the lower sheet resistance,and the higher collection efficiency at the anode of the device,the solvent treated PEDOT∶PSS would contribute to the higherJscof PSCs.TheJscof isopropanol treated device reaches 9.17 mA/cm2and the PCE rises from 2.95% to 3.50%,gaining an enhancement of 18.6% with the combined effect of optical scattering from the island structure and improvement of the hole transport.
4 Conclusions
In summary,the optical and electrical properties of PEDOT∶PSS film have been significantly improvedviathe low boiling-point solvent treatment,including isopropanol,ethanol,and acetone.After the treatment,theJscof the corresponding P3HT∶PC61BM PSCs with the PEDOT∶PSS film as the HTL shows a significant improvement.TheJscimproves from 8.15 mA/cm2to 9.17,8.99,9.06 mA/cm2respectively.Especially the isopropanol treatment achieves an increased PCE from 2.95% to 3.50%with an enhancement of 18.6%.The absorption of the treated PEDOT∶PSS film all declines while the absorption of the P3HT∶PC61BM active layer instead enhances from 400 nm to 600 nm.The film becomes more hydrophobic,the improved electrical conductivity and the AFM phase images both imply a significant phase separation occurring in the treated film.This leads to a regular island distribution on the film surface and a change in the carrier transport path.FDTD simulation indicates that the nanostructured PEDOT∶PSS layer enhances the light absorption of P3HT∶PC61BM.The sheet resistance of PEDOT∶PSS declines by 1-2 orders of magnitude,simultaneously the hole transport capability increases,especially after the isopropanol treatment.These results manifest that the solvent treatment process is more suitable to modify the photoelectric properties of the PEDOT∶PSS film especially with low boiling-point solvents.The corresponding PSCs overcome the drawback of significant leakage currents from the addition of high-boiling solvents to PEDOT∶PSS dispersion.
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20220108.
- 发光学报的其它文章
- Preparation of Molecularly Imprinted Polymer Fluorescence Probe Modified by Lanthanide Eu3+Complex and Hemoglobin Sensing Detection
- 共掺Rb+和Zn2+蓝光钙钛矿量子点及其发光二极管
- A Stable UV Photodetector Based on n-ZnS/p-CuSCN Nanofilm with High On/Off Ratio
- 不同功率O2或N2等离子处理TiNx阳极表面对硅基OLED 发光性能的影响
- 四苯乙烯类聚集诱导发光探针在生物分子检测领域的应用
- 一种比色/荧光增强型碳点基纳米探针用于环境中苯硫酚的高选择性检测

