Current outcomes and treatment of tetralogy of Fallot
Manish Juneja , Pankaj Raut, Harshawardhan Dhanraj Ramteke*, Vaishnavi Jayant Walke
Abstract One of the most common types of cyanotic congenital heart disease is Tetralogy of Fallot (ToF). Treatment has constantly increased since the first surgical repair in 1954. Excellent treatment having long-term survival (30 years survival ranges from 68.5% to 90.5%)is available for the ToF. However conventional and frequently required re-interventions include residual issues like right ventricular outflow tract obstruction, pulmonary regurgitation and (ventricular) arrhythmia. Right ventricular dysfunction might lead to longstanding pulmonary regurgitation and/or stenosis. It is important to perform pulmonary valve replacement or relief of pulmonary stenosis prior to irreversible right ventricular dysfunction, though determining optimal timing of pulmonary valve replacement is a problematic task due to various reasons. As seen in longstanding pulmonary regurgitation, the biological mechanisms underlying dysfunction of the right ventricle is often unclear. Various techniques of assessing the right ventricle are used to predict imminent dysfunction. The interventricular, ventriculo-arterial and atrioventricular interactions of right ventricle are not completely explained but play significant role in right ventricle performance. This review focuses on providing a brief overview of the history of ToF,describing the current strategies for treatment and describing the long-term survival, residual lesions and re-interventions following repair. Remaining related challenges and present condition of the art regarding these challenges are also illustrated.
Keywords: Tetralogy, Fallot, congenital heart disease, survival, outcomes
Introduction
Most commonly occurring cyanotic congenital heart disease,Tetralogy of Fallot (ToF) occurs 0.34 per 1000 live birth [1].The classic tetrad (Figure 1) was first styled in 1673 by bishop and anatomist Nicolas Steno, but the anatomy was more broadly defined by the French physician Étienne-Louis Fallot in 1888 [2,3]Depending upon the severity of pulmonary artery (PA) anatomy and right ventricular outflow tract (RVOT), varying degrees of cyanosis is experienced by patients with ToF. Variation from mild to more severe phenotypes is expressed as anatomical abnormalities in ToF.Pulmonary atresia and Fallot-type double outlet right ventricle (RV)are some severe forms and might require diverse management and treatment strategies. This review illustrates the “classic” ToF with right ventricular outflow (pulmonary) stenosis excluding atresia and double outlet right ventricle.
Pathophysiology
The normal physiological process of development of heart starts around 20th day of gestation. Different outer endotracheal tubes fuses into single tube known as cardiac tube. Later, this cardiac tube folds and loops over, with the development of cranial and dorsal regions of atrium, and a primitive ventricle moves downward to the right. As a result, the right ventricle, which is formed first becomes dominant chamber and supplies to the lower part of the body, placenta and lungs. The right ventricle has three components:the inlet, the trabeculated apical myocardium and infundibulum or conus. The inlet consists of tricuspid valve contains chordae tendineae and papillary muscles. The exact embryological and physiological process is not known regarding development of tetralogy of Fallot. A few associations had been observed like anterior and cephalad deviation of infundibular septum. As a result,ventricular septal defect could be seen, with aortic root causing obstruction in right ventricular outflow obstruction. The ventricular septal defects observed in patients having tetralogy of Fallot have perimembranous structure, which can extent up to the muscular septum. Various different factors contribute for right ventricular outflow obstruction like pulmonary valve which is usually bicuspid and stenotic, hypoplastic pulmonary annulus, deviation of infundibular septum that causes sub valvular obstruction, and hypertrophy of muscular bands in the same region.
The degree of the overriding aorta usually varies and the part receives blood from both ventricles. The episodes of hypercyanotic episodes or Tet spells usually vary with decrease in systemic valvular resistance or increase in pulmonary resistance which eventually contributes to right-to-left shunt in the ventricular septal defect,which causes desaturation [4]. The pathophysiological process has been shown in the Figure 2.

Figure 1. Schematic overview of the defects seen in tetralogy of Fallot. A)Pulmonary stenosis; B) Overriding aorta; C) Malalignment ventricular septal defect; D) Right ventricular hypertrophy [175].
Surgical approaches to repair
Lillehei et al. [5] first described surgical repair of ToF in 1995. The right ventricular outflow tract obstruction (RVOTO) was reached by ventriculoctomy into the right ventricular anterior wall and relief was obtained by inserting a transannular patch (TAP) as per requirement(Figure 3, left). Aggressive RVOTO relief was encouraged as early results had confirmed that residual RVOTO was predicted of early mortality [6] which resulted in relatively good long-term survival[7]. Residual lesions after repair were common and investigatory studies show that these resulted in late mortality and morbidity [8-11].Majority of victims showed, pulmonary regurgitation (PR), most commonly in those with TAPs [12]. Initially PR was misinterpreted as benign hemodynamic residual lesion but later, it was found to be predictive of reduced exercise performance and advancing RV dilation, which further was associated with biventricular dysfunction and ventricular arrhythmia [13-15]. As a result, patients were found to be at high risk of sudden cardiac death [8, 9, 11, 16, 17].
Various surgical methods were established, reducing the degree of ventriculotomy and attempting to preserve competence of pulmonary valve without causing significant residual RVOTO. The need for ventriculotomy can be minimized via Trans atrial and transatrialtranspulmonary approach (Fig. 3, right). Trans atrial and transatrialtranspulmonary methods offer excellent long-term outcomes and are practiced in most centres [12, 19-22]. TAP is necessary for optimum RVOTO relief in patients with small pulmonary valve annulus.Other procedures to substitute pulmonary valve competence are implantation of monoscup valve [25, 26], valvuloplasty with patching limited to infundibulum [23, 24], a valved RV-to- PA conduit [27,28] and a homograft valve [27]. No demonstration of valve-sparing and valve-replacing techniques has been done yet [29-32].
In the recent discoveries of surgical treatment of patients with tetralogy of Fallot, there can be significantly reduce in load of right ventricle which furthermore improve the hypoxic state, and thereby protecting cardiovascular and cerebrovascular function [33]. There can be a major contradiction in the neonatal period to have a surgery as the neonates may require transpulmonary valve annulus [34]. In a study, it was noted that neonates can have the acute hypoxic attack once in 3 to 6 months period post-surgery [35]. This can increase the chances of ventricular septal defect and dredging or widening of right ventricular outflow tract [36].
To reduce the chances of surgery for post complications,physicians can look forward for four various different techniques.Systemic arterial-pulmonary shunt can reduce the chances of hypoxemia, promotes the growth of pulmonary artery and reduces the chances of mortality by 3% to 5% [37]. Another procedure that has been suggested is Stent Placement of arterial conduits. It is Surgical strategy to ensure pulmonary blood flow by directing arterial blood flow to the pulmonary artery. Another surgery called Balloon dilatation of the pulmonary valve can Increase oxygen saturation in patients with tetralogy of Fallot and promote pulmonary angiogenesis, but the ultimate goal of this treatment strategy is to reduce the transpulmonary valve. Right ventricular outflow tract stent placement surgery can be Palliative care strategies for patients with tetralogy of Fallot to improve patient outcomes quality of survival, but the current experience with this treatment strategy is relatively limited. Patients treated with stenting have increased reoperation rates [38]. Still More research is needed to determine which treatment option is best for tetralogy of Fallot.Early intervention in patients with co-morbidities, and for optimal outcomes, may require to personalized management strategy.
The complications of tetralogy of Fallot can be residual remnants of Right ventricular outflow tract obstruction, which can be the most common complication. This can be caused by progressive right ventricular hypertrophy. Recent studies have shown that right ventricular hypertrophy can lead to ventricular tachycardia and may increase the chances of death [39]. Another major complication is Pulmonary valve regurgitation. Studies have found that 40% to 85% of patients suffer from moderate to severe pulmonary valve replacement within 5 to 10 years of the surgery. Pulmonary valve regurgitation can induce right ventricular volume overload, leading to progressive right ventricular dilatation, which may include tricuspid regurgitation and right ventricular dysfunction [40].
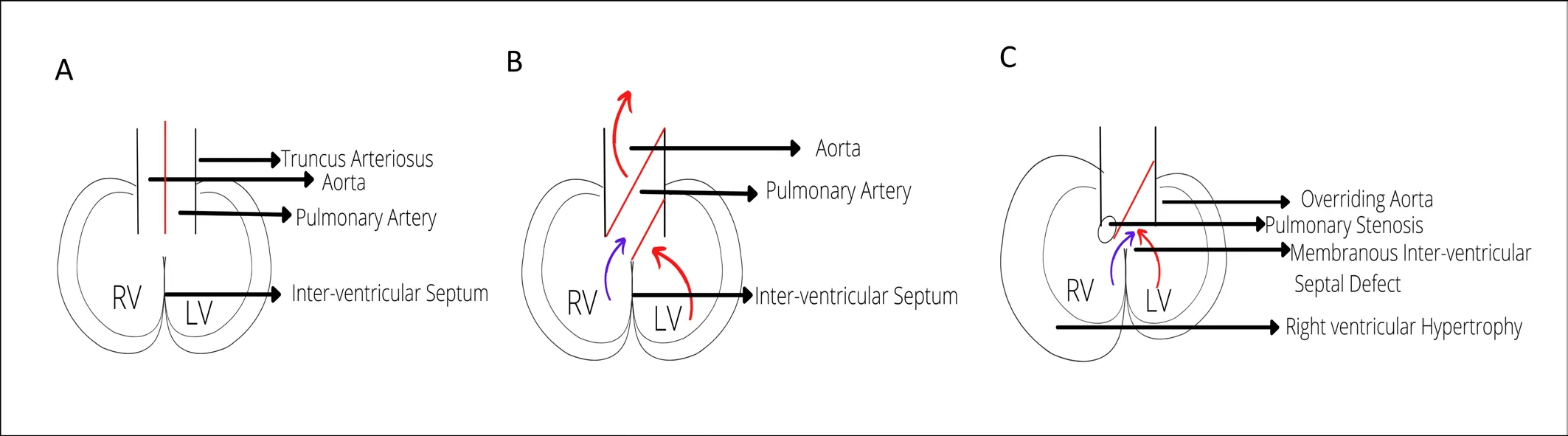
Figure 2. Pathophysiological Process of Development of Tetralogy of Fallot. A) Normal embryonic heart; B) Developed normal embryonic heart.; C) Tetralogy of Fallot.
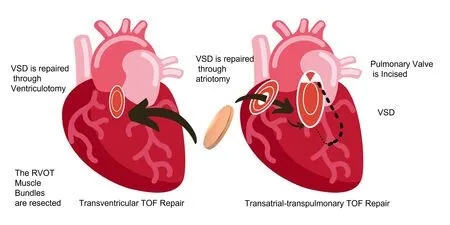
Figure 3. Transventricular (left) and transatrial-transpulmonary (right) approach to tetralogy of Fallot (ToF) repair. VSD : ventricular septal defect.
Variations in current treatment strategies
In general, it was assumed that early primary repair of ToF could limit extended exposure to RV pressure loading and oxygen saturations would be minimized, preserving the cardiovascular[41] and brain [42] function. Nonetheless the definition of “early”versus repair is not determined yet. Neonatal-repair (repair before 1 month of age) is practical with acceptable outcomes; however,it is not widely practiced due to better short-term results of nonneonatal repair [43]. Neonatal repair often necessities TAP relative to repair after the neonatal period, causing worse event-free survival[43]. Primary repair can be postponed to 3 to 6 month’s age in most patients presenting remarkable outcomes [44, 45].
Intervention in the neonatal period might be required by symptomatic ToF patients. If primary repair is not found to be the best choice, then various other methods can be used. A systematic pulmonary shunt, typically a modified Blalock- Taussing (mBT)- has been used, since early times to increase pulmonary flow, decrease hypoxemia and gain time for PA development. This allows repair to be executed at an older age having less or no extensive use of TAP.Even then palliative shunt is associated with 3% to 5% mortality rate [46, 47]. Illustration of superiority of a staged approach versus primary neonatal repair is not achieved yet [48, 49].
Stenting of the ductus arterious (DA) is an alternative strategy to permit pulmonary blood flow after birth by inducing a systemictopulmonary shunt. Whereas in cyanotic CHD, the anatomy of the DA difficult and inappropriate for stenting [50], procedural success is predicted to be 83% [51,52]. Results after DA stenting and mBT shunting using propensity score-adjusted models [53,44] have been compared in a recent multicentre study. DA stenting was more favourable as compared to mBT shunting [53,54] in terms of clinical status, assessed by saturation, haemoglobin levels, and PA size. Better survival (hazard ratio 0.25, 95% confidence interval(CI) 0.07-0.85) for DA stent than mBT shunt was discovered by Bentham et al. unlike Glatz et al. who found no difference in survival (hazard ratio 0.64, 95% CI 0.28-1.47) [54]. A trend towards greater re-intervention rate in DA stent was witnessed in both studies[53, 54]. DA stenting appears to be a practical strategy for selected cases. Moreover, in particular patients, palliative balloon dilation of pulmonary annulus can be used to escalate the oxygen saturation and support development of pulmonary vasculature and as a bridge to later complete repair [55, 56]. This method improves long-term outcomes or it eventually reduces TAP use remains a controversy[55,56].
Likewise palliative strategy or bridge to repair in neonatal life are some RVOT stenting techniques that can be used [57,58]. Use of this technique is not widespread but has been demonstrated to be comparatively safer, promoting growth of pulmonary arteries as a bridge to repair [59-60]. No dissimilarity was obtained when Quandt et al. compared medium-term results of RVOT stent with systemic-to-pulmonary shunt [59]. More favourable options for RVOT stenting like intensive care and hospital duration and preoperative but the re-interventions rate was higher for this group [59],most common re-interventions being re-stenting and re-ballooning.In comparison to patients who had undergone primary mBT, the(Re) shunt surgery or early repair was less common than those in this group. Short-term and long-term outcomes were obtained on comparison of neonatal repair and RVOT stenting [61, 62]. During 10 years of investigations of Wilder et al. similar increased rate of catheter-based re-interventions in RVOT stent group was observed in comparison to neonatal repair [62]. More analysis is needed for determination of best technique for patients’ group requiring early intervention. Management strategies likely needed to individualized for optimal outcome.
Overall survival
Overall survival, later to ToF repair had remarkably improved in recent times. Figure 4 demonstrates the survival in various large studies. Reports within the two last decades investigation was up to 40 years foe cohorts [12,63-76]. A decline in early mortality is seen in recent era and European and American congenital cardiothoracic surgery registries report that preoperative mortality is below 3%in current years [76-78]. The severity of ToF determines the preoperative outcomes such as, pre-operative size of pulmonary size and pulmonary arteries, oxygen saturation and RV-PA pressure gradient [61, 79-81]. Greater pre-operative mortality is observed in patients with repair, including TAP [76]. Most centres consider TAP only when pulmonary annulus z-score is below -2 and -3 which shows more severe ToF [21, 82]. Moreover, pre-operative mortality is associated with some co-morbidities like coronary abnormalities,genetic abnormalities and small body size- associated lesions [71,79-81, 83].
Medium term mortality rates data hasn’t shown much modification across various surgical eras (Figure 4) [75]. 68.5% to 90.5% of Survival is observed at 30 years of age [64,87,78,72-75].Long-term (20 to 30 years) of survival from large masses of patients who had undergone recent surgical modifications of ToF repair(for example, valve-sparing and valve-replacing techniques) is still deficient. Residual RVOTO and severity of PR are significant factors for defining long-term outcomes [64].
Patients who underwent ToF are presenting survival in adulthood resulting into growing populations of people with corrected ToF,thus requiring specialized medical are [84-87]. Re-interventions are customary in these patients. As per Cuypers et al. about 44% of patients went through surgical or re-intervention at least once after 35 years of follow up [73]. According to D’Udekem et al. about 24±5% of patients had their re-operation done after 30 years of follow-up [74]. Lower rates of re-interventions have been reported later to trans trial transpulmonary repair. 80% of freedom of reintervention and death after 10 years was reported by Luijten et al.whereas D’Udekem et al. observed 75% of freedom or re-operation after 25 years. Low pulmonary valve replacement (PVR) rate after trans-trial repair was obtained in comparison with trans-ventricular repair in a case-control study. Use of TAP is linked with high reintervention rate [12,64], so is the severity of ToF at repair [66,75].Specific indications fie re-intervention is later stated in the review.
Residual problems and re-interventions
Residual right ventricle outflow tract obstruction
Residual RVOTO commonly follows repair resulting in residual or progressive concentric hypertrophy of the RV. According to data from the INDICATOR study, RV hypertrophy due to increased mass-volume ratio is more significant long-term risk factor for ventricular tachycardia (VT) and death than severity of RV dilation(RV-end diastolic volume index) [88]. Current guidelines deliver clear suggestions for re-interventions for residual RVOTO (Table 1) [85-87]. In case of valvular pulmonary stenosis (PS) balloon valvuloplasty or PVR can be performed. PA branch stenosis can be relieved by balloon dilation or PA reconstruction [89]. About 1% to 7% of patients have undergone PA dilation or stenting in long term follow up (median of 5.8 to 36 years), as reported in several large studies [71, 73, 74, 90, 91]. 1% to 5% patients underwent surgical relief of RVOT and PA plasty at a long-term follow-up [71,73,90,91].
Pulmonary regurgitation
PR is very common at medium- to long-term follow-up.Approximately 40% to 85% od patients have moderate to severe PR later five to ten years after repair [63,83,92-94]. RV volume overload of RV, often with progressive RV dilation is induced by PR which might include tricuspid regurgitation (TR) and RV dysfunction.It is often accompanied by accompanied by prolongation of QRS complex and RV dysynchrony could lead to progression of dysfunction [95-97]. RV function is maintained during a long period in compensated state, which might fail in some cases resulting in RV dysfunction [95, 96]. Molecular events contributing to transition from a compensated to a decompensated state and the mechanisms of RV adaptation and remodelling are still unclear. The progressive adverse RV modelling leading to RV dysfunction as seen in chronic PR can be stopped by timely restoration of pulmonary valve competence.
PV would be performed in 40% of the patients, later 30 years of ToF repair [73,75,98].mild residual PS seems to reduce the risk[99] whereas TAP and staged repair act as risk factors for late PVR[12,64,73,90]. Increasing survival of ToF patients in adulthood works simultaneously with increasing use of PVRs [100].
PVR decreases RV volume, QRS duration, and TR, however it increases left ventricle ejection reaction (EF), thus improving functional status [101,102]. As a matter of fact, no improvement in survival after PVR compared with medical management has been illustrated till date [103,104].
Most preferred valves for PVR include homograft and bioprosthetic valves [105]. The current 10-year re-PVR-free survival of ToF patients enduring homograft PVR varieties from 74% to 89%[105, 106].
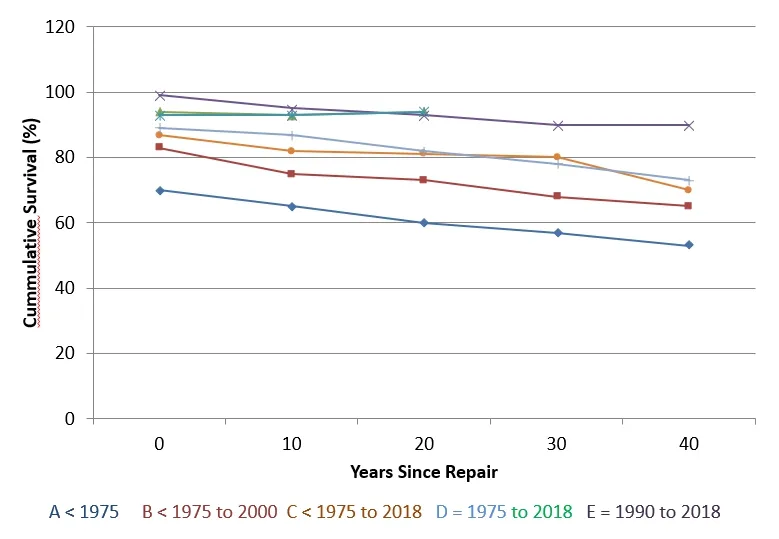
Figure 4. Survival following tetralogy of Fallot (ToF) repair. Each colored line represents a single study, and dots represent Kaplan-Meier survival estimates at different time points [12,53-65].
Lifelong valve replacement capacity is observed in tissueengineered valves with a non-synthetic and non-immunogenic surface [107]. One of the most preferred ones are in situ engineering methods which use a de-cellularized “starter scaffold” of polymers to provide shape and structure to the valve. This scaffold is subverted by endogenous cells to offer a regenerating functional valve. As the scaffold would be non-immunogenic, this could deliver a relatively cheap “off the shelf” valve. Positive results are by recent studies about evaluation of tissue-engineered animals and humans [108].
Increased use of various kinds of trans catheters is seen in clinical(trial) setting [109], whereas clinical experience relative to (surgical)homograft PVR is limited [109]. Transcatheter PVR generally shows positive results (>95%) [110]. 0.4% to 5.9% per patient-year of hazard rate for re-intervention after transcatheter PVR is seen [110].High rates of infective endocarditis during follow-up have been explained [111]. Infective endocarditis risk is reported to be 2.3% per patient-year by the MELODY registry [112], whereas, the infective endocarditis risk in surgical PVR has been predicted to be 0.3% per patient-year [113]. Enhanced potential and improved quality of life for 6 months after the procedure is shown by the transcatheter PVR[114,115]. No direct comparison with surgical PVR is done yet.
Arrhythmla
Ventricular tachycardia (VT): is a common arrhythmia in the repaired ToF patients. A 5% cumulative incidence of sustained VT after a median of 35 years after ToF rpaie by Cuypers et al. and these figures similar to majority of reports [68,116], 15% of cumulative incidences have been reported in adult population [117]. Higher age,number of prior cardiac surgeries, presence of a TAP, LV diastolic dysfunction, and QRS width are some of the predictions of sustained VT [73,116-118]. Implantable cardioverter defibrillators (ICDs) are preferred for majority of patients who have had sustained VT or cardiac arrest [[86, 87]. Primary prevention cases often include ICD whereas selecting high-risk patients who can get benefitted remain a challenge [86,87]. ICD prevalence in adult ToF populations and pacemaker both range from 5% to 10% [73,117,119].
Underlying substrate can be determined by Electrophysiological studies and radiofrequency ablation can be performed. 18% patients who had a mean follow-up after 34 months show excellent outcomes with recurrent VT after the ablation of monomorphic VT substrate is performed [120]. Another analysis found a related recurrence rate(19%) 10 years after ablation [121].
Supraventricular tachycardia: The prevalence or collective incidence of supraventricular tachycardia (SVT) in adult patients’varieties from 4% to 20% [117-119, 122]. SVT is relatively uncommon in fist 10 to 15 years after ToF but its occurrence rises over a period [117]. Most common type of SVT in patients with ToF is intra-atrial re-entrant tachycardia, typically involving the right atrium. [117]. As per two large studies discovered that SVT was an independent predictor of death or VT [88,118]. Some reports suggest the efficiency of ablation of atrial arrhythmias in improved ToF, andlong-term development is deficient [123-125].
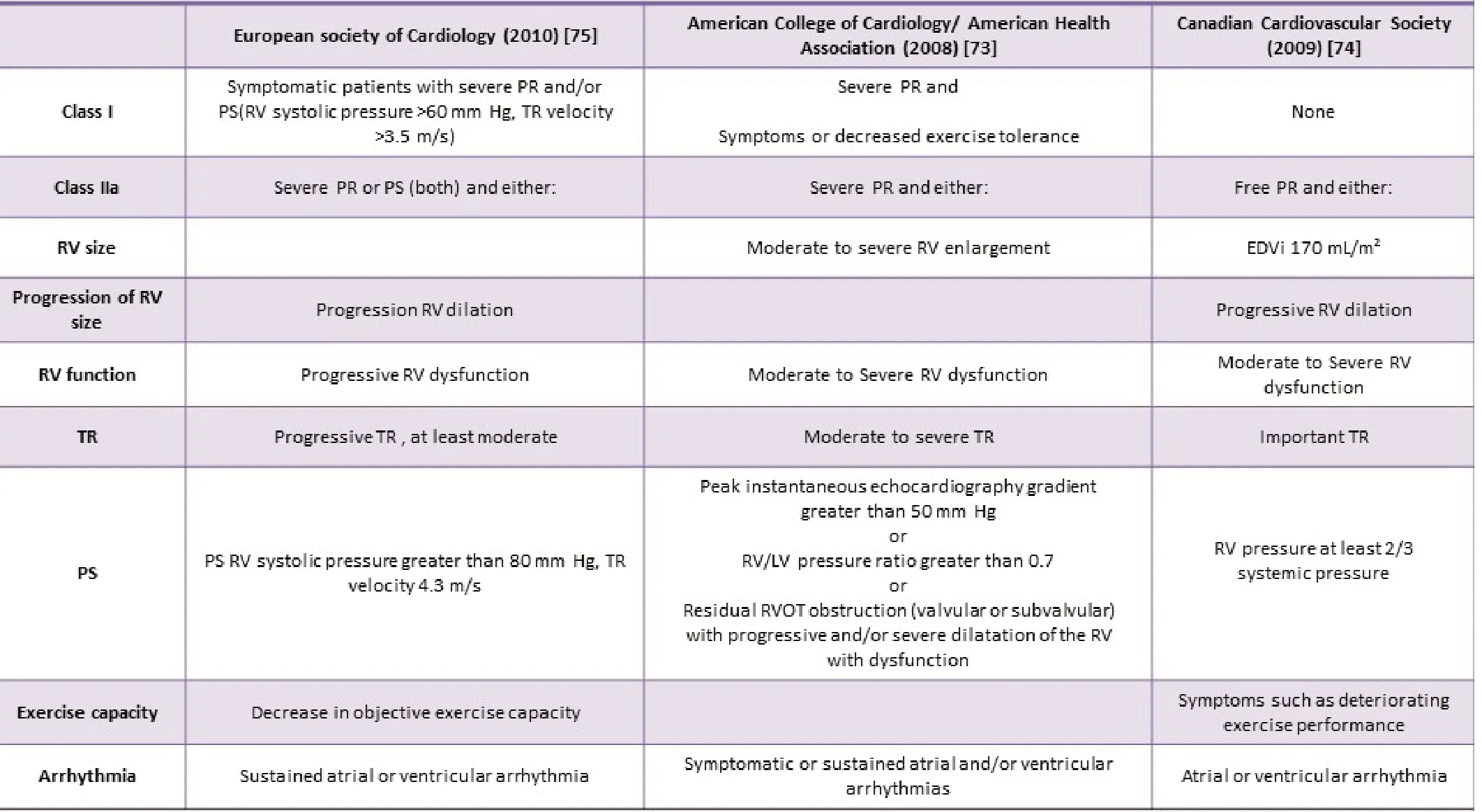
Table 1. Indications for pulmonary valve replacement in current guidelines.
Aortopathy
12% to 24% of adult patients of ToF suffer from dilation of aorta[126-127]. Aortic root size seems to increase over years in patients with aortic dilation. A rare complication appears in aortic dissection following ToF [128]. No increased risk of thoracic aortic dissection for patients with ToF relative to general population was stated in population-based study in Texas [129]. Increasing aortic root dilation can result to maladaptation of the aortic valve and aortic regurgitation. Furthermore, the elasticity of the dilated aortic root was revealed to be condensed in patients with ToF, possibly hindering circulatory function [130]. The significance of aortopathy in circulatory function and mortality remains partly understood.
Knowledge gaps
Right ventricular adaptation and remodelling
Unclear understanding of mechanisms of RV adaptation and remodelling in response to chronic RV volume becomes overloaded,resulting in PR [131]. Chronic PR affects biventricular systolic function, RV myocardial contractility and LV diastolic performance in young pig models [132]. Early hypertrophy of chronically volumeloaded RV and later myocardial fibrosis is visible in histopathology of several animal models [131]. The molecular responses to elevated volume or pressure loading of the RV are unlike those in the LV[131,133-135]. RV hypertrophy and dilation were found after 23 weeks in a pig model of repaired ToF with induced PR, PS, and an RVOT scar. In myocardium, Decreased impulse conduction velocity and dispersion was caused due to increased collagen deposition[136]. Related findings were found in the LV, despite preserved LV function at this stage, which shows biventricular adverse effects are present early in the adverse remodelling process [137].
Basic research into RV remodelling, focuses primarily on the response to increased pressure loading rather than the predominantly volume-loaded RV as seen in PR [134,135]. Pressure loading and volume loading increases the myocardial metabolic demand.Increased amount of reactive oxygen species is induced by this metabolic rate. Compensatory anti-oxidant manufacture in the RV is weakened as compared with the LV [135]. Thus, RV is more prone to oxidative stress in abnormal loading conditions.
In volume-loaded RV mouse models, a clinical course like to RV dysfunction with volume-loaded RV in humans is observed. RV function is preserved during a recompensed phase, followed by RV dysfunction [138]. Gene expression patterns of the cardiomyocyte,in the compensated state, is different from healthy controls. Several molecular pathways, like p53 signalling, cytoskeleton-related pathways and transforming growth factor beta (TGF-β) are down regulated in the early compensated state but show late up regulation as the RV progressively remodels [138]. However, the precise cellular and molecular mechanisms of transition from a remunerated to a decompensated state of the volume-loaded RV have not been fully explicated [135,139].
Assessing the right ventricle in patients with tetralogy of fallot
Early detection of failure in earlier stages is caused due to the limited understanding of pathophysiology of RV failure. Imaging techniques are practiced for investigating RV and follow patients consecutively with a focus to detect early changes in biventricular size and performance. Cardiovascular magnetic resonance (CMR) imaging is regularly used to constantly measure RV volumes and function,wall mass, and PR [140]. Some relatable adverse events are large RV volumes, PR severity, biventricular EF, and mass-to-volume ratio [88,141,142]. Elevated RV volumes mostly end-diastolic volume index (EDVi), is considered a sign of prolonged high PR burden and, thus a predictor of RV dysfunction. Compensatory mechanisms can still be adequate to maintain performance of large RVs and exercise capability can be conserved even in severely dilated ventricles [143]. Increased RV wall mass-to-volume ratio was found to be independent predicting factor whereas RV EDV and end-systolic volume are not predictive end points according to report by INDICATOR cohort [88].In patients with residual PS the RV hypertrophy can act as sensitive marker of pending dysfunction as compared with EDV.
Strain imaging studies can analyse the regional myocardial performance and mechanical synchrony. RV function is assessed by Global circumferential or longitudinal strain. Normally RV primarily ejects longitudinal shortening whereas with better RV pressure loading, circumferential contraction is increased [144].The prognostic value of global longitudinal or circumferential strain in ToF is still tentative. According to Orwat et al., RV global longitudinal strain evaluated by CMR was a superior independent predictor for death, cardiac arrest, or VTs compared with RV volumes [145]. A similar relation for LV global longitudinal strain assessed by echocardiography was found by Diller et al. [146].
Mechanical desynchrony has been proved to relate to extended or fragmented (QRS complex containing additional spikes without bundle branch block) QRS complexes [147]. Studies assessing mechanical desynchrony report conflicting results because the contributions of this mechanic-electrical interaction to RV function remain uncertain [145,146, 148-151]. One of the studies show negatively predict exercise capacity of RV circumferential desynchrony [150], but this association is not confirmed by other studies [148,151]. Increasing use of Cardiac resynchronization therapy is done in ToF. According to a recent study 12 out of 15 adult patients with ToF had an improved NYHA (New York Heart Association) class or LV function after 2.6 years (median) of cardiac resynchronization therapy [152], Procedural success being high and adverse events were rare.
Right ventricular interactions in tetralogy of fallot
Atrioventricular interactions: amount of PR can be determined by diastolic function after ToF repair. In some patients, enddiastolic forward flow (EDFF) in the main PA during right atrial contraction can be visible [153]. As the non-compliant RV acts as a conduit during atrial contraction as RV diastolic pressure exceeds PA diastolic pressure, this is considered a sign of “restrictive RV physiology” [154,155]. Restrictive physiology could limit the amount of PR as elevated diastolic RV pressure decreases the amount of PR. No relationship between the presence of EDFF and other markers of diastolic dysfunction (that is, RV hypertrophy,atrial dilatation, reduced stroke volume, or reduced PR), according to a recent study [156]. Important roles are played by various mechanisms, such as pulmonary arterial capacitance and atrial function [157] in occurrence of EDEF. According to Luijnenburg et al. found that bi-atrial function, but not diastolic ventricular function,differed between patients with EDFF and those without it [157].In that study, abnormal atrial function was about worse exercise capacity and higher N-terminal pro brain natriuretic peptide (NTproBNP). Kutty et al. discovered that right atrial longitudinal strain predicted RV performance but not exercise capacity [158].Controversy is seen when speaking of effects of EDEF and studies mention conflicting results concerning the relationship between EDFF and the amount of PR [153,154,156], exercise capacity [154-157], and EDV [154-157].
Ventriculo-arterial interactions: Energetic and efficient transfer of blood through heart requires adequate atrio-ventricular coupling and ventricular-arterial (VA) coupling, which has not been studied deeply in ToF. Latus et al. evaluated VA coupling as the relationship between pulmonary arterial elastance and ventricular end-systolic elastance in adult patients with ToF by using CMR and catheterderived measurements both in resting stateand during dobutamine stress [159]. VA coupling gets enfeebled during resting conditions.EF and load-independent parameters of RV contractility amplified during dobutamine stress. Pulmonary arterial elastance increased consequently and the hampered VA coupling resulting in dobutamine stress which was like to that under resting conditions.
Interventricular interactions: RV and LV are broadly explained.LV and RV have common myocardial fibres, the anatomic space confined by the pericardium, the inter-ventricular septum and a common neurohumoral system [160]. Not surprisingly the mechanisms of this ventriculo-ventriculo interaction in chronic PR remain unclear whereas the effects of chronic PR are not limited to the RV. A linear correlation between LV and RV EF has been explained [160,161]. Severe RV dilation causes abnormal diastolic septal positioning, manipulating LV filling [162]. LV function has been associated with increased mortality and increased risk of VT[146,163] thus the role of the LV in outcomes in ToF is progressively appreciated. As per the INDICATOR report, LV EF was one of three independent predictors of mortality and VT [164]. Geva et al. states that LV EF as independent of RV parameters and anticipated poor functional status [161]. Strangely, parameters of LV function are not considered in present guidelines for the timing of PVR (Table 1).
Drug theraoy for right ventricular failure
Pharmacotherapy is significant in the treatment of LV failure and improves consequences. Conversely, the effects of the use of heart failure medication for RV failure have been unsatisfactory [165-167].RAAS (renin-angiotensin- aldosterone system) inhibitors do not appear to influence RV EF or exercise capacity [168] in patients who underwent ToF repair. Beta blockers showed no beneficial possessions after 6 months of treatment and an surge in NT-pro BNP was observed [169] in a controlled trial of 33 patients with ToF. New goals for medical treatment which are unique to the RV might be revealed, increasing our understanding of the pathophysiology of RV failure.
Current guidelines on the tining of pulmonary velve replacement
Prevention of RV failure can be achieved by restoring pulmonary valve function before irreversible RV dysfunction occurs. However,the durability of presently used pulmonary prosthetic valves is restricted. As a result, the timing of PVR is always a compromise.It should be arranged early enough to avert irreversible adverse remodelling but late enough to limit the number of re-interventions.Predicting decline in RV function is difficult, and the optimal timing of PVR is controversial because of the problems in evaluating RV function. Procedures by the European Society of Cardiology, the Canadian Cardiovascular Society (CCS), and the American College of Cardiology/ American Heart Association offer some references on indications for performing PVR [85-87]. These indications are summarized in Table 1.
The indication in guidelines, differ from actual situation and have limitations. Most guidelines are statistical constructs which do not benefit ever individual patient like; the 2009 CCS guideline provides cut-off points for EDVi but difference in normal (indexed) RV volumes between gender and age gets ignored [170]. End-systolic volume index and RV mass-volume ratio are better predictors than EDV [78,171]. PVR can be detected by progressive RV dilation but ‘too much progression’ is not yet defined [172-182]. Several studies mark the longitudinal changes in RV size and function after ToF repair [176-182]. RV volume shows non-linear graph until adolescence, which warns about determining results of adolescent or younger population.
Careful interpretation of current guidelines, are vital and individual patient parameters and views must be considered. Clinically, using information from various sources might prove beneficial like medical history, electrocardiogram, physical examination, exercise testing, imaging techniques and biomarkers [182].
Conclusions
Short-term and long-term mortality can repair ToF, causing a demographic shift which promotes better survival in adulthood.Long-term follow-up in older groups have showcased detrimental effects of PR in the long term, surgical lesions cause significant morbidity. Limited use of TAP and surgical modifications,to preserve pulmonary valve function like, transatrial (and transpulmonary) methodologies have extensively been implemented.In spite of development in morbidity, the follow up time for these methods is much less for demonstration of survival.
Early-stage detection in heart failure and prediction of future decline in RV function is hindered by limited understanding of RV adaptation and pathophysiology of RV heart failure. Adult ToF survivors need one or more PVRs in their lifetime and selection of optimal candidates and optimal timing for PVR remains challenging.These problems can be overcome by improving our understanding of RV failure, which is capable of providing treatment options to gain long-term health outcomes for patients with ToF.
- Life Research的其它文章
- The pharmacological mechanism of quercetin as adjuvant therapy of COVID-19
- Exploratory study of highly susceptible to SARSCoV-2 populations
- Epidemiological characteristics of syphilis in Nanchong City from 2017 to 2021
- MiR-93-5p in BM-MSCs-derived exosomes ameliorates renal fibrosis by affecting macrophage polarization
- Upregulation of MEG3 inhibits neuroblastoma progression via decreasing proliferation and promoting apoptosis
- Research progress in the causes of intracranial aneurysms

