MiR-93-5p in BM-MSCs-derived exosomes ameliorates renal fibrosis by affecting macrophage polarization
Anqi Nie, Jiaqi Shi, Xuerong Wang, Yuqing Lu, Wufei Dai, Jianhua Wu, Jing Liu, Xiaolan Chen*
Abstract MiRNAs and macrophages play important roles in renal fibrosis. The exosomes secreted by bone marrow mesenchymal stem cells(BM-MSCs) can alleviate renal fibrosis. What is not clear, however, is whether a type of miRNAs in the BM-MSCs exosomes can alleviate renal fibrosis by modulating macrophage polarization. First,we take a high-throughput sequencing of miRNAs in exosomes of BM-MSCs from chronic kidney disease (CKD) and normal people. Then we used the UUO mouse model and injected exosomes into the tail vein. The macrophages were stimulated with lipopolysaccharide (LPS). MSC-Exo or exosomes from BMMSCs transfected with miR-93-5p inhibitor (Inhi- Exo) were added to the culture medium. The macrophages were transfected with miR-93-5p inhibitor or miR-93-5p mimic alone. The expression of miR-93-5p in exosomes of CKD patients was significantly decreased compared with normal people and in the LPS-stimulated macrophages and UUO mice kidneys. After stimulation with LPS, the macrophages polarized toward M1 subtype. MSC-Exo or miR-93-5p mimic promoted macrophages from M1 to M2 subtype. Inhi- Exo or miR-93-5p inhibitor blocked the differentiation from M1 to M2 subtype. Significant fibrotic changes occurred in the kidneys of UUO mice, and M1 macrophages were significantly increased. After injecting exosomes into the tail vein of UUO mice, the degree of renal fibrosis was alleviated, the expression of miR-93-5p in the kidney was significantly increased, and the renal macrophages differentiated from M1 to M2 subtype. These results demonstrated that miR-93-5p in the exosomes derived from BM-MSCs can improve renal fibrosis by inducing macrophage differentiation from M1 to M2 subtype.
Keywords: BM-MSCs exosomes; miR-93-5p; macrophages; renal fibrosis
Introduction
Chronic kidney disease (CKD) involves structural and functional disorders of the kidneys caused by various reasons. Irrespective of the cause of CKD, the disease will eventually develop into renal interstitial fibrosis. Therefore, it is important to study the mechanism of renal fibrosis and identify the target of intervention (1-4). Macrophages play an important role in the development of renal fibrosis.When the kidney is injured, macrophages are recruited in the renal interstitium, and according to the local tissue microenvironment,they differentiate into classical activated macrophages (M1) and non-classical activated macrophages (M2). The M1 macrophages release tumor necrosis factor-α (TNF-α), interleukin (IL)-12, IL-6,reactive oxygen species (ROS) and other inflammatory mediators,which cause tissue inflammation and renal fibrosis. The M2 macrophages (M2a, M2b and M2c) release anti-inflammatory mediators,which can inhibit the inflammatory response and promote tissue repair at specific stages of injury (5,6). Bone marrow mesenchymal stem cells (BM-MSCs) possess the biological characteristics of self-replication, high proliferation potential and multi-directional differentiation. MSCs can improve the microenvironment of the injured area, promote angiogenesis, reduce inflammatory reaction,thus accelerate the recovery of renal function and repair of the damaged area, and prevent further damage to the kidney (7, 8). MSCs can reduce the secretion of pro-inflammatory factors from macrophages and promote M1 to M2 type transformation in vivo and in vitro (9, 10). However, the underlying mechanism is unclear. Studies have shown that homing of MSCs in the circulatory system to the injured site is not the main mechanism for their continuous therapeutic effect. BM-MSCs mainly change the microenvironment through paracrine or autocrine release of cytokines, and regulate the damage of tissues and cells due to inflammation and fibrosis (11, 12). The exosomes secreted by BM-MSCs are rich in bioactive molecules such as proteins, lipids, mRNAs and miRNAs, which can regulate tissues or organisms. MSC-exos have a similar function to MSC and MSC-exos have emerged as a favorable alternative to mesenchymal stem-based therapy strategies, which are safer, stable, and easy to store. Many genetic materials, neurotrophic factors and proteins to axons by MSC-exos to restore microenvironment homeostasis, regulate axon regeneration, and promote neurogenesis. Moreover, previous studies have indicated that MSCS-exos may not only effectively suppress inflammatory responses, but also preferably deliver genetic information between cells through miRNAs (13-17). MiRNAs can play various mediating effects on the kidneys, including alleviating the degree of renal fibrosis in CKD patients (18, 19). In addition,BM-MSC-derived exosomes (MSC-Exo) can promote tissue and organ recovery from injury by regulating macrophage polarization.For example, MSC-Exo alleviates myocardial ischemia-reperfusion injury through miR-182 by promoting the polarization of M2 macrophages (20). However, whether MSC-Exo can alleviate renal fibrosis by promoting macrophage polarization is unknown.
This study showed that the expression of miR-93-5p was significantly decreased in the MSC-Exo of CKD patients. After transfection with miR-93-5p mimic, LPS-stimulated macrophages differentiated from M1 to M2 subtype. In addition, the degree of renal fibrosis in UUO mice was relieved after injecting MSC-Exo into their tail vein. These findings suggested that miR-93-5p in MSC-Exo ameliorates renal fibrosis by affecting macrophage polarization.
Materials and Methods
Extraction and culture of BM-MSCs
The human bone marrow was diluted with an equal volume of phosphate buffer saline (PBS, PH7.4) and gently mixed. Thereafter, an equal volume of lymphocyte separating fluid (Cat: 07851, STEMCELL, Norway) was added to the bone marrow, and the diluted bone marrow was gently placed on the lymphocyte separator. After centrifugation, it was divided into four layers. The second layer (white transparent layer) was aspirated and mixed with 2-3 ml PBS. After centrifugation, the supernatant was discarded and the precipitate was resuspended in 2-3 ml PBS. This step was repeated twice. After centrifugation, the supernatant was discarded and low glucose medium(89% DMEM/low glucose + 10% FBS + 1% penicillin-streptomycin solution) was added to resuspend the cell pellet. After culturing for three days without discarding the culture medium, 3-5 ml low glucose medium was directly added and the culturing was continued.The medium was changed when the cells were completely attached after 5-7 days. Before we collected the exosomes of BM-MSCs transfected with miR-93-5p inhibior (RiboBio, Guangzhou, China)(Inhi-Exo) or negative control inhibitor (NC inhibitor,RiboBio,Guangzhou, China) (NC Inhi-Exo), we transfected BM-MSCs with miR-93-5p inhibior (200nM) or NC inhibitor (200nM) for 48 h(37°C, 5% CO2).
Exosome isolation and identification
When P3-P5 MSCs reached 70-80% confluency, the culture medium was replaced with DMEM/low glucose and cultured for 48 h (37°C,5% CO2). Exosomes were isolated using differential centrifugation based on previously described methods with minor modifications(21). The morphology of exosomes was observed under a transmission electron microscope. After isolation, 10 μL exosomes was diluted in 1 mL of filtered PBS, and size distribution was measured by Zetaview (PMX120-Z, Germany). Exosomes were then identified by the marker proteins CD9 and CD63 using western blot. The quantity of exosomes was determined by BCA assay by measurement of total protein. The uptake of PKH-67-labelled MSC-Exo was determined by confocal microscopy. For in vitro experiments, the amount of exosomes used was 160 μg/ml. For in vivo experiments,the amount of exosomes used was 200 μg/mouse. Exosomes from BM-MSCs of normal people were used in the in vivo and in vitro experiments.
Macrophage culture and treatment
RAW.264.7 cells (macrophages) were provided by Professor Yang Bin at the University of Leicester, UK. The cells were cultured in high glucose medium (89% DMEM/high glucose + 10% FBS + 1%penicillin-streptomycin solution) in a 5% CO2atmosphere at 37℃.Macrophages were seeded in a 6-well plate at a density of 1x10^5/well for 24 h. Then LPS (500 ng/ml) was added to the medium. After 24 hours, the cells were incubated with MSC-Exo, Inhi-Exo or NC Inhi-Exo (160 μg/ml) or transfected with miR-93-5p inhibitor(200 nM) or NC inhibitor (200 nM) and miR-93-5p mimic (RiboBio,Guangzhou, China) (50 nM) or negative control mimic (NC mimic)(RiboBio, Guangzhou, China) (50 nM). After 48 hours, the levels of IL-10 and IL-12 were detected in the supernatant of the cells. Cell morphology was observed, and iNOS and Arg1 expression were detected in the cells. M1 macrophages (CD86+/F4/80+) and M2 macrophages (CD206+/F4/80+) were labeled, and the change of M2/M1 ratio was detected by flow cytometry.
Animal model
Ethical approval number of animal experiments is S20191202-403(Affiliated Hospital of Nantong University). Healthy male BALB/c mice (6-8 weeks old, weighing 20 ± 2 g) were purchased from the Experimental Animal Center of Nantong University School of Medicine (license number: 220191465). 10% chloral hydrate was used at 0.08 mL/10 g body weight to anesthetize the mice. The mice were fixed and sterilized, and the abdominal wall was cut about 5-6 mm on the left side of the abdominal midline above the pubic symphysis.The left ureter was isolated and ligated with 4-0 silk suture, and then the entire layer was sutured. The left ureter of the sham operation group was isolated but not ligated. Eight hours before the operation,the mice in the MSC-Exo group were injected with normal human MSC-Exo (200 μg exosomes dissolved in 0.1 ml PBS/mouse) into the tail vein. The PBS group was injected with PBS (0.1 ml/mouse)into the tail vein. After the operation, the animal was put back into the cage, and allowed free access to food and water. The mice were sacrificed seven days later. Blood samples were collected to determine BUN and serum creatinine (Scr). Kidney tissues were collected and snap-frozen in liquid nitrogen, and stored at -80 °C for RNA and protein extraction.
Fluorescent labeling of exosome
BM-MSCs were labeled with cell tracker Dil-C18 (Beyotime, Beijing, China) , a fluorescent lipophilic cationic indocarbocyanine dye,for 1 h and washed three times with PBS, the molarity of fluorescent dyes was 10ummol/L. Exosomes were harvested from the conditioned media of Dil-labeled BM-MSCs and injected into mice via tail vein (200 μg exosomes dissolved in 0.1 ml PBS/mouse) eight hours before the operation, and then detected by immunofluorescence.
High-throughput sequencing of miRNAs
Bone marrow samples were collected from normal individuals and CKD patients. The inclusion criteria for participants are listed in Table 1. The mesenchymal stem cells were extracted for culture, and the exosomes in the cell supernatants were grouped as Normal-Exo and CKD-Exo, respectively. RNA from the Normal-Exo and CKDExo groups were extracted using miRNeasy kit (Qiagen, Hilden,Germany). RNA yield was assessed using the Qubit® 2.0 (Life Technologies, USA). In total, 20 ng RNA was used to prepare small RNA libraries by NEBNext® Multiplex small RNA library prep set for Illumina (NEB, USA) according to the manufacturer’s instructions. The RNA libraries were sequenced by HiSeq 2500 (Illumina,USA) with single-end 50 bp reads at Ribobio Co. Ltd, Guangzhou,China. Differential expression was calculated between two sets of samples with the edgeR algorithm based on the significant criteria of|log2 (Fold Change) | ≥ 1 andP< 0.05.
Exosome uptake
Normal-Exo was extracted and resuspended in 100 μL PBS. Then 0.5 ml Diluent C was added and mixed well, followed by addition of 4 μL PKH67 (Sigma, USA) in 0.5 ml Diluent C (Sigma, USA).The solution was mixed and kept at room temperature for 4 min.Then, 1 ml BSA was added to stop the staining, and the exosomes were re-extracted by ultracentrifugation. The exosomes were re-suspended and added to a 24-well plate of inoculated macrophages, and incubated at 37 °C for 12 h. Thereafter, the culture medium was discarded, and the cells were washed with PBS for three times. Finally,DAPI (Beyotime, Beijing, China) was added to the cells for about 10 min, and the cells were observed and imaged using a fluorescence microscope.
MiRNA
MiR-93-5p inhibitor and mimic were used to suppress or over-express miR-93-5p. The inhibitor and mimic, and the corresponding negative control miRNA were purchased from RiboBio (Guangzhou,China). According to the manufacturer’s protocol (RiboBio), miRNA inhibitor, mimic and the corresponding negative control were transfected into macrophages or BM-MSCs using the riboFECT™CP transfection kit (RiboBio) at an effective concentration of 200 nM (inhibitor and its negative control miRNA) or 50 nM (mimic and its negative control miRNA). MiRNA transfection efficiencies were monitored by qRT-PCR at 48 h after transfection.
RNA extraction and real-time quantitative RT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad,CA, USA) according to the manufacturer’s instructions. The firststrand cDNA synthesis using 1 μg of RNA was performed with the reverse transcription system kit (Vazyme, Nanjing, China), according to the manufacturer’s instructions. Quantitative RT-PCR was performed with 2 × SYBR Green PCR master mix reagents (Roche,Germany) using 1 μL of cDNA and specific primer pairs in the Roche Luo Shi LightCycler® 480II (Switzerland). The reverse-transcription and amplification of miR-93-5p were completed using Bulge-Loop TM miRNA qRT-PCR starter kit (RiboBio, Guangzhou, China), Bulge-Loop TM miRNA qRT-PCR primer (RiboBio,Guangzhou, China), according to the instruction manual. All primer sequences are shown in Table 2. Quantitative RT-PCR reaction was conducted using standard conditions, including a single denaturation cycle at 95 °C for 3 min, an amplification protocol of 40 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 60 s. A post-amplification melting-curve analysis was run to assess the efficiency of primer pairs. The relative levels of RNA were calculated using the 2 (-ΔΔCt) method.
Western blotting
RAW.264.7 cells and kidney tissue proteins were extracted with RIPA strong lysis buffer (Beyotime, Beijing, China) and 100 mM PMSF (Beyotime, Beijing, China), and the protein concentration was determined by BCA method. The total protein content at various volumes was 100 μg.Next, 5×SDS-PAGE protein loading buffer(Beyotime, Beijing, China) was added to the samples and heated at 100℃ for 10 minutes. Immunoblotting was performed with primary antibodies against α-SMA (1:2000, Abcam, USA), fibronectin(1:2000, Abcam, USA) and α-Tubulin (1:2000, Abcam, USA), followed by the addition of horseradish peroxidase-labelled secondary antibodies (Cat: BL003A, Biosharp, China). The blots were visualized with Amersham ECL Detection Systems (Amersham, UK).Densitometric analysis was performed using Quantity One Software(Bio-Rad).
Immunohistochemistry
The paraffin slices were placed in a 60℃ oven and baked for 2-4 h. The paraffin sections were put into xylene and gradient alcohols for dewaxing and hydration, and then put into citric acid buffer for microwave antigen repair for 15 min, cooled at room temperature,and washed with PBS three times. Thereafter, 3% H2O2-methanol was added and the slices were kept at room temperature for 15 min followed by washing with PBS three times. Next, goat serum (Cat:SL038, Solarbio Beijing, China) was added drop-wise for blocking at room temperature for 60 min, and washed with PBS three times.The primary antibody (anti-α-SMA 1:500, anti-fibronectin 1:2000)was added drop-wise at 4°C overnight. Next day, the slices were rewarmed for 1 hour and washed with PBS three times. Then the secondary antibody (1:1000) was added drop-wise, incubated at 37 °C for 30 min, and washed with PBS three times. DAB color developing solution was added drop-wise for 1 min and rinsed with running water for 10 min. The slices were stained with hematoxylin(Beyotime, Beijing, China) for 3 min and rinsed with running water.After dehydration with gradient alcohols and dewaxing with xylene,the film was sealed with neutral gum and read.
HE, Sirius Red and Masson staining
Kidney tissue was fixed in 4% paraformaldehyde at 4°C overnight.The thickness of the whole kidney section was 6 mm. After dewaxing in xylene and rehydration with graded alcohol solution, the placental section was sent to Servicebio (Wuhan) for HE, Sirius Red,Masson staining. Images were taken under FSX100 Bio Imaging Navigator (Olympus, Tokyo, Japan). At least five random areas were analyzed for each sample, and the density of collagen per field was evaluated. Histology staining was analyzed using Image J 6.0 software.
ELISA
The serum samples were collected. Samples and standard markers were added respectively incubated 90 min at room temperature.Then biotin-labeled antibody was added for reaction 60 min at 37℃.After 1X buffer wash 3 times, ABC was added to culture 30 minutes and then TMB was added for reaction 20 minutes. The levels of IL-10 and IL-12 were determined by ELISA following the manufacturer’s recommendations, and immediately quantified using Varioskan LUX microplate reader at 450 nm.
Flow cytometry
The cells were resuspended and incubated with Alexa Fluor 647 anti-mouse F4/80 (Cat: 123122, BioLegend, USA) and FITC anti-mouse CD86 (Cat: 105006, BioLegend, USA). For intracellular staining, the cells were immobilized and permeated with a fixative and permeabilization solution (BD Biosciences). Then a PE anti-mouse CD206 (Cat: 141706, BioLegend, USA) was added,washed three times, incubated at 4 °C in a dark chamber for 30 minutes, and analyzed by flow cytometry. The data was analyzed using FlowJo software.
Statistical analysis
Data are presented as means ± SE. Statistical analysis was performed using ANOVA followed by a Bonferroni posttest or unpaired Student’s t-test. AP< 0.05 was considered statistically significant.
Results
Characterization of MSC-Exo
BM-MSCs are long spindle-like adherent cells (Fig. 1a). We purified exosomes from the culture supernatant of MSCs by continuous centrifugation and ultracentrifugation, and then identified the morphology and phenotype of the isolated particles based on the known characteristics of exosomes. First, transmission electron microscopy showed that the isolated MSC vesicles had a typical goblet-like foreign body shape, with a bilayer membrane structure (Fig. 1b).Nanoparticle Tracking Analysis (NTA) showed that the concentration and the diameter of the particles were within 50-150 nm (Fig.1c). In addition, Western blot analysis confirmed the expression of CD9 and CD63, which are recognized molecular markers of exosomes (Fig. 1d). Therefore, the above findings indicated that the MSC particles collected were exosomes.
High-throughput sequencing
There were several differences in the expression of miRNAs in exosomes derived from BM-MSCs between normal people and CKD patients. There were 23 down-regulated miRNAs in CKD patients,of which miR-93-5p was significantly down-regulated (Fig. 2a, b).
MSC-Exo were phagocytosed by macrophages
PKH-67-labeled MSC-Exo were added to the serum-free medium of macrophages stimulated by LPS, and the macrophage nuclei were labeled with DAPI. The MSC-Exo were phagocytosed by macrophages as observed under a fluorescence microscope ×400 (Fig. 2c).
MSC-Exo and its miR-93-5p promote the macrophages from M1 to M2 subtype
After macrophages were stimulated by LPS, the expression of miR-93-5p in the cells decreased significantly. After MSC-Exo incubation, the expression of miR-93-5p in macrophages increased significantly, confirming that MSC-Exo was taken up by macrophages, but the expression of miR-93-5p in macrophages decreased if the MSC was transfected with miR-93-5p inhibitor, which was consistent with the expression of miR-93-5p in exosomes of these MSCs. In addition, when we transfected macrophages with miR-93-5p inhibitor,the expression of miR-93-5p in macrophages decreased (Fig. 3b,c).After LPS stimulation, the macrophages became significantly larger and deformed. The proportion of malformed macrophages incubated with MSC-Exo decreased significantly. However, there was no such change in the proportion of malformed macrophages in the culture medium added with Inhi-Exo, suggesting that miR-93-5p in exosomes played an important role in affecting the morphology of macrophages. In addition, the proportion of malformed macrophages transfected with miR-93-5p inhibitor was increased, suggesting that miR-93-5p played an important role for macrophages (Fig. 3a).
The M1 type macrophages were labeled with CD86+/F4/80+ and M2 type macrophages with CD206+/F4/80+. The results of flow cytometry showed that macrophages differentiated to M1 type after LPS stimulation, while macrophages incubated with exosomes underwent M2 type differentiation. In order to explore whether miR-93-5p in exosomes played an important role in this process, we added Inhi-Exo, and found that the ability of exosomes to promote M2 type differentiation of macrophages was significantly weakened,and the addition of NC Inhi-Exo had no significant effect on the ability of exosomes, proving that miR-93-5p in exosomes played an important role in this process. In addition, miR-93-5p inhibitor directly reduced the polarization of macrophages to M2 type, confirming that miR-93-5p inhibitor and Inhi-Exo have similar effects on polarization of macrophages (Fig. 3d).
After macrophages were stimulated by LPS, the expression of iNOS increased significantly, and the level of IL-12 in the cell supernatant increased significantly, proving that the cells were transformed to the M1 subtype. After MSC-Exo incubation, the expression of iNOS in the cells decreased significantly, the expression of Arg1 was significantly increased, the level of IL-12 in the cell supernatant decreased, and the level of IL-10 increased, which proved that the cells were transformed from M1 to M2 type. When we added Inhi-Exo to cell culture, the expression of iNOS in the cells had increased, the expression of Arg1 had decreased, the level of IL-12 in the cell supernatant had increased, and the level of IL-10 had de-creased, proving that the transformation of cells from M1 to M2 type was inhibited. In addition, after transfected with miR-93-5p inhibitor, the expression of iNOS increased, and the level of IL-12 in the cell supernatant increased, proving that the cells were transformed to the M1 subtype even if the effect was not particularly obvious (Fig.3e,f).
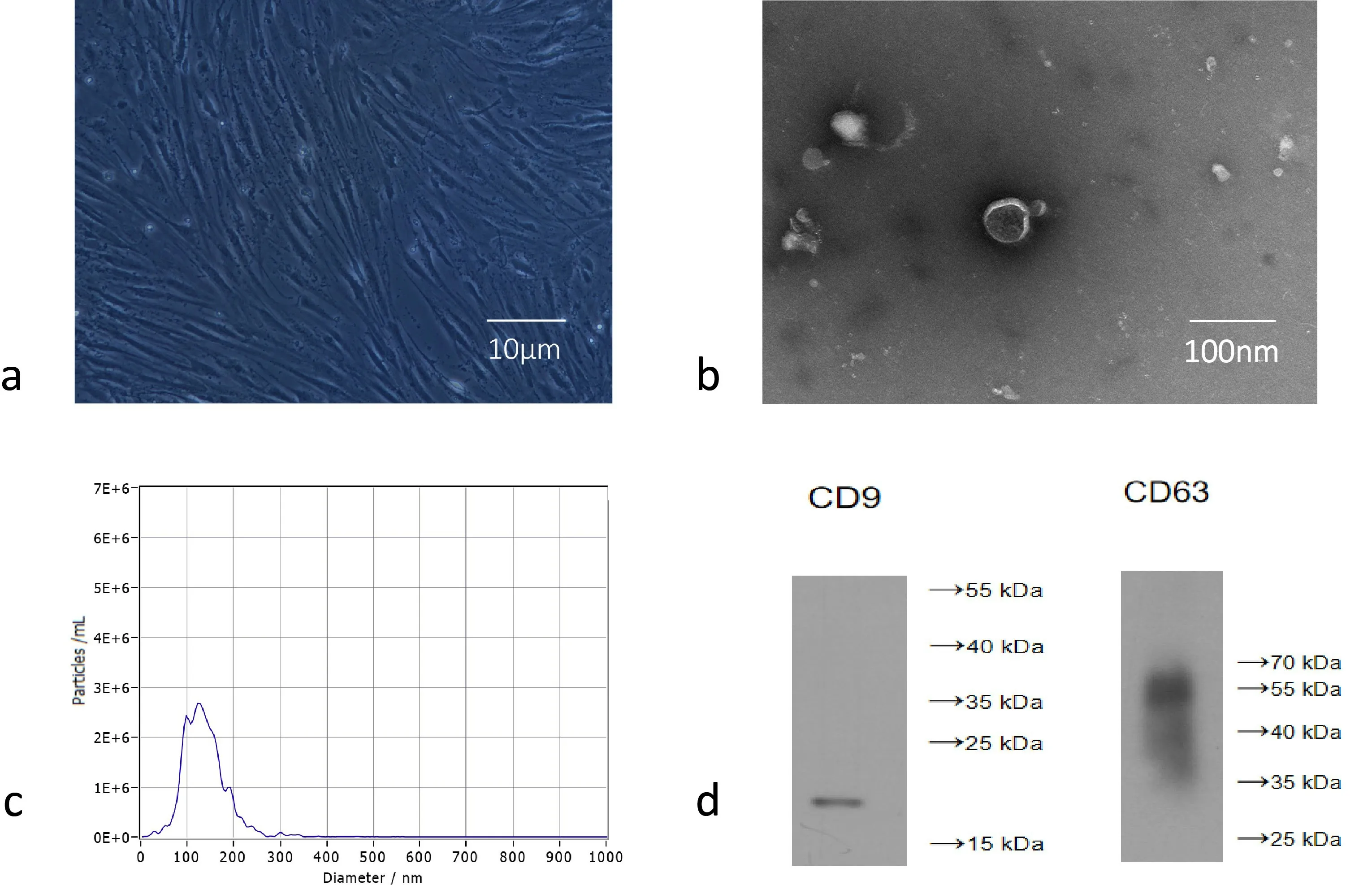
Figure 1. BM-MSCs and MSC-Exo identification. a) Observe the P3 generation of BM-MSCs under a microscope. b) Observe the shape of MSC-Exo by transmission electron microscope. c) NTA analysis of MSC-Exo diameter. d) Western blot analysis confirmed the expression of CD 9, CD 63.
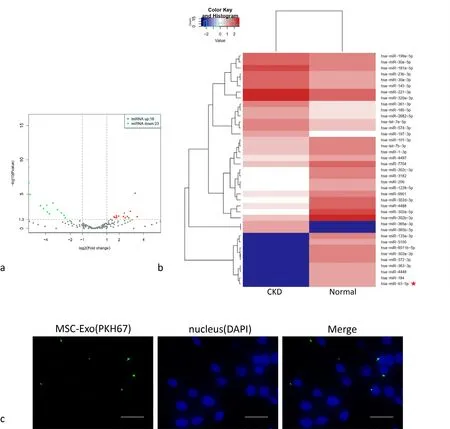
Figure 2. High-throughput sequencing result. a) Two groups of miRNA differential expression analysis Svolcano graphs (the abscissa represents the fold change of miRNA expression in different samples; the ordinate represents the statistical significance of the difference in miRNA expression). b) The two groups of miRNA differential expression analysis heat map (color represents log10 (COUNT+1) value; red star mark the miRNA that we choose to study). c) PKH-67 labeled MSC-Exo are phagocytosed by macrophages. Scale bar = 20μm.
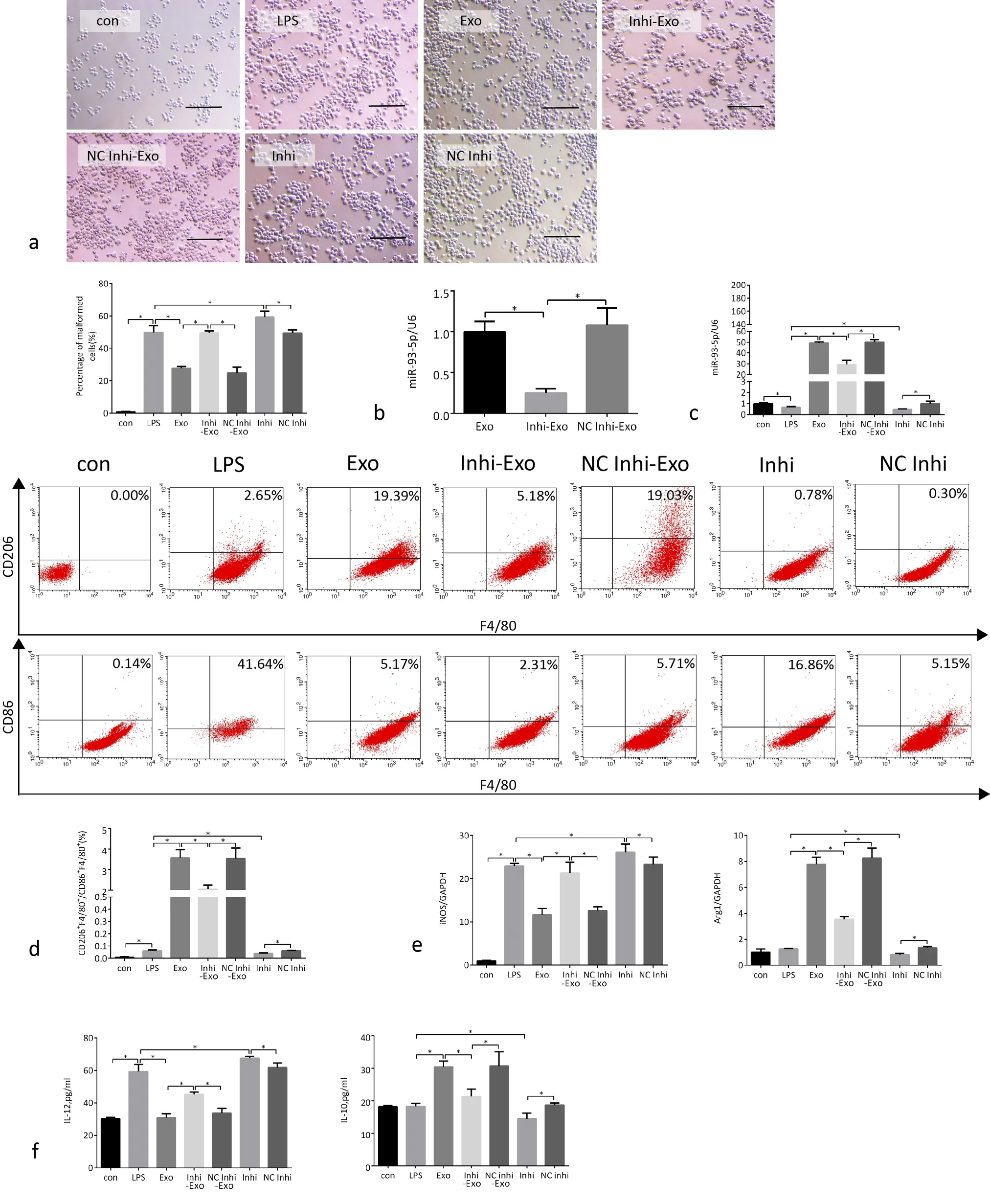
Figure 3. MSC-Exo and its miR-93-5p promote the macrophages from M1 to M2 subtype. a) Macrophage morphology in each group. Scale bar, 100μm. Quantitative enumeration of the proportion of malformed macrophages to total cells in each group. At least five random fields were calculated in different groups. b) The expression of miR-93-5p in exosomes in 3 groups of MSCs. c) The expression of miR-93-5p in 7 groups of macrophages. d) Representative flow cytometry plots showing the percentages of M1 (CD86+/F/4/80+) and M2 (CD206+/F/4/80+)phenotype and quantification of M2/M1 (%) in 7 groups macrophages. e) The expression of iNOS and Arg1 in 7 groups of macrophages. f)Levels of IL-12 and IL-10 in cell supernatants of 7 groups. Con, macrophages were cultured without any treatment; LPS, macrophages were cultured under LPS (500ng/ml) stimulation for 24 h; Exo, after LPS stimulation for 24h, macrophages cultured medium were added with MSC-Exo (160μg/ml) for 48h; Inhi-Exo, after LPS stimulation for 24h, macrophages were cultured in the presence of exosomes from BMMSCs transfected with miR-93-5p inhibitor for 48h; NC Inhi-Exo, after LPS stimulation for 24h, macrophages were cultured in the presence of exosomes from BM-MS Cs transfected with NC inhibitor for 48h; Inhi, after LPS stimulation for 24h, macrophages were cultured in the presence of miR-93-5p inhibitor for 48h; NC Inhi, after LPS stimulation for 24h, macrophages were cultured in the presence of NC inhibitor for 48h.*p <0.05, n = 5.
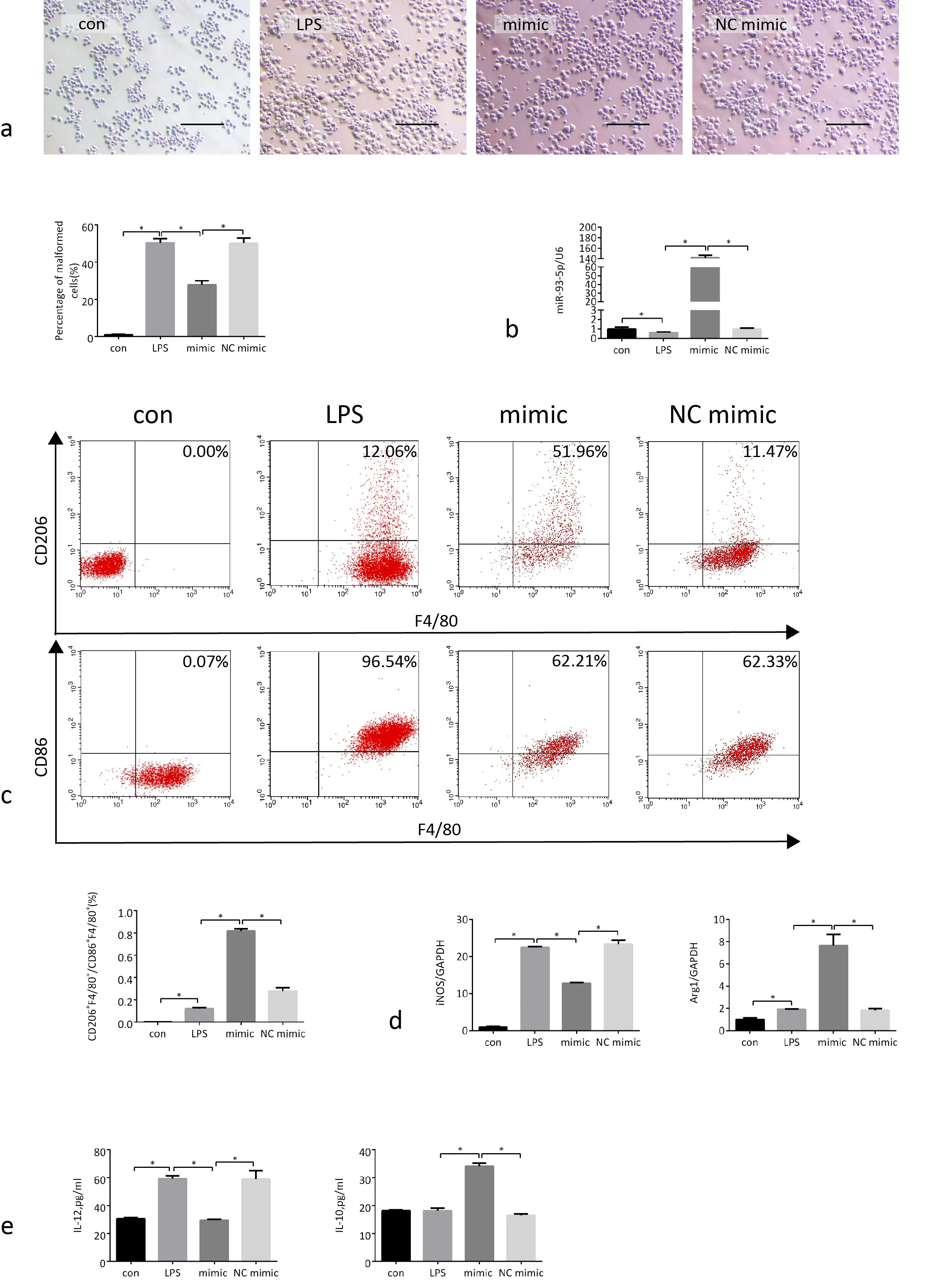
Figure 4. MiR-93-5p mimic promote the polarization of macrophages M1 to M2 type. a) Macrophage morphology in each group. Scale bar,100μm. Quantitative enumeration of the proportion of malformed macrophages to total cells in each group. At least five random fields were calculated in different groups. b) The expression of miR-93-5p in 4 groups of macrophages. c) Representative flow cytometry plots showing the percentages of M1 (CD86+/F/4/80+) and M2 (CD206+/F/4/80+) phenotype and quantification of M2/M1 (%) in 4 groups macrophages. d) The expression of iNOS and Arg1 in 4 groups of macrophages. e) Levels of IL-12 and IL-10 in cell supernatants of 4 groups. Con, macrophages were cultured without any treatment; LPS, macrophages were cultured under LPS (500ng/ml) stimulation for 24 h; mimic, after LPS stimulation for 24h, macrophages were cultured in the presence of miR-93-5p mimic for 48h; NC mimic, after LPS stimulation for 24h, macrophages were cultured in the presence of NC mimic for 48h.*p <0.05, n = 5.
MiR-93-5p mimic promote the polarization of macrophages M1 to M2 type
After macrophages were stimulated by LPS, the expression of miR-93-5p in the cells decreased significantly, the macrophages became significantly larger and deformed. After transfected with miR-93-5p mimic, the expression of miR-93-5p in macrophages increased significantly, the proportion of malformed macrophages decreased (Fig. 4a, b). These phenomenon confirming that miR-93-5p mimic has similar direct effect on macrophages as MSCExo. The results of flow cytometry showed that macrophages differentiated to M1 type after LPS stimulation, while macrophages transfected with miR-93-5p mimic underwent M2 type differentiation (Fig. 4c), confirming that miR-93-5p mimic has similar direct effect on polarization of macrophages as MSC-Exo.
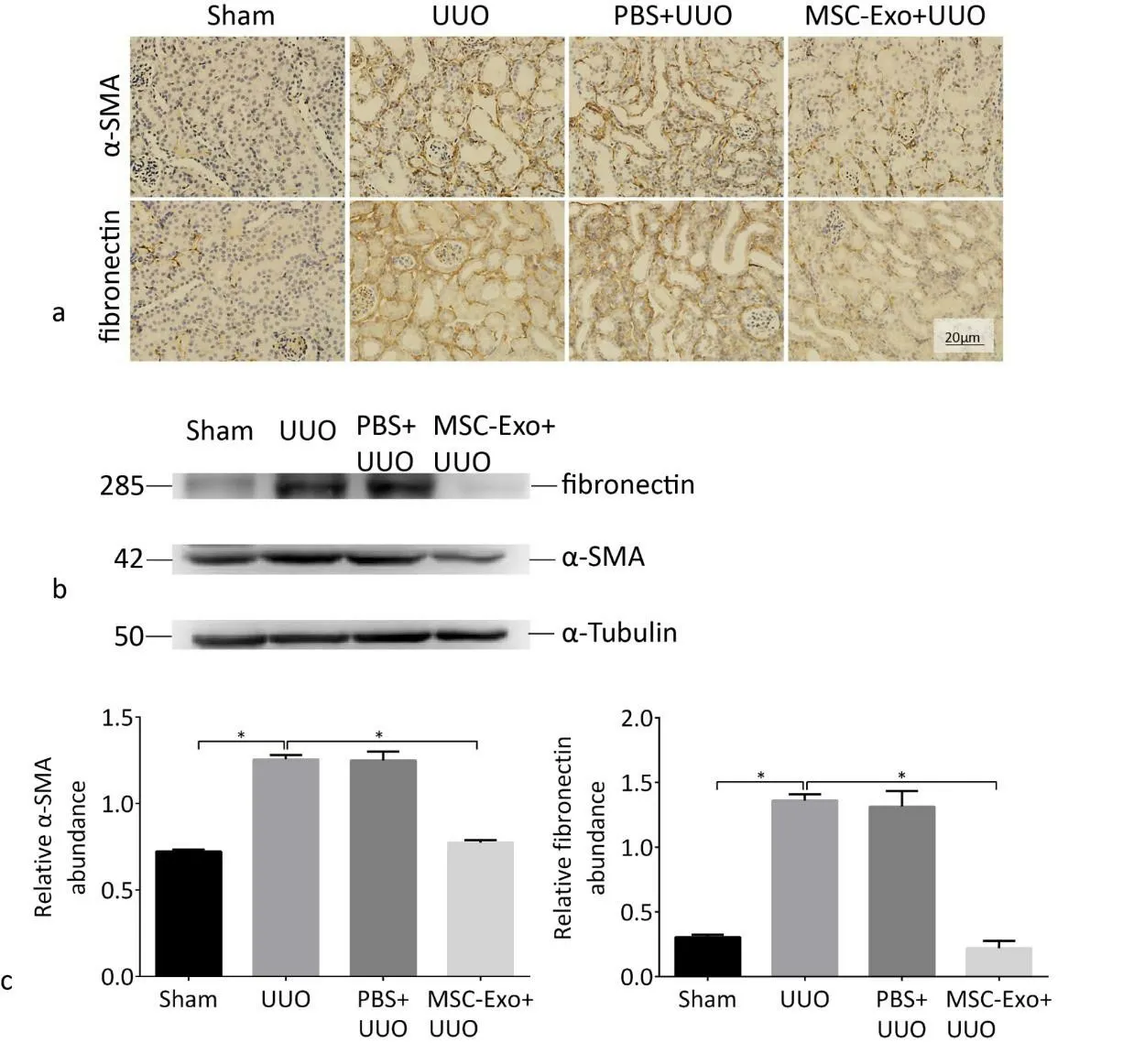
Figure 5. MSC-Exo can reduce the degree of kidney fibrosis in UUO mice. a) Serum creatinine and BUN in four groups mice. b) Representative images of HE staining, Sirius red and Masson staining of kidneys in four groups mice. Collagen is stained red in Sirius red staining and is stained blue in Masson staining. Scale bar, 20 μm. c) Quantification of the collagen content. At least five random fields were calculated in different sections. *P <0.05, n = 4.
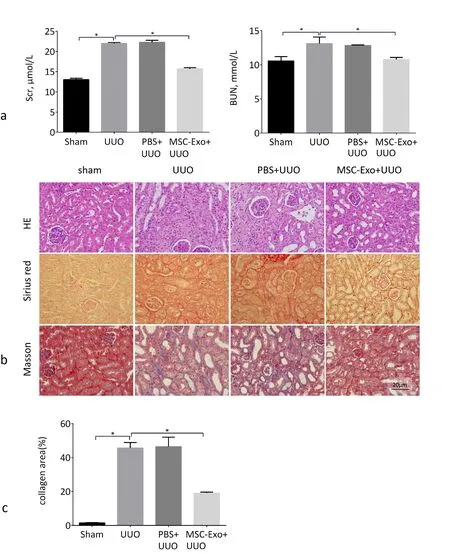
Figure 6. MSC-Exo treatment reduced fibrotic-related gene expression induced by UUO. a) Immunohistochemical detected α-SMA and FN expression in kidneys of four groups of mice. Scale bar, 20 μm. b) Western blot detected α-SMA and FN expression in kidneys of four groups of mice. c) Quantitative analysis of protein expression is shown.*p <0.05, n = 4.
After macrophages were stimulated by LPS, the expression of iNOS increased significantly, and the level of IL-12 in the cell supernatant increased significantly, proving that the cells were transformed to the M1 subtype. After miR-93-5p transfection, the expression of iNOS in the cells decreased significantly, the expression of Arg1 was significantly increased, the level of IL-12 in the cell supernatant decreased, and the level of IL-10 increased, which proved that the cells were transformed from M1 to M2 type. (Fig. 4d, e). All the results proved that miR-93-5p mimic has similar direct effect on macrophages as MSC-Exo, which suggest miR-93-5p could promote macrophages from M1 to M2 subtype.
MSC-Exo reduced the degree of kidney fibrosis in UUO mice
Compared with the sham group, the serum Scr and BUN values of the UUO group were increased, indicating that the renal function was damaged. Compared with the UUO group, the serum Scr and BUN levels of the mice in the MSC-Exo+UUO group were decreased, indicating that the renal function was improved (Fig. 5a).The results of HE staining showed that the UUO group mice had glomerular sclerosis, increased extracellular matrix, and deposition of collagen fibers in the renal interstitium. Sirius red and Masson staining showed severe collagen deposition in the kidneys of UUO mice. Compared with the UUO group, the kidney tissue structure of the MSC-Exo+UUO group was improved, the renal interstitial tissue was destroyed and collagen fibers were significantly reduced. The kidney tissue structure of the PBS+UUO group showed no significant changes (Fig. 5b).
UUO-induced fibrosis-related gene expression was reduced by MSC-Exo treatment
To further determine the protective effect of MSC-Exo on renal fibrosis, we studied the expression changes of fibrosis-related proteins, α-SMA and fibronectin in the kidneys of mice in each group.The expression of α-SMA and fibronectin in the kidneys of mice in the MSC-Exo group was significantly decreased. These results indi-cated that MSC-Exo inhibited the development of renal fibrosis (Fig.6a, b, c).
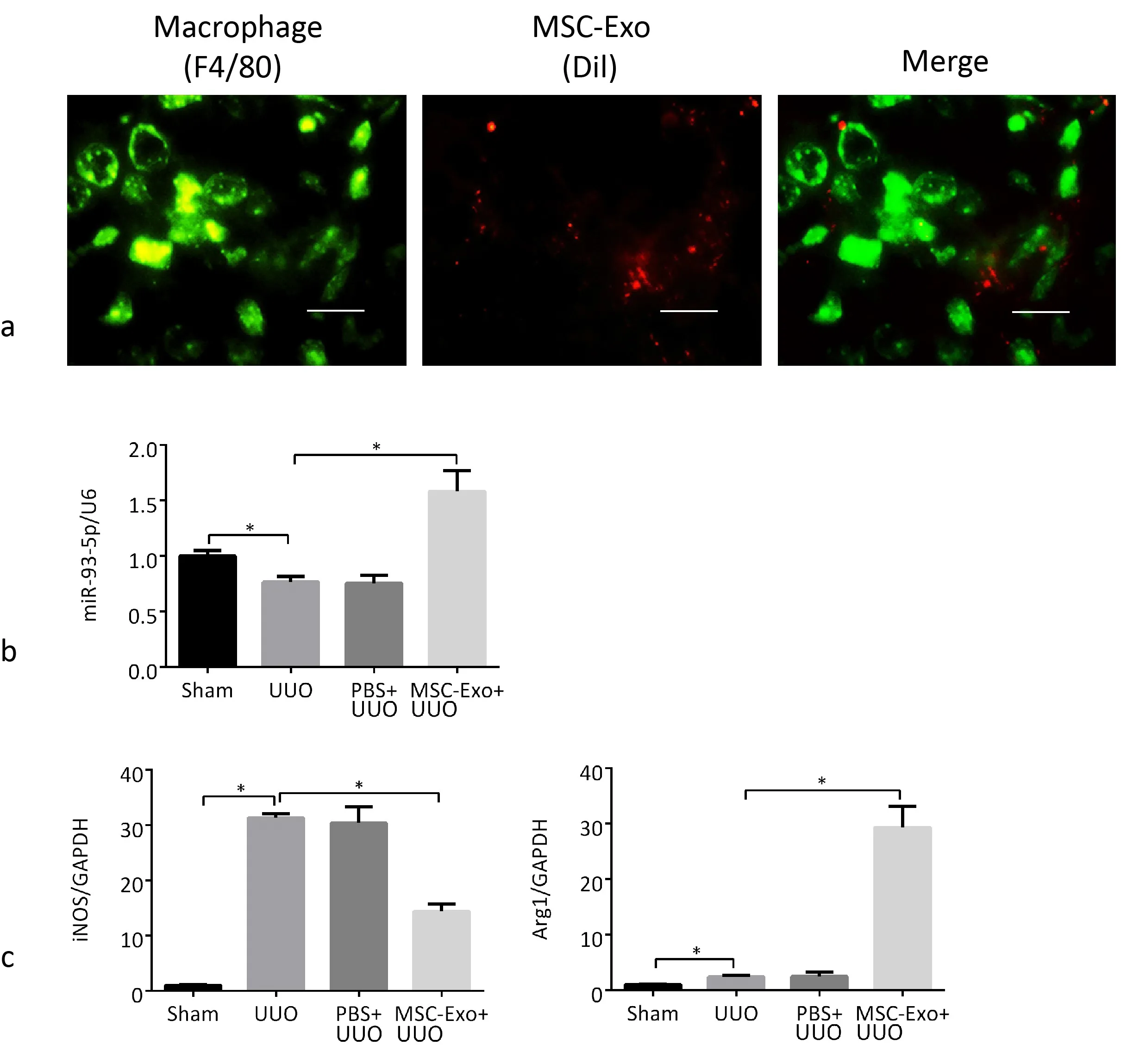
Figure 7. MiR-93-5p promotes macrophage transformation from M1 type to M2 type. a) Dil labeled MSC-Exo are phagocytosed by macrophages in kidneys of mice. Scale bar, 20μm. b) QRT-PCR analysis of miR-93-5p in the kidneys. c) QRT-PCR analysis of iNOS and Arg1 in the kidneys.*p <0.05, n = 4.
MiR-93-5p promoted the transformation of macrophages from M1 to M2 type in UUO mice kidneys
Exosomes were harvested from the conditioned media of Dil-labeled (10ummol/L) BM-MSCs and injected into mice via tail vein before operation, and then detected by immunofluorescence. We marked macrophages with F4/80 antibody.Observed under a confocal microscope, Dil-labeled MSC-Exo were phagocytosed by macrophages in kidney (Fig. 7a×400).
During the establishment of UUO model and the tail vein injection of exosomes into mice, the expression of miR-93-5p decreased significantly and the expression of iNOS increased significantly in the kidneys of UUO mice, which demonstrated that renal macrophages transformed to M1 type. After the injection of exosomes, the expression of miR-93-5p increased significantly, the expression of iNOS decreased significantly, and the expression of Arg1 was significantly increased, demonstrating that miR-93-5p in exosomes promoted the transformation of renal macrophages from M1 to M2 type (Fig.7b,c).
Discussion
BM-MSCs have the characteristics of high proliferation and multidirectional differentiation, which can improve the microenvironment of the injured site, promote angiogenesis, and reduce the inflammatory response to accelerate the repair of damaged kidneys.Like MSCs, MSC-derived exosomes can promote repair of tissue damage and facilitate immune regulation, which reduce the risk of immune rejection and teratogenic tumors of MSC transplantation(22). MSC-derived exosomes alleviate kidney fibrosis in UUO mice by inhibiting the epithelial-mesenchymal transition process (23).
MiRNAs are endogenous non-coding RNAs that regulate cell differentiation, proliferation and other processes by regulating the expression of target genes. They are widely present in exosomes from various cell sources. MiRNAs can control different phenotypes of macrophages to regulate the development of inflammation. For example, miR-199a-5p induces polarization of M1 macrophages by targeting the Klotho/TLR4 pathway, and accelerates the progression of CKD (24). During renal injury, macrophages accumulate in the renal interstitium and differentiate into classical activated macrophages (M1) and non-classical activated macrophages (M2) according to the local tissue microenvironment. Type M1 macrophages release IL-12, which induces inflammation and renal fibrosis, and type M2 macrophages release IL-10, which promotes tissue repair.
In order to study whether exosomes derived from BM-MSCs can improve renal fibrosis by regulating the polarization of macrophages and identify the specific components of exosomes that play a major role, we conducted high-throughput sequencing and found that compared with normal people, miR-93-5p was significantly decreased in exosomes derived from BM-MSCs of CKD patients. MiR-93-5p is related to inflammation and can control the secretion of various inflammatory chemokines. The level of miR-93 in kidneys of patients with diabetic nephropathy is significantly lower than that in normal people, and the over-expression of miR-93 can inhibit renal fibrosis by regulating the CDKN1A and TGF-β1 signaling pathways, further suggesting that miR-93-5p has therapeutic significance in renal fibrosis (25).
In order to study whether miR-93-5p in exosomes regulates the development of renal fibrosis by regulating the polarization of macrophages, we used LPS to stimulate macrophages and simulate the impact of the microenvironment on macrophages during renal injury, and then added MSC-Exo or Inhi-Exo to cells or transfect cells with miR-93-5p inhibitor or miR-93-5p mimic alone to study its effect. The results showed that after LPS stimulation, the macrophages showed significantly enlarged malformation. After adding exosomes and transfecting miR-93-5p mimic, the proportion of malformed macrophages decreased significantly, but there was no significant change in the proportion of malformed macrophages after adding Inhi-Exo to macrophage, confirming that miR-93-5p in exosomes played an important role in affecting the morphology of macrophages. It is suggested that exosomes and miR-93-5p mimic have a similar effect on macrophages stimulated by LPS, reducing the damage of macrophages, and miR-93-5p inhibitor can increase the proportion of malformed macrophages. In order to further explore the mechanism of the effect on macrophages, we used qRTPCR to detect the expression of miR-93-5p, iNOS and Arg1 mRNAs in the cells, and ELISA to detect the levels of IL-12 and IL-10 in the cell supernatants. The results showed that MSC-Exo promoting their transformation from M1 to M2 type, this effect could be blocked if the MSCs are pretreated with miR-93-5p inhibitor, suggesting that miR-93-5p in exosomes played an important role in affecting the polarization of macrophages. This finding was verified by flow cytometry results. In addition, miR-93-5p mimic has similar effect with MSC-Exo to macrophages, but miR-93-5p inhibitor has reverse effect. After stimulation of LPS, Inhi-Exo has few effcet on polarization of macrophages, however,miR-93-5p inhibitor exacerbates macrophages to M1 subtype, suggesting that instead of miR-93-5p in MSC-Exo, it can promote macrophages to M2 subtype alone. This finding was verified by miR-93-5p mimic transfection to macrophages. This can be a point for us to continue to study in depth in the future. All in all, these data confirmed that miR-93-5p in MSC-Exo can promote the transformation of macrophages from M1 to M2 type, which is responsible for its anti-fibrosis effect.
The UUO model is extensively used to study the development of renal fibrosis (26). In this study, we established a mouse UUO model and observed the effect of MSC-Exo on renal fibrosis by tail vein injection. Serum creatinine and urea nitrogen increased significantly in UUO mice, indicating impaired renal function. The pathological section staining and the test of fibrosis index showed that the renal fibrosis was serious. Moreover, the expression of miR-93-5p decreased significantly and the macrophages polarized to M1 subtype. After tail vein injection of exosomes, the renal function was improved, the degree of renal fibrosis was reduced, and the expression of miR-93-5p was significantly increased. Macrophages were polarized from M1 to M2 type. In order to confirm the MSC-Exo were phagocytosed by macrophages in kidney, we labeled the exosomes with Dil,which were found in macrophages in mice kidney. These results suggested that miR-93-5p in MSC-Exo can improve renal fibrosis by promoting the transformation of macrophages from M1 to M2 type.
This study had some limitations. The target genes of miR-93-5p and the signaling pathways involved were not identified. Identifying the specific target genes and signaling pathways will help us to better understand this regulatory mechanism, which is our future research goal.
In summary, miR-93-5p in the MSC-Exo can improve renal fibrosis by promoting the transformation of M1 macrophages to M2 type.MiR-93-5p could become an innovative therapeutic target to prevent renal fibrosis.
- Life Research的其它文章
- The pharmacological mechanism of quercetin as adjuvant therapy of COVID-19
- Exploratory study of highly susceptible to SARSCoV-2 populations
- Epidemiological characteristics of syphilis in Nanchong City from 2017 to 2021
- Upregulation of MEG3 inhibits neuroblastoma progression via decreasing proliferation and promoting apoptosis
- Current outcomes and treatment of tetralogy of Fallot
- Research progress in the causes of intracranial aneurysms

