Preventive and therapeutic effects of Aerva lanata (L.) extract on ethylene glycolinduced nephrolithiasis in male Wistar albino rats
Ankul Singh S,Chitra Vellapandian,Gowri Krishna
Department of Pharmacology,SRM College of Pharmacy,SRM Institute of Science and Technology,Kattankulathur,Tamil Nadu 603203,India
ABSTRACT Objective Nephrolithiasis is a common urological disease. This study aims to evaluate the preventive and therapeutic effects of hydro-alcoholic extract of Aerva lanata (L.) roots (HAEAL) on ethylene glycol-induced nephrolithiasis in rats.Methods Fifty grams of shade-dried coarsely powdered Aerva lanata (L.) root was successively extracted with organic solvents in increasing order of polarity [petroleum ether (60 -80 °C),chloroform,and ethanol] using a Soxhlet apparatus,and then concentrated. Physical tests including nature,color,odor,and texture were performed on the herbal suspension. In vitro nephrolithiasis assessment was performed by nucleation assay,aggregation assay,and crystal growth assay. Thirty adult male Wistar albino rats were randomly divided into five groups (six rats in each group). Group 1: negative control group without induction or treatment till day 28. Group 2: positive control group receiving a daily oral solution of 0.75% ethylene glycol till day 14,and mixed with distilled water till day 28. Group 3: standard group receiving a daily oral solution of 0.75% ethylene glycol till day 14 and Cystone (750 mg/kg) from day 15 to day 28. Group 4: low dose HAEAL group receiving a daily oral solution of 0.75%ethylene glycol till day 14,and 400 mg/kg HAEAL from day 15 to day 28 (1 mL per day). Group 5: high dose HAEAL group receiving a daily oral solution of 0.75% ethylene glycol till day 14,and 800 mg/kg HAEAL from day 15 to day 28 (1 mL per day). Urine (urine volume,pH value,appearance,odor,and turbidity) examination and serum test were performed. On day 29,the kidneys were dissected,and histopathology examination was performed to determine the degree of tubular injury.Results The suspension showed stability and aroma with no turbidity at room temperature.The suspension did not show changes in color and odor until day 3,indicating that the preparation was stable for 72 h. Body weight decreased in the positive control group indicating stone formation and changes in water intake. Both standard and HAEAL treatments restored the body weight to normal levels after treatment,indicating the beneficial effects of the treatment. Histopathological examination revealed no significant findings in the negative control group,whereas the positive control group showed inflammation in the kidney parenchyma.Compared with positive control group,there was increase in urine volume and excretion of urinary constituents such as calcium and oxalate (P < 0.01) as well as improved clearance rate(P < 0.05) in HAEAL treatment groups,in addition,the urine pH value of HAEAL groups was increased.Conclusion HAEAL reduced nephrolithiasis formation and had a diuretic effect,which could be used to promote the expulsion of stones. Further studies are needed to enhance the stability of the suspension for the production of better pharmaceutical formulations.
Keywords Aerva lanata (L.) Nephrolithiasis Ethylene glycol Antioxidant Tubular injury Diuresis Suspension
1 Introduction
The claims of the antiurolithiatic activity have only been examined for a few plants and herbal formulations,includingAerva lanata(L.) in both India and China[1]. Kidney stone formation is asymptomatic in the early stage.As it progresses,signs and symptoms such as flank pain(backside pain),hematuria (blood in urine),obstructive uropathy,urine blockage,hydronephrosis (kidney dilation),and renal colic (intense cramp) begin to appear,resulting in associated suffering from nausea and vomiting[2]. The annual incidence of nephrolithiasis is eight cases per 1 000 adults[3]. The extensive obligation to establish convenient alternative therapy put an end to the uncertainty regarding treatment costs and clinical management of the patients. Owing to supersaturation,the solutes in urine precipitate and coalesce into crystals (i.e.,when the concentration strength of two ions surpasses their point of saturation in the solution,crystallization occurs)[3]; this process relies on the thermodynamics (nucleation) and kinetics (crystal growth) of supersaturated solutions[4]. Calcium oxalate and uric acid calculi constitute 85% and 15% of all kidney stones in developed countries,and 75% and 5% in developing countries,respectively; struvite stones represent 7% and 14% of all kidney stones in developed and developing countries,respectively[5]. In the current study,we focused on the preventive effect ofAerva lanata(L.) plant extract on kidney stone formation.
Aerva lanata(L.) is spread throughout India and also used in China[1].Aerva glabrata,Aerva hainanensis,Aerva sanguinolentaare the species of Aerva found exclusively in China with its usage in various health problems[6]. Its extract is used in several regions for jaundice,liver congestion,dyspepsia,typhoid,pneumonia,biliousness,and prolonged fevers[7]. It has been scientifically studied for its antimicrobial[8],diuretic[9,10],nephroprotective[11],anti-asthmatic[12],anti-fertility[13],anti-hyperglycemia[14],hepatoprotective[15],hypolipidemic[16],immunomodulatory[17,18],anti-tumor[19],and anti-diarrheal[20]activities.
Inhibitors of stone formation include multivalent metallic cations (magnesium),macromolecules (glycoproteins,glycosaminoglycans,osteopontin,tammhorse fall proteins,urine prothrombin fragment-1),small organic anions like citrate,and small inorganic anions like pyrophosphates[21,22]. Several promoters incorporate cell membrane lipids (cholesterol,glycolipids,and phospholipids)[23]. Parathyroid hormone stimulation leads to the enhancement of calcitriol hormone and increase in stone formation[24]. The risk of stone formation is increased by high sodium intake,which leads to delayed calcium reabsorption in the renal tubules and promotes high calcium levels in the urine[25]. A high protein intake decreases urine pH value and citrate levels,thereby enhancing the excretion of calcium in urine through bone resorption. To avoid acidic urine formation,higher risk of urine formation,one must eat less fish,meat,and poultry,and avoid vitamin D rich foods[26]. Alternatively,an increased consumption of potassium-rich fruits and vegetables is suggested for wellbeing[27].Aerva lanata(L.)belongs to the familyAmaranthaceaeand has various traditional uses,including the treatment of hemorrhage arrest during pregnancy[28],healing of burns[29],inflammation[30],nasal bleeding[31],cough[32],scorpion stings[33],diarrhea[34],fractures and spermatorrhoea[35],dysentery[36],and bronchitis[37]. Here,we sought to determine whetherAerva lanata(L.) root extract could prevent kidney stone formation. To achieve that aim,we examined the capacity of this formulation to reduce oxalate stone formation in male Wistar albino rats chronically exposed to ethylene glycol,which induces oxalate precipitation.
2 Materials and methods
2.1 Identification,collection,and extraction of Aerva lanata (L.)
The wholeAerva lanata(L.) plant was procured from Kochi,Kerala,India. The roots were identified by Professor P. Jayaraman,Ph.D.,from the Plant Anatomy and Research Center located at Sakthi Nagar,West Tambaram,Chennai,India. The authentication ID is PARC/2019/4131. Fifty grams of theAerva lanata(L.) root was shade dried,finely powdered,and then subjected to extraction by a maceration process with 300 mL of ethanol and distilled water in a ratio of 3∶1,yielding the extract ofAerva lanata(L.) root named HAEAL. After extraction,the solute was strained and vaporized via rotary evaporation at 35 °C[38]. The concentrated product was reconstituted to prepare a suspension in distilled water before the experiments were commenced. The extract had a hygroscopic nature and was stored in the refrigerator in an air tight glass container for the entire study. The yield was 15% w/w in terms of dried product.
2.2 Preparation and stability parameters of the suspension
The 100-mesh size fine particles of the plant extract were properly mixed by triturating. After that,the drug was mixed in water with different additives,including Tween-80 (SRL,India),sodium-CMC (SRL,India),flavoring agent (SRL,India),sweetening agent (SRL,India),and sodium benzoate (SRL,India) as a preservative for better stability of the formulation during the shelf-life period.Physical tests including nature,color,odor,and texture were performed on the herbal suspension. Accelerated stability testing was carried out,and sedimentation volume,redispersibility,and rheology (flow rate in milliliter per second) were determined[39]. The viscosity was measured using a Brookfield viscometer at 50 rpm,the pH value of the suspension was measured using a pH meter,and crystal growth was observed for changes in appearance and stability. The concentration of the suspension used was 2 g/mL.
2.3 Preliminary phytochemical study
Phytochemical constituents in HAEAL were identified using a standard method[40]. Fifty grams of shade-dried coarsely powderedAerva lanata(L.) root was successively extracted with organic solvents in increasing order of polarity [petroleum ether (60 - 80 °C),chloroform,and ethanol] using a Soxhlet apparatus,and then concentrated. Further hydroalcoholic extraction was carried out,and the extract was subjected to qualitative tests for the identification of plant constituents such as alkaloids,terpenoids,saponins,tannins,carbohydrates or glycosides,phenols,steroids,and flavonoids.
2.4 In vitro assessment of nephrolithiasis
2.4.1 Nucleation assay A buffer containing Tris(0.05 mol/L) and sodium chloride (0.15 mol/L) at pH 6.5 was used as a medium for 3 mmol/L calcium chloride and 0.5 mol/L sodium oxalate solutions. Both solutions were filtered through a 0.22 μm membrane[41]. 33 mL of the calcium chloride solution was mixed with 3.3 mL of the test solution at various concentrations. Then,33 mL of the sodium oxalate solution was added to initiate crystallization. The final solution was placed in a magnetic stirrer at 800 rpm and 37 °C,and absorbance was monitored at 620 nm at 1 min intervals. Cystone was used as standard to compare the effects of nucleation in the solution.
2.4.2 Calcium oxalate crystal assay Calcium chloride and sodium oxalate (each at 4 mmol/L) were mixed with 1.5 mL of 90 mmol/L sodium chloride. Tris HCl buffer(10 mmol/L) at pH 7.2 and 30 μL of a calcium oxalate monohydrate crystal slurry was mixed with 1.5 mg/mL of acetate buffer with pH 6.5. The consumption of oxalate commences immediately; free oxalate ions get depleted and decrease upon addition of the test (HAEAL) into the solution,and calcium oxalate crystal growth is inhibited by the test (HAEAL)[42]. The free oxalate reduction rate is calculated using the baseline value and value for over 30 s after incubation both with and without the test (HAEAL).
2.4.3 Crystal aggregation assay Calcium oxalate monohydrate crystals were seeded into 96-well plates (containing saturated aggregation buffer) at various concentrations (20,40,60,80,and 100 μg/mL)[43]. The plates were shaken in an incubator at 150 rpm and 25 °C for 1 h.Crystal morphology was imaged using Nikon Inverted Microscope Eclipse Ti-S (Nikon Instruments Inc.).Absorbance was measured at optical density (OD) 620 nm(UV-visible spectrophotometer) and 10 s intervals over 300 s.
2.5 Ethylene glycol-induced nephrolithiasis in Wistar albino rats
Thirty adult male Wistar albino rats were randomly divided into five groups (six rats in each group). The animal experiments were approved by the Institutional Animal Ethics Committee (IAEC) (Approval No. IAEC/216/2019). All rats received standard pellet feed with drinking water ad libitum. HAEAL was prepared and administered orally after kidney stone induction. Group 1: negative control group without induction or treatment till day 28.Group 2: positive control group receiving a daily oral solution of 0.75% ethylene glycol till day 14,and mixed with distilled water till day 28,which induced acidosis,hyperoxaluria and led to pronounced retention in the kidneys as well as oxalate,calcium,and phosphate excretion. Group 3: standard group receiving a daily oral solution of 0.75% ethylene glycol till day 14,and Cystone(750 mg/kg) from day 15 to day 28. Group 4: low dose HAEAL group receiving a daily oral solution of 0.75%ethylene glycol till day 14,and 400 mg/kg HAEAL from day 15 to day 28 (1 mL/rat per day). Group 5: high dose HAEAL group receiving a daily oral solution of 0.75%ethylene glycol till day 14,and 800 mg/kg HAEAL from day 15 to day 28 (1 mL/rat per day). On day 29,the kidneys were dissected,and histopathology was performed to determine parenchyma injury.
2.6 Collection and analysis of urine samples
The analysis of 24 h urine samples was performed by collecting the urine in metabolic cages on days 1,7,14,21,and 28[44]. Before urine collection,rats were provided with water but no feed,and the urine was collected the next day in a 50 mL beaker. Total urinary excretion of calcium,oxalate,and phosphorous was measured,and the remaining of the samples was used for microscopic analysis. The volume and pH value of the urine samples were also measured.
2.7 Collection and analysis of serum samples
Serum samples were obtained by retro-orbital collection of blood from the eyes of the rats using 5% isoflurane on day 28[45]. Rats were later sacrificed by cervical dislocation for histological analysis. The collected blood was placed in a cooling centrifuge at 10 000 rpm for 10 min to separate the serum from the blood,and the samples were labeled accordingly. The serum was placed in a refrigerator for further analysis of creatinine and uric acid levels[34].
2.8 Microscopic analysis of urine samples
Crystalluria analysis was performed using the collected fresh urine samples from each group without the use of any preservatives[46]. Microscopic analysis of crystal deposition was performed by adding 1 mL fresh urine samples to the centrifugation chamber at 3 000 rpm for 10 min,and the supernatant layer was discarded promptly. The bottom layer was placed on a slide with a cover slip on the top,and visualized under a light microscope (Siscon Olympus Microscope,CH20i) to identify the type and number of crystals.
2.9 Analysis of HAEAL on urine volume and pH value
The fresh samples of urine were collected separately in a metabolic cage of various groups the following day,and the volume was measured for the days 1,7,14,21,and 28,and similarly pH value was also recorded for the urine sample by using pH meter.
2.10 Analysis of HAEAL on body weight
Rats were weighed on the same days of urine sample collection. In addition to the standard feed and water provided,a multivitamin syrup (Zincovit) purchased from apex India was administered to the animals to withstand the given dose of extract and maintain proper body weight. Any changes in body weight were recorded promptly from day 1 to day 28,and the health of the animals was monitored[47].
2.11 Histopathological analysis of kidneys
Rats were given anesthesia,and sacrificed by an incision on their abdomen,and both kidneys were located and removed on day 29. The isolated kidneys were cleaned to remove the fatty deposits,and rinsed with a cold solution of normal saline[48]. Then,the kidney tissue portion was fixed in 10% neutral formalin,and sections were embedded in 5 μm thick paraffin films. The sections were stained with a hematoxylin and eosin solution to study the histopathological changes and crystal deposition.
2.12 Statistical analysis
IBM SPSS Statistics 26.0 was used for data analysis. The results were presented as mean ± standard deviation(SD). The data were analyzed by Analysis of Variance(One-way ANOVA) followed by Turkey’s post hoc test for multiple comparisons. Statistical significance was set atP< 0.05.
3 Results
3.1 Preliminary phytochemical screening
The phytochemical composition of HAEAL is shown in Table 1. Alkaloids,carbohydrates,tannins,saponin,flavonoids,terpenoids,steroid,phenols,and glycosides were identified as the active constituents.
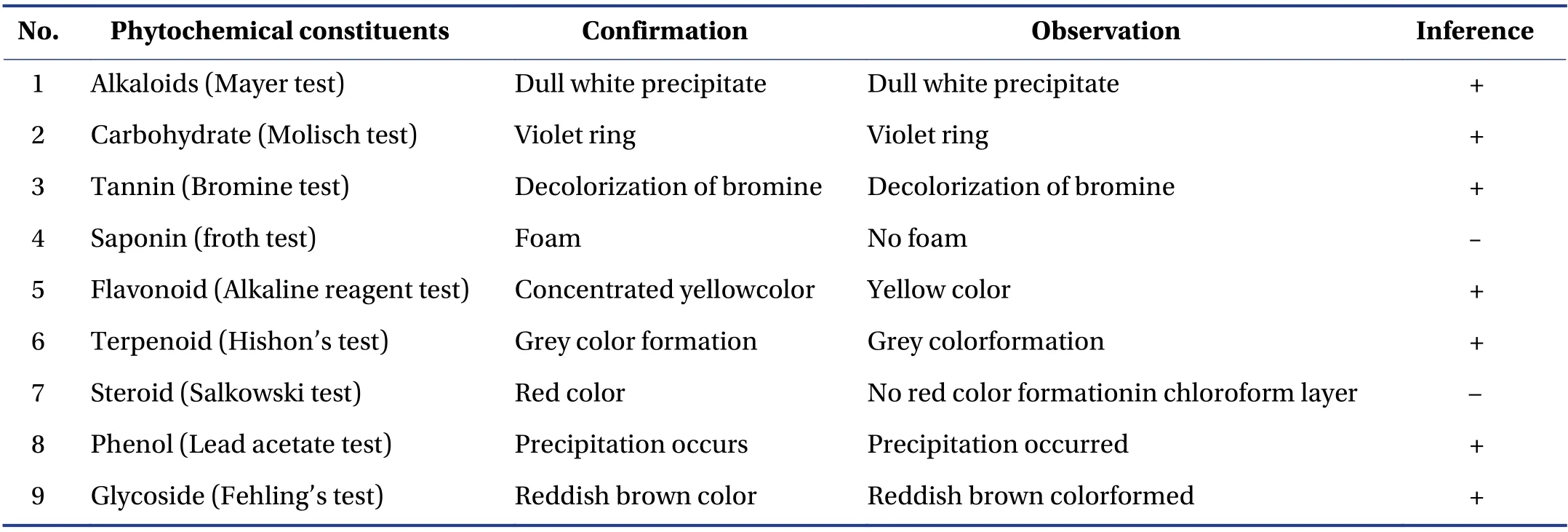
Table 1 List of phytoconstituents of HAEAL
3.2 In vitro studies
3.2.1 Nucleation of calcium oxalate crystals The rate of calcium oxalate crystal nucleation was determined by a previously reported method[41],and the results are shown in Table 2. The nucleation assay is based on turbidity formation and dissolution,wherein absorbance increases on the increasing concentration,and is measured by spectrophotometry at 620 nm. The solution with HAEAL at 100 μg/mL showed the lowest turbidity and the highest inhibition of calcium oxalate monohydrate crystals (P< 0.05). HAEAL at various concentrations significantly inhibited stone formation,as indicated by the increases in the rate of absorbance.

Table 2 Nucleation of calcium oxalate crystals
3.2.2 Aggregation of calcium oxalate crystals The rate of calcium oxalate crystal aggregation was determined as described previously[42],and the results are shown in Table 3. The absorbance in the presence of HAEAL at various concentrations was measured at 620 nm. Thein vitroinhibitory effect of HAEAL on various phases of calcium oxalate crystallization was determined by measuring the turbidity and absorbance rate. Figure 1 shows crystal aggregation formed as calcium oxalate crystals.
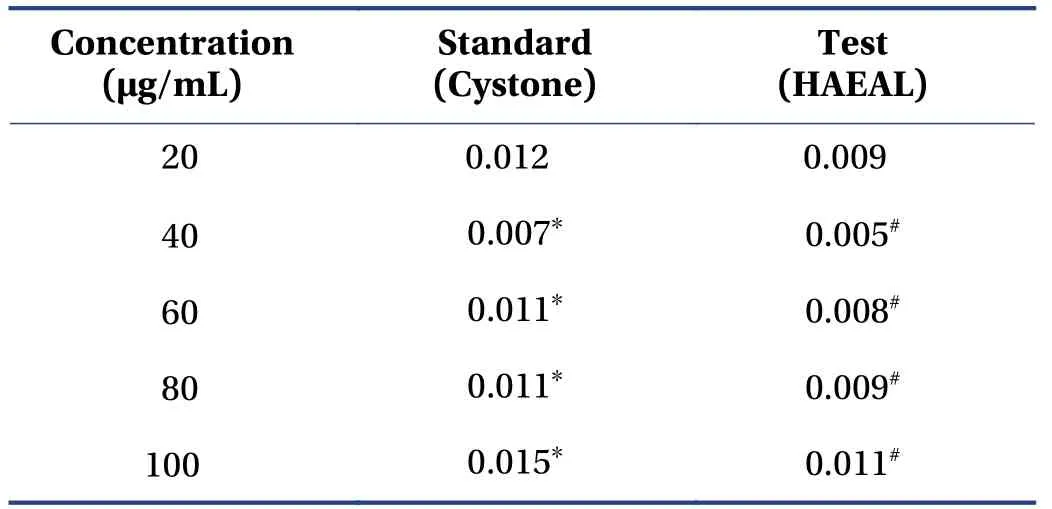
Table 3 Aggregation of calcium oxalate crystals

Figure 1 Crystal aggregation assay showing crystal morphology
3.2.3 Growth of calcium oxalate crystals The rate of calcium oxalate crystal growth was determined as described previously[43],and the results are shown in Table 4. The absorbance in the presence of HAEAL at various concentrations was measured at 214 nm. Thein vitroinhibitory effect of HAEAL on various growth phases was determined by measuring the turbidity and absorbance rate.HAEAL at 100 μg/mL concentration resulted in the lowest turbidity and highest absorbance,and inhibited crystal formation.
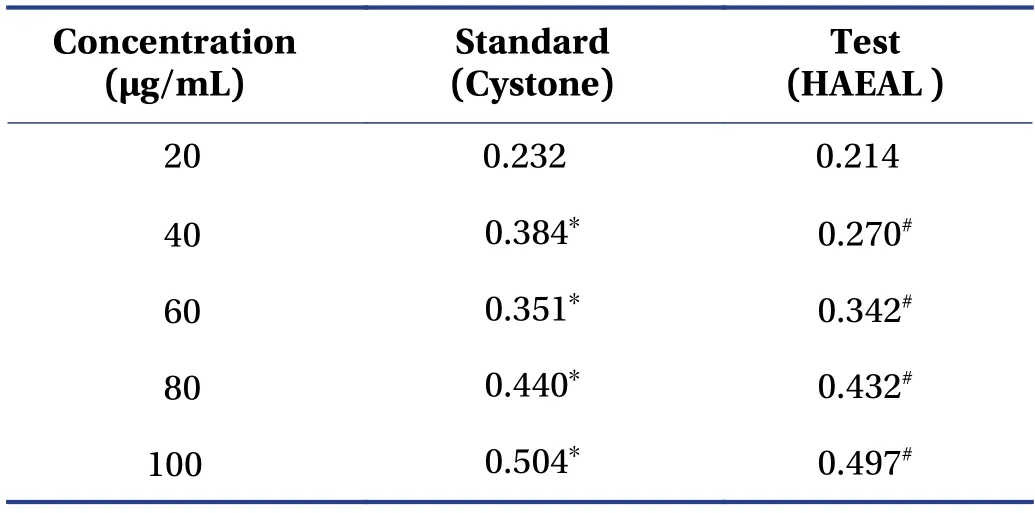
Table 4 Growth of calcium oxalate crystals
3.3 Effects of HAEAL on ethylene glycol-induced nephrolithiasis
Ethylene glycol was administered to rats to induce formation of oxalate stone,and it showed changes in urinary and serum parameters. Successful induction of stone was confirmed by reduction in body weight and changes in urine volume and pH value.
3.3.1 Biochemical parameters of urine and serum samples The results of urine and serum analyses are shown in Table 5. Uric acid concentration was higher in the positive control group than that in the negative control group (P< 0.000 1). Furthermore,the concentration was reduced upon treatment with either Cystone or HAEAL,but HAEAL could be further isolated for compounds to show better results than standard drug. Oxalate and phosphorous levels were the highest in the positive control group. The high dose HAEAL group had lower calcium levels than the positive control group [(1.19 ± 0.02) mg/dL vs. (6.30 ± 0.02) mg/dL] at the end of the study period(day 28). From the analysis,we concluded that,compared with the positive control group,the HAEAL had reduced levels of uric acid,oxalate,phosphorous,and calcium. Effects of HAEAL on urine and serum parameters are mentioned in Figure 2.
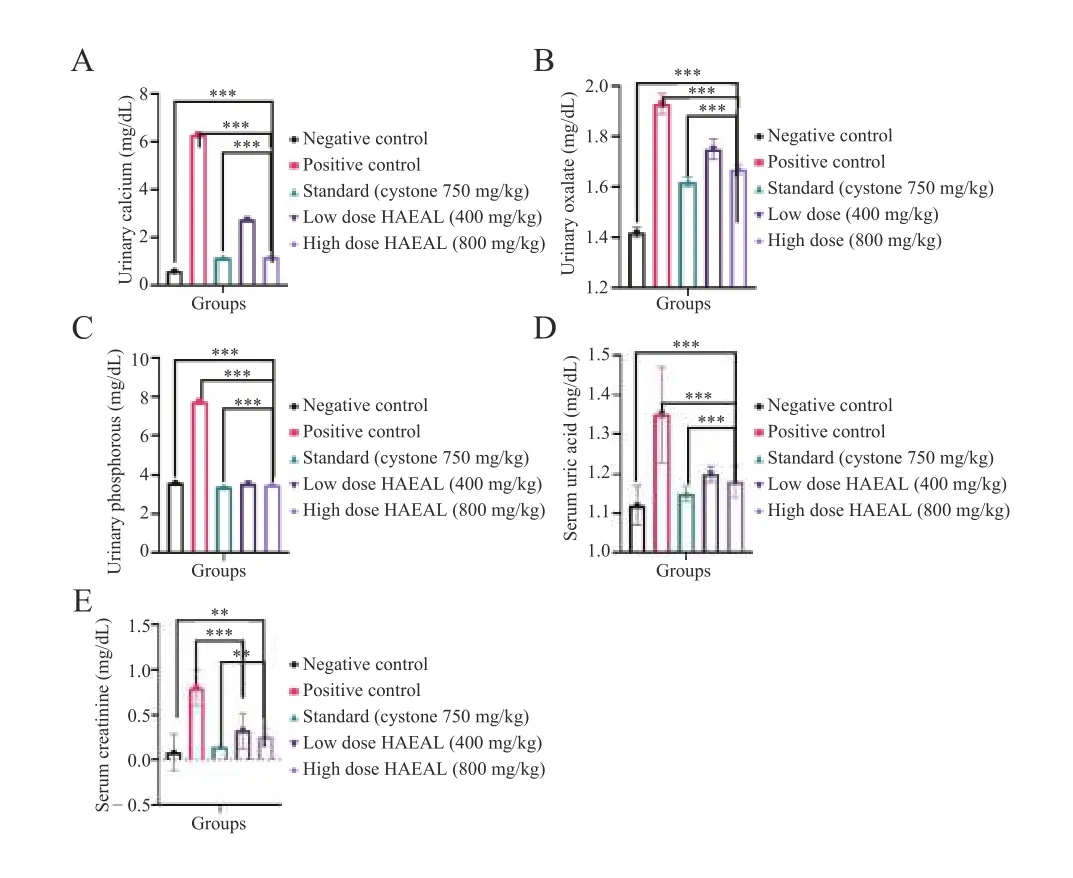
Figure 2 Effects of HAEAL on urine and serum parameters
Serum analysis revealed higher creatinine levels in the positive control group than tlat in the negative control group,indicating that ethylene glycol induced kidney obstruction. Treatment with HAEAL at low and high doses inhibited crystal deposition and was found to be statistically significant (P< 0.000 1). Uric acid levels wereincreased in the positive control group,causing crystal sedimentation,but were decreased by Cystone or HAEAL treatment. Thus,the plant extract significantly inhibited the increase in uric acid levels induced by ethylene glycol.An image of the calcium oxalate crystals is shown in Figure 3.
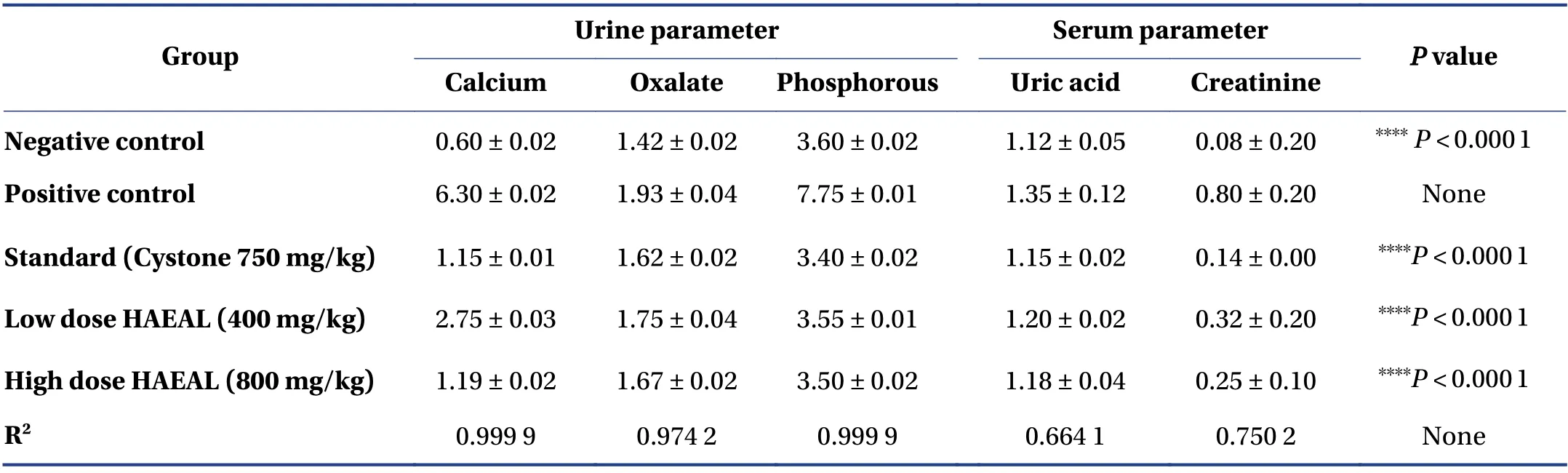
Table 5 Analysis of urine and serum parameters

Figure 3 Microscopic analysis of calcium oxalate crystals
3.3.2 Analysis of urine samples Urine samples were collected in a centrifuge tube and centrifuged at 2 000 rpm for 10 min. The supernatant was discarded after centrifugation and the sediment was examined under the microscope. The negative control group did not show evidence of crystal formation. In contrast,the urine from the positive control group had abundant and large calcium oxalate crystals. HAEAL treatment decreased crystal formation and the size of the crystals formed. The samples from the standard treatment group showed less turbidity and were less malodorous than those of the positive control group,which had very bad odor due to bacterial infection. The differences between the HAEAL treatment groups and the positive control group were significant,indicating that HAEAL reduced crystal formation and improved urine odor. Effects of HAEAL on urine volume and pH value are mentioned in Table 6.
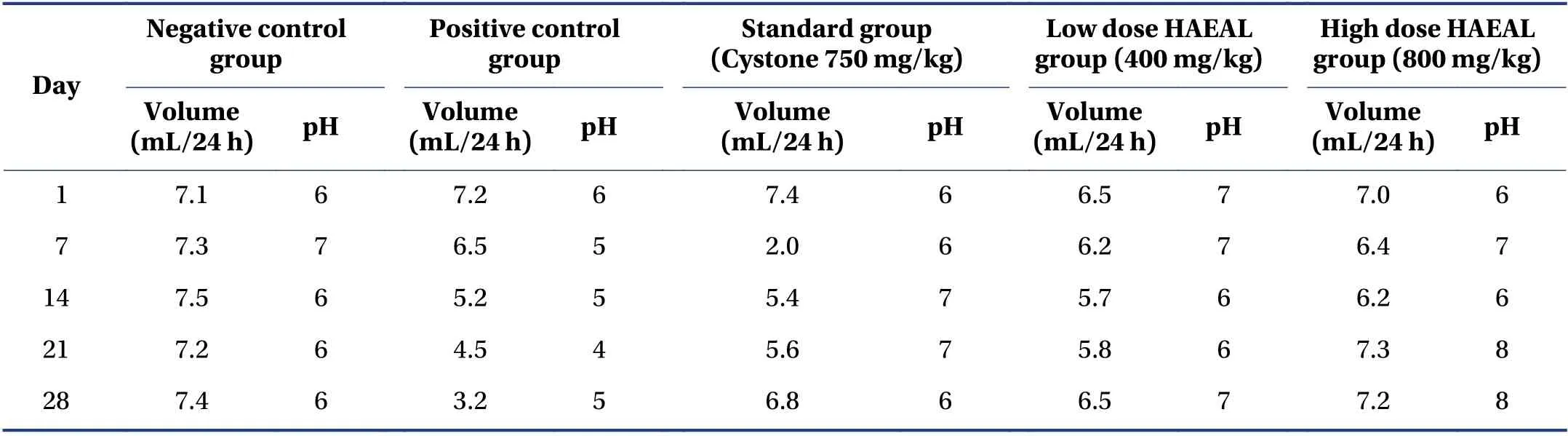
Table 6 Effects of HAEAL on urine volume and pH value
3.4 Effect of HAEAL on body weight
The extract was prepared as suspension and given to the rats. The suspension showed stability and aroma with no turbidity at room temperature. The suspension did not show changes in color and odor until day 3,indicating that the preparation was stable for 72 h. The high dose HAEAL was subjected to observe any changes on body weight,and showed increase in body weight compared with positive control group (P< 0.001) from day 14 to day 28,indicating the effectiveness of the suspension in changing baseline parameters of animals employed in the study. The body weight of the animals was observed in consistency to understand the changes occurring in animals. Illustration of the effects of HAEAL on body weight changes from day 1 to day 28 is shown in Figure 4.
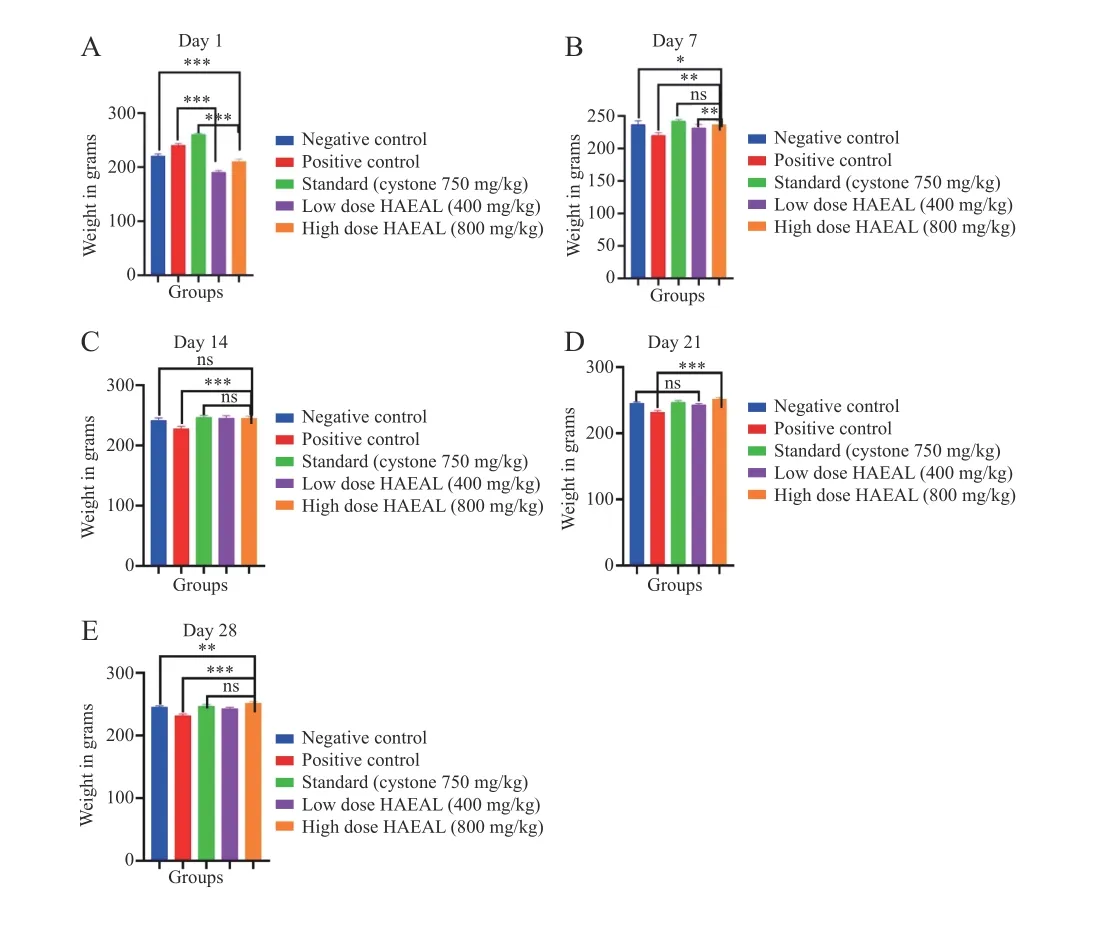
Figure 4 Effects of HAEAL on body weight changes from day 1 to day 28
3.5 Histopathdogy analysis of kidney tissues
Photomicrographs of kidney tissue sections are shown in Figure 5. No significant pathological findings were found in the kidney parenchyma of the negative control group.In the positive control group,no evidence of tubule interstitial inflammation/acute tubular injury was noticed on day 28; however,the kidney parenchyma in this group showed signs of chronic interstitial inflammatory infiltration.Mild chronic interstitial inflammation was observed in the standard and HAEAL treatment groups,with no evidence of acute tubular injury. The low dose HAEAL group showed slight evidence of interstitial inflammation with lymphocytic infiltration. In contrast,the kidney parenchyma of the high dose HAEAL group showed no significant pathological findings,indicating that 800 mg/kg HAEAL ameliorated kidney injury,controlling or preventing the disease.

Figure 5 Photomicrographs of kidney tissue sections from the experimental rats
4 Discussion
In the current study,we investigated the antiurolithiatic activity of HAEAL bothin vitroandin vivo,and compared it with the activity of a standard treatment (Cystone). The animals were treated with HAEAL to dissolve the stones induced by the administration of ethylene glycol. Treatment with HAEAL reduced the production and size of kidney stones. This effect was likely the result of the action of various phytochemical constituents,such as flavonoids,phenols,terpenoids,glycosides,and tannins. Stone formation is a multifactorial process with various stages,including nucleation,crystal growth,and crystal aggregation[39].
A report has revealed the urinary and serum parameters that play a role in the prevention of kidney stone development[49]. The key factor in preventing stone formation is alkalization of the urine and reduction of ion formation of various salts[21]. HAEAL at 800 mg/kg showed significant changes (P< 0.001) when compared with standard treated rats,and dose of 400 mg/kg was found to show better activity (P< 0.001) when compared with positive treated rats.
In vitrostudies,nucleation,aggregation,and growth assays were performed to measure absorbance and the rate of stone inhibition. In the nucleation assay,the rate of absorbance was increased when 100 μg/mL HAEAL was used,which indicated lower turbidity of the solution and higher nucleation inhibition required to prevent stone formation[40]. Similarly,the inhibition in aggregation and growth assays was more pronounced at the 100 μg/mL concentration,indicating a less turbid solution.In vivostudies related to citrate,oxalate,calcium,uric acid,phosphate,and creatinine levels enhances their excretion rate and improves the clearance rate[33,50]. The urine volume was lower in the positive control group,indicating a reduction in the glomerular filtration rate. An increase in urine output dilutes urine concentration and prevents precipitation. Ethylene glycol administration leads to hyperoxaluria,and high calcium concentration leads to reduced urine inhibitor activity. According to the previous study established,the high extract dose significantly reduces the calcium concentration and the oxalate concentration was increased with the administration of ethylene glycol,resulting in the formation of calcium oxalate precipitates[33].
Oxalate levels were much more decreased by the high dose than the low dose of the extract,indicating a therapeutic dose. The lithiasis-induced groups had increased phosphate concentrations in the urine,providing a favorable environment for the formation of calcium phosphate and calcium oxalate. Treatment with high dose HAEAL decreased the phosphate levels,but to a lower extent than treatment with the standard drug,Cystone[33].Uric acid and creatinine levels were reduced by treatment with high dose HAEAL,but not to the levels after treatment with the standard drug[45].
Additionally,Aerva lanata(L.) has significant antioxidant properties that prevent the oxidative stress causing tubular cell injury. Thus,pharmacological preparations ofAerua. lanata(L.) may be beneficial for treating nephrolithiasis and other disorders related to oxidation because of their antioxidative effects. The body weight in the induced group decreased progressively when compared with that in other groups,indicating that the disease was induced and was accompanied by changes in urine parameters. Changes in behavioral patterns in the ethylene glycol induced group,where rats became less active and drank more water,was considered as a protective mechanism that improved the excretion rate which was seen in urine volume changes[46]. Urine volumes from day 1 to day 28 were measured and found to be higher in both the HAEAL treatment groups(P< 0.01) when compared with positive control groups.These findings indicate that high urine output dilutes the urine concentration,and demonstrates the diuretic activity of the plant extract,which reduces the acidic nature of the urine by enhancing the concentration of inhibitors,thus reducing the chances of stone formation[51]. Changes in urine volume were observed after induction,and a foul smell of the urine indicated that certain microbial infections may also be responsible for stone formation by changing the concentration of urine. Microbial growth can be averted by providing antimicrobial drugs along with the plant extract.
Histopathological examination revealed no significant findings in the negative control group,whereas the positive control group showed inflammation in the tubular area[47]. In the standard and HAEAL treatment groups,there were relevant changes in interstitial inflammatory cells with reduction in interstitial inflammation with lymphocytic infiltration,and a reduction in crystal accumulation was apparent. Urine pH value plays a significant role in kidney stone formation; acidic urine favors crystal retention through adhesion,resulting in stone formation in the distal part of the kidney[46]. The pH value of the urine from the positive control group was in the range of 4 to 6,indicating chances of kidney stone formation due to the acidic nature of the urine. In contrast,the treatment groups had a pH ≥ 6,suggesting that treatment with the extract is efficient at treating kidney stone formation.
5 Conclusion
Urine output decreased in the positive control group.Both standard and HAEAL treatments significantly increased the urine volume,indicating the diuretic activity of HAEAL. The data revealed that the excretion levels of oxalate,phosphorous,and calcium were increased in the positive control group,whereas the high HAEAL treatment group showed lower excretion rates. Furthermore,creatinine clearance was restored to normal levels in the standard and HAEAL treatment groups. Thus,HAEAL significantly reduced kidney stone formation. Our findings suggest that HAEAL has beneficial diuretic activity,which can be used to promote the passage of stones. Further studies are needed to enhance the stability of the suspension for the production of better formulations.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2022年2期
Digital Chinese Medicine2022年2期
- Digital Chinese Medicine的其它文章
- Visualization analysis of the international standard ISO/TC 249 for traditional Chinese medicine
- MEDICLOUD: a holistic study on the digital evolution of medical data
- Ancient and modern medication laws of aromatic Chinese medicines in treating angina pectoris based on data mining
- Intra-set correlation analysis of medical records of thyroid cancer treated by traditional Chinese medicine Master ZHOU Zhongying
- Traditional Chinese medicine Master XIONG Jibo’s medication experience in treating arthralgia syndrome through data mining
- Screening influencing factors of blood stasis constitution in traditional Chinese medicine
