Analysis of bacterial spectrum,activin A,and CD64 in chronic obstructive pulmonary disease patients complicated with pulmonary infections
INTRODUCTION
Chronic obstructive pulmonary disease(COPD)is a chronic respiratory disease,characterized by progressive and persistent airflow obstruction,along with high morbidity and mortality.COPD is often complicated by a collection of underlying diseases.This disease,coupled with poor nutrition and immunologic function,can often lead to a high incidence in pulmonary infections[1,2].In turn,pulmonary infections can worsen COPD and promote acute exacerbations of COPD(AECOPD),which further aggravates patient prognosis.This is the main cause of death in COPD patients[1,3].
Under normal conditions,CD64 is scarcely expressed.Following infection,however,CD64 Levels rise due to the direct stimulation of pathogenic microorganisms or the indirect stimulation of inflammatory cytokines.Therefore,the neutrophil CD64 index can act as an early diagnostic marker for infection[4-6].Moreover,multiple reports correlated the CD64 index with the severity associated with COPD and bacterial infections.Qian and Huang[7],for instance,observed that the CD64 index is higher in patients with AECOPD than those with stable COPD and healthy volunteers,and that the CD64 index is higher in AECOPD patients with positive bacterial sputum cultures than in those with negative cultures.This suggests that the CD64 index can be a guiding marker that offers better therapeutic implications,compared to conventional diagnosis,for the use of antibiotic treatments in AECOPD patients.Similarly,Titova[8]also demonstrated that the neutrophil CD64 index possesses approximately the same level of diagnostic accuracy as CRP in diagnosing pneumonia in patients hospitalized with AECOPD.
66. Get more restful sleep67. Forgive and move on68. Prepare food ahead69. Fix it or get a new one70. Be prepared to wait71. Don t always be right72. Focus on the moment73. Take a lunch break74. Read a book75. Shift your attitude76. Laugh each day77. Develop self-esteem78. Take vitamin supplements79. Stop the shoulds 80. Avoid excess81. Plan a special outing82. See through illusions83. Relax your muscles84. Slow down and notice85. Nurture15 good friends86. Be in nature87. Listen to music88. Limit caffeine and sugar89. Go on a fast or cleanse1690. Be spontaneous91. Love your partner92. Get some fresh air93. Be pampered1794. Volunteer95. Join help networks96. Maintain good posture1897. Respect your limits98. Exercise routinely99. Go dancing100. Sigh occasionally101. Do yoga
Between March 2015 and January 2018,a total of 85 patients with COPD,who also suffered from pulmonary infections,and a total of 96 COPD patients,without pulmonary infection,were enrolled from the First Affiliated Hospital of Chongqing Medical University,and were assigned to either the pulmonary infection or control group.Baseline characteristics,such as,age,gender,forced expiratory volume in the first second(FEV1),and FEV1/forced vital capacity(FVC)ratio were collected for comparisons.All participants signed informed consent before entry into the study,and all clinical practices were consistent with our institution’s code of ethics.This study conformed with the 2013 revised Helsinki Declaration and was approved by the Medical Ethics Committee of The First Affiliated Hospital of Chongqing Medical University.
MATERIALS AND METHODS
General information
Activin A is a glycoprotein that promotes follicle-stimulating hormone secretion from pituitary gland.It is a member of the transforming growth factor beta superfamily and participates in the regulation of proliferation,chemotaxis,and apoptosis of neutrophils,macrophages,fibroblasts and other cells.Activin A also plays pathological roles in a series of respiratory diseases like COPD,asthma,and pulmonary fibrosis[9-11].In 2014,Verhamme[12]first reported that activin A plays a key role in regulating inflammation in COPD patients and that the expression of activin A is significantly increased in the airway smooth muscle cells,bronchial epithelial cells,and alveolar macrophages of COPD patients.These conclusions were further confirmed in animal models whereby cigarette smoke exposure induced a significant increase in activin A levels in the lungs and bronchoalveolar lavage fluid of mice.Moreover,the cigarette smoke-exposed bronchial epithelial cells exhibited higher levels of activin A and lower levels of its endogenous inhibitor follistatin.Nevertheless,there are few reports on the effects of pulmonary infections on the CD64 index and activin A in patients with COPD,and the underlying mechanism remains unclear.Therefore,this study analyzed the bacterial spectrum and expressions of the CD64 index and activin A in COPD patients with pulmonary infection,and discussed the relevant mechanisms.
Inclusion and exclusion criteria
(1)COPD: Diagnostic criteria consistent with the Global Strategy for the Diagnosis,Management,and Prevention of COPD(2016 revised edition): Patients who had dyspnea,chronic cough or sputum production,and/or a history of exposure to risk factors,such as,smoking.The diagnosis was further confirmedpost-bronchodilator spirometry(FEV1/FVC ratio <0.7)[13];(2)AECOPD: Patients with COPD,who experienced a sustained increase in cough,shortness of breath,sputum production or purulence of sputum,and/or dyspnea;and(3)Pulmonary infections: COPD patients who experienced fever,produced abnormal sounds like crackles or rhonchi in lungs,and exhibited pulmonary infiltrates on chest X-ray.Pathogenic bacteria were isolated from the cultures of their sputum samples.
(1)COPD patients who received antibiotics,hormones,or immunosuppressive drugs within 1 mo prior to admission;(2)COPD patients who suffered from other infections,such as abdominal,skin,soft tissue,bone,and cartilage infections;(3)COPD patients with pulmonary infection,but pathogenic bacteria could not be isolated from sputum culture;(4)COPD patients who also suffered from other respiratory diseases like asthma,bronchiectasis,pneumothorax,hemothorax,or comorbidities;(5)COPD patients who also suffered from tumors,autoimmune diseases,cardiovascular and cerebrovascular diseases,or renal and hepatic dysfunction;(6)COPD patients with other diseases that could lead to acute exacerbations,such as,heart failure,spontaneous pneumothorax,pulmonary embolism,or pleural effusion;and(7)COPD patients who died or suffered worsening of condition due to un-related diseases during hospitalization.
Identification of pathogenic bacteria
The sputum samples were collected from the lower airways of patients in the pulmonary infection group and were cultivated for bacterial identification before these patients were given antibiotics.Patients rinsed their mouths with normal saline before sputum collection to avoid contamination by oral flora.In case of difficulty in sputum collection,due to coughing,samples were takenfiberoptic bronchoscopy.Gram staining was initially performed on the sputum samples.Sputum samples containing <10 squamous epithelial cells and >25 Leukocytes per low-power field(squamous epithelial cells/leukocytes <1:2.5)were considered qualified,otherwise the sputum sample was re-collected.The qualified samples were then inoculated on blood agar and MacConkey agar plates,and cultured at 37 °C for 24 h before the fully automated VITEK 2 Compact bacterial identification system(BioMérieux)was applied for the identification of pathogenic bacteria.All samples were processed according to the National Guide to Clinical Laboratory Procedures[14].
Assessment of CD64 index
Even the summer when Dad got laid off from the mill, and Mama had to serve dried beans several times a week, not a single dime10 was taken from the jar
Enzyme-linked immunosorbent assay
Upon hospital admission,we collected 2 mL of venous blood from all patients.Following a 20 min incubation at room temperature,the blood samples were centrifuged at 6000 r/min for 15 min.Subsequently,the supernatants were collected and analyzed for serum activin A levels using the Elisa kit(Shanghai Renjie Biological Technology Co.,Ltd.RJ12742),following manufacturer’s instructions.
Western blot
Upon hospital admission,we collected 2 mL of heparinized venous blood from all patients.The blood samples were then centrifuged at 2000 r/min for 10 min to separate the blood components into three layers: Blood plasma,a buffy coat containing platelet cells,and red blood cells.The blood plasma was collected in sterile centrifuge tubes containing 1 mL of 1.090 g/mL Percoll solution(Solarbio,P8370)and an additional 1mL of 1.077 g/mL Percoll solution were successively added to the tubes.The buffy coat layer of centrifuged samples was next pipetted into a Percoll density gradient solution,and was followed by another centrifugation at 2000 r/min for 15 min,which separated the components into four layers: Blood plasma,Percoll solution,neutrophils,and Percoll solution.The neutrophils were then pipetted into a new centrifuge tube,mixed with blood plasma and centrifuged again at 1000 r/min for 10 min.Following this,the cells were rinsed three times,and re-suspended in appropriate amount of plasma.After the cell count,10cells were collected,completely lysed by adding 100 μL of lysis buffer(Beyotime Biotech,China,P0013),and centrifuged at 12000 r/min for 5 min.The supernatants were then collected for BCA protein quantification(Beyotime Biotech,China,P0012).For each test sample,20 μg of total protein was obtained for polyacrylamide gel electrophoresis.The proteins were then transferred onto PVDF membranes,and blocked in 5% non-fat dry milk at room temperature for 2 h.After the blocking process,the membranes were incubated with primary antibodies for either anti-activator A(1:500,Abcam,ab89307),anti-Smad3(1:500,Abcam,ab40854),anti-TLR4(1:500,Abcam,ab13556),anti-MyD88(1:500,Abcam,ab2064),anti-NFκB(1:1000,Abcam,ab32360),or anti-GAPDH(1:1000,Abcam,ab8245)at 4 °C overnight.The membranes were then rinsed three times and incubated with HRPconjugated goat anti-rabbit IgG(Boster,BA1056,1:2000)at room temperature for 1 h.The membranes were then rinsed three times,followed by incubation with the ECL substrate solution(Beyotime Biotech,China,P0018),and exposure and imaging using gel doc(Bio-Rad,GelDoc XR).The protein band gray values were determined with the Image Pro Plus 6.0 software,and the intensity of the target protein band divided by the intensity of GAPDH in the control group was adjusted as 1[15].
Statistical analysis
All data were statistically processed using the SPSS 20.0 software.Data are expressed as mean ± SD.Inter-group comparisons were made with the independent-sample-test.Enumeration data are expressed as cases/percentage(/%).Inter-group comparisons were performed using the chi-squared test.<0.05 was considered statistically significant.
RESULTS
Comparison of baseline characteristics
Cannot you give the little girl a drink so that she may have the strength of twelve men and overcome the Snow-queen? The strength of twelve men! said the Finland woman; that would not help much
Bacterial spectrum of patients with COPD,complicated with pulmonary infection
The CD64 index was 0.91 ± 0.38 in the pulmonary infection group and 0.23 ± 0.14 in the control group.Based on our statistical analyses,the pulmonary infection group had significantly lower CD64 index than the control group(<0.001)(Figure 1A).
Baseline patient characteristics are summarized in Table 1,including gender,age,course of disease,smoking history,FEV1,and FEV1/FVC.A total of 181 cases met our inclusion criteria.Among these cases,46 patients had pulmonary infection,and 96 patients were included in the control group.The gender,patient age,disease,and smoking history between the two groups were comparable(all>0.05).Patients in the pulmonary infection group presented with a lower level of FEV1(%),compared to the control group(41.30 ± 9.9147.65 ± 10.07,<0.001).Similarly,the level of FEV1/FVC(%)was lower in the pulmonary infection group,compared to the control group(50.48 ± 9.1358.24 ± 8.62,<0.001).
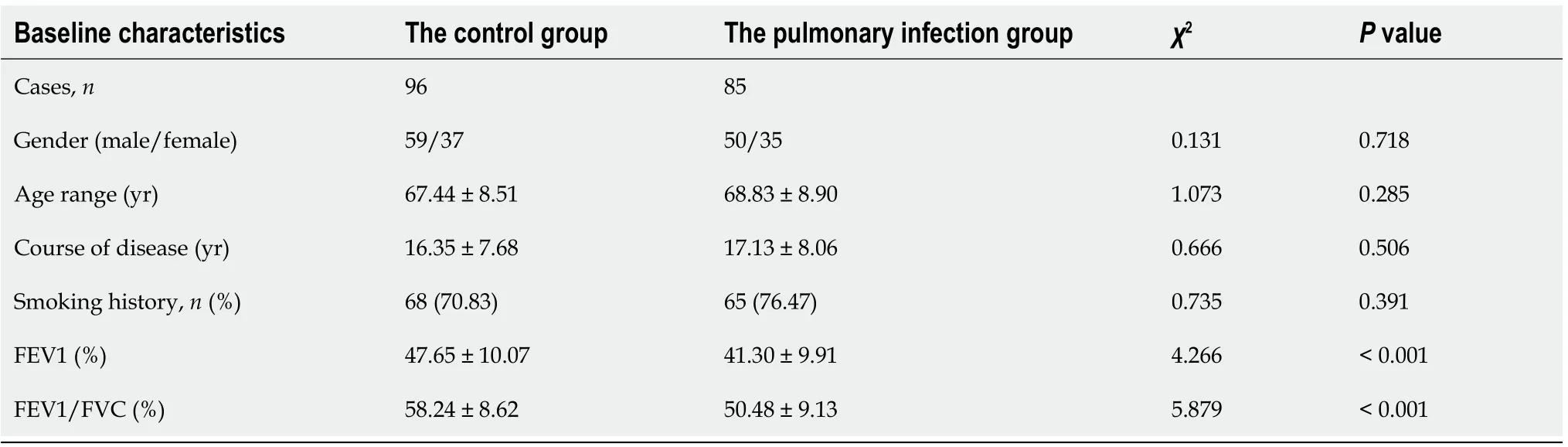
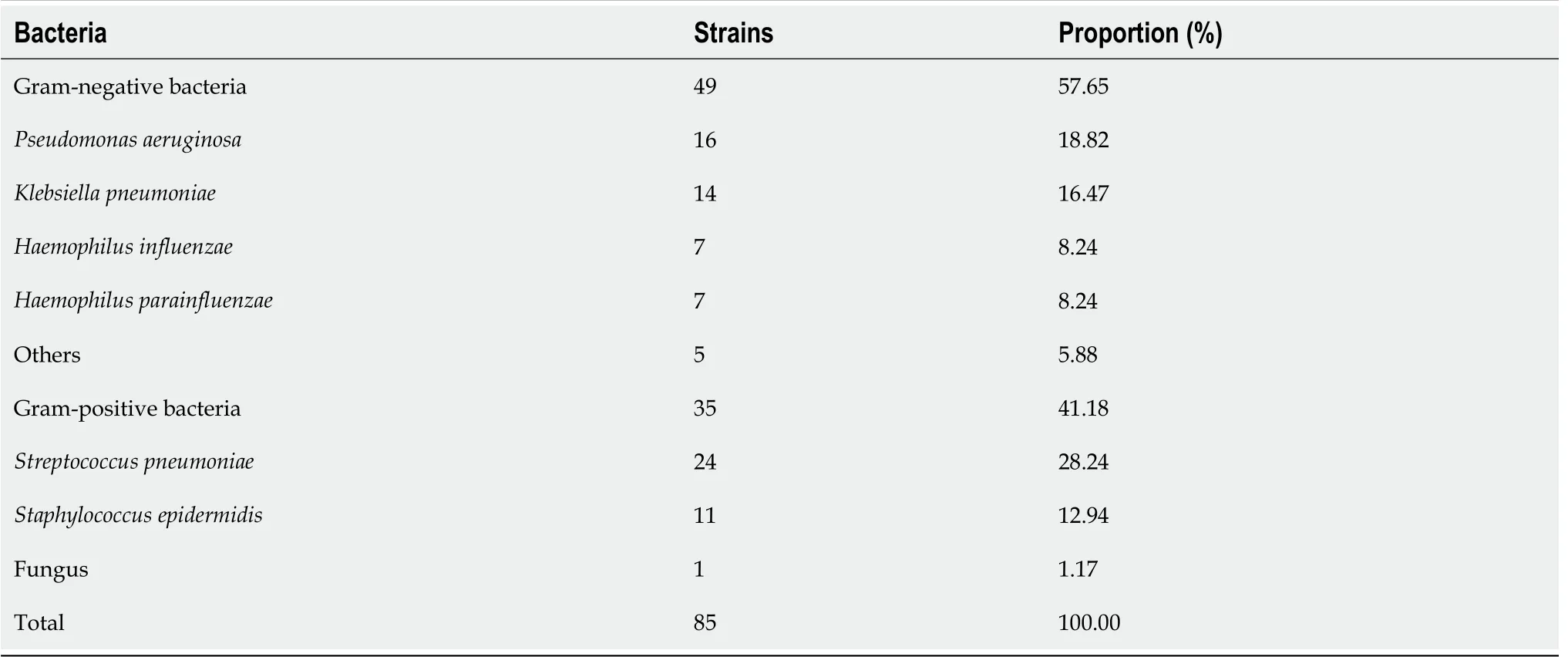
Comparison of CD64 index
The bacterial spectrum of patients in the pulmonary infection group is shown in Table 2.Among the 85 strains,49(57.65%)were gram-negative bacteria and 35(41.18%)were gram-positive bacteria.The mostprevalent gram-negative species were(16,18.82%),followed by(14,16.47%),(7,8.24%),and(7,8.24%).Among the gram-positive bacteria,24(28.24%)were,and 11(12.94%)were staphylococcus epidermidis.Apart from these,fungi were identified in 1.17% sputum samples.
Comparison of serum levels of activin A
Figure 1B illustrates the activin A levels in the pulmonary infection and control groups.The activin A levels were 43.50 ± 5.22 ng/mL in the pulmonary infection group,and 34.82 ± 4.16 ng/mL in the control group(<0.001).Hence,the pulmonary infection group had significantly higher activin A levels than the control group(<0.001).
She soon breathed again and came to herself; but the young King had watched the proceeding38, and not knowing why Trusty John had acted as he did, he flew into a passion, and cried: Throw him into prison
Pulmonary infection promotes activation of neutrophil activin A/Smad3 signaling pathway
Activin A is a member of the transforming growth factor beta superfamily that participates in multiple physiological and pathological processes like embryogenesis,neuroprotection,apoptosis,and fibrosis[9,19].Several studies reported that activin A is significantly increased during infections and inflammatory diseases,including sepsis,inflammatory bowel diseases,and rheumatoid arthritis[10,11,20].Moreover,activin A plays critical roles in regulating inflammation during COPD.Likewise,Verhamme[12]reported that the expression of activin A is significantly increased in the airway smooth muscle cells,bronchial epithelial cells,and alveolar macrophages of COPD patients.These conclusions were further confirmed in animal models.The administration of follistatin in cigarette smoke-exposed mice was shown to significantly decrease accumulation of monocytes,macrophages,neutrophils,as well as CD4and CD8T-lymphocytes.This suggests that the significant increase in activin-A is not caused by pulmonary inflammation in COPD models,but is,in fact,a mediator of COPD development.Likewise,the results of our study demonstrated that the level of serum activin A is significantly higher in the pulmonary infection group than in the control group.This indicates that pulmonary infections are consistent with other infections or inflammatory diseases in stimulating a significant increase of activin A levels in COPD patients.
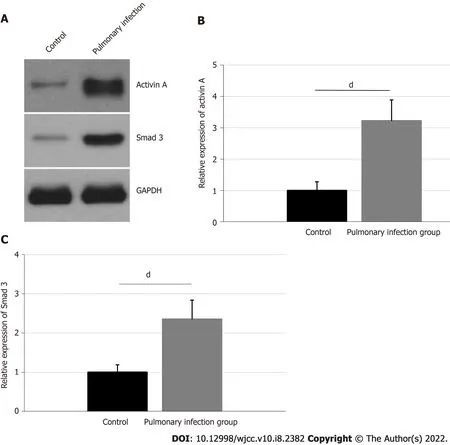
Comparisons of TLR4,MyD88,and NFκB expressions in neutrophils
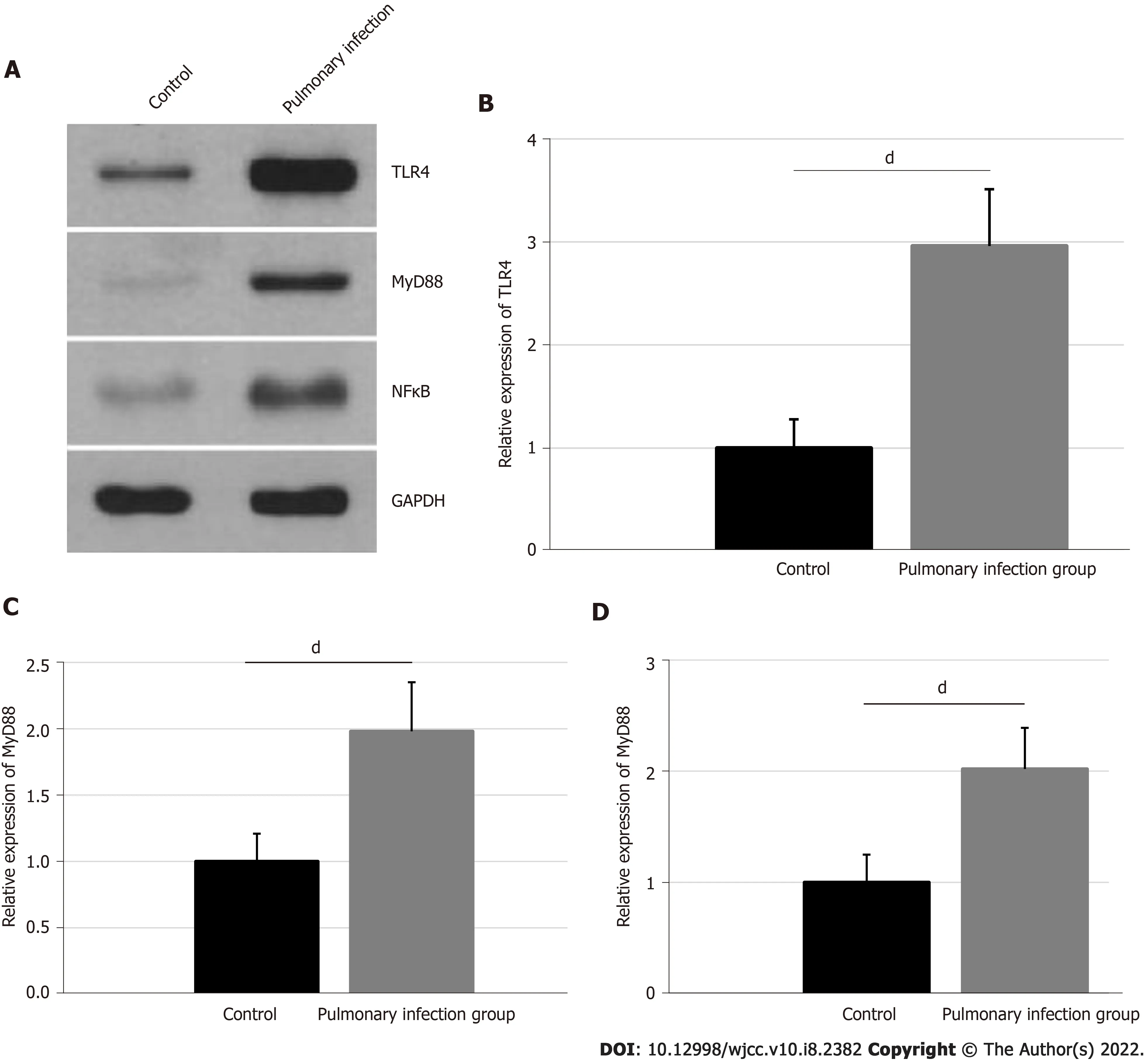
The relative expressions of neutrophil TLR4,MyD88,and NFκB were confirmed,using western blot,and are presented in Figure 3.The TLR4 expression was 2.96 ± 0.55 in the pulmonary infection group,and 1.00 ± 0.28 in the control group.The MyD88 expression was 1.98 ± 0.37 in the pulmonary infection group,and 1.00 ± 0.21 in the control group.Lastly,the NFκB expression was 2.02 ± 0.37 in the pulmonary infection group,and 1.00 ± 0.25 in the control group.Based on our statistical analyses,the levels of neutrophil TLR4,MyD88,and NFκB were significantly higher in the pulmonary infection group than the control group(all<0.001).
DISCUSSION
COPD often occurs in the elderly,due to multiple underlying diseases,weak cough reflex,and low immune function.This ultimately leads to high incidences of pulmonary infections[1,2].This study found that pulmonary infections in COPD patients are mainly caused by Gram-negative bacteria likeand.Our conclusion is consistent with the results of the Qu[16]study.In another study,Zhou[17]reported that COPD patients with pulmonary infections are more likely to have diabetes,elevated risk of ventilator usage,and prolonged bed rest,compared to COPD patients without pulmonary infection.Moreover,the study demonstrated that pulmonary infections are mainly caused by Gram-negative bacteria including,,and.Nevertheless,our study revealed that the pulmonary infections in COPD patients are mainly caused by Gram-negative bacteria,such as,and.This inconsistency may be related to the collection and treatment of samples and the isolation and identification of bacterial strains.
CD64 is usually expressed in low quantities in neutrophils,but a significant rise in expression occurs with the stimulation of pathological factors or inflammatory cytokines.Therefore,the neutrophil CD64 index is used as a diagnostic and prognostic biomarker in multiple diseases like systemic lupus erythematosus,neonatal sepsis,bacterial peritonitis,and inflammatory bowel diseases[4-6].Previous studies found that CD64 is also an important diagnostic and prognostic biomarker in COPD patients[8,18].In fact,Qian and Huang[7]found that the level of CD64 is significantly increased in patients with AECOPD or COPD with positive bacterial sputum culture.Our study also showed that the CD64 index is significantly higher in the pulmonary infection group than in the control group,which is consistent with prior publications.
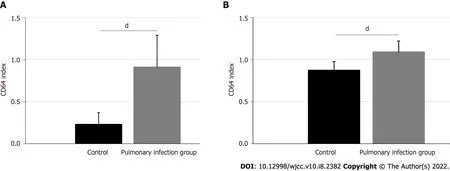
The activin A and neutrophil Smad3 protein expressions and their corresponding statistical analyses are shown in Figure 2.The activin A expression was 3.22 ± 0.67 in the pulmonary infection group,and 1.00 ± 0.28 in the control group.The expressions of neutrophil Smad3 in the pulmonary infection group was 2.35 ± 0.49,and in the control group was 1.00 ± 0.19.Hence,patients in the pulmonary infection group presented with higher levels of activin A and neutrophil Smad3,compared to the control group(<0.001).
Upon hospital admission,we collected 2 mL venous blood from the median cubital vein in the antecubital fossa of all patients and treated each sample with the anticoagulant agent,ethylenediaminetetraacetic acid.Subsequently,to each 50 μL of anti-coagulated blood,5 μL of anti-CD64-PE(Invitrogen,MA5-16436)and 5 μL of anti-CD45-PerCP(Invitrogen,MHCD4531)were added,mixed thoroughly,and the mixture was incubated at room temperature in the dark for 30 min.This was followed by red cell lysis with 500 μL of hemolytic agent(Beijing Tongsheng Shidai Biotech Co.,Ltd.,Z6910001S)incubated at room temperature in the dark for an additional 15min.The test samples were then centrifuged at 3000 r/min for 5 min.Subsequently,the supernatants were removed and the cell pellets were resuspended in 300 μL PBS for flow cytometry analysis using instrument from Becton-Dickinson,FACS Calibur.Monocytes,lymphocytes,and neutrophils were identified by an established gate,based on the forward and side scatters,as well as CD45-PerCP.For each test sample,the fluorescence signals of 10000 cells were collected,and the average fluorescence intensities of sub-populations were measured.The lymphocyte CD64 Levels were used as the internal negative control(<1.0),whereas the monocyte CD64 Levels were set as the internal positive control(>8.0).The CD64 index was calculated as follows: CD64 index =(CD64 average fluorescence intensity on the neutrophil/CD64 average fluorescence intensity on the lymphocyte)/(CD64 average fluorescence intensity on the monocyte/CD64 average fluorescence intensity on the neutrophil).
Smad3 is a downstream key effector of transforming growth factor-β1 and it plays a significant role in COPD patients,particularly,in terms of regulating inflammation,airway remodeling,and fibrosis[21,22].Mahmood[23]observed that the activation of the Smad3 signaling pathway is linked to the epithelial mesenchymal transition and loss of lung function.Furthermore,a recent study reported that the Smad3 signaling pathway is also a major effector of the activin A biological activity[24].In our research,we employed neutrophils protein expression analysis to reveal that pulmonary infection significantly promotes expressions of activin A and Smad3 in neutrophils,which indicates that the activin A/Smad3 signaling pathway is strongly activated during this time.In another study,the TLR4/MyD88/NFκB signaling pathway activation in neutrophils was shown to be an important contributor to the significant activin A secretion during the pathophysiology of endotoxemia[25].In our study,we also demonstrated that pulmonary infection can significantly promote expressions of TLR4,MyD88,and NFκB in neutrophils,which indicates that the elevated serum activin A levels and activation of the activin A/Smad3 signaling pathway may be strongly related to the activation of the TLR4/MyD88/NFκB signaling pathway.
She appeared suddenly, in all her splendour, and cried: Stay, Grumedan; this Princess is under my protection, and the smallest impertinence will cost you a thousand years of captivity29
This study focused on the bacterial spectrum,and expressions of the CD64 index and activin A in patients with COPD,complicated with pulmonary infections.But,there are still many scientific aspects that have not been discussed.First,this article only discussed the effects of pulmonary infections in COPD patients,however it remains unclear whether infections caused by other types of bacteria produces similar or different results.Second,previous studies reported that the activin A antagonist follistatin effectively alleviates pathological conditions associated with pulmonary fibrosis.But,the role of follistatin in patients with COPD,complicated with pulmonary infections,remains unclear[26-28].Third,this study explored alterations within the TLR4/MyD88/NFκB signaling pathway after separation of the patients’ neutrophils.However,the relationship between this signaling pathway and activin A expression still lacks strong evidence.Therefore,cytological experiments are warranted for verification of this correlationtargeted inhibition and/or overexpression investigations.
CONCLUSION
In conclusion,pulmonary infections in COPD patients are mainly caused by,,and.Pulmonary infections can result in a significant increase in the neutrophil CD64 index and serum levels of activin A,and,in turn,activate the activin A/Smad3 signaling pathway,which may be positively regulate the TLR4/MyD88/NFκB signaling pathway.
ARTICLE HIGHLIGHTS
Research background
A sharp exacerbation of chronic obstructive pulmonary disease(COPD)is often triggered by a lung infection and often has a poor prognosis.
Research motivation
Since COPD induces complex inflammatory events,Activin A and CD64 may collectively contribute to the development and progression of this disorder.
Research objectives
To analyze the bacterial profile of COPD patients with pulmonary infections and to assess activin A levels,CD64 index,and the underlying mechanisms involved in disease development.
Research methods
The whole data set consisted of 85 COPD patients with pulmonary infection and 96 COPD patients without pulmonary infection.Sputum samples were obtained from patients with pulmonary infections for further bacterial culture.The levels of CD64 index,activin A,Smad3,TLR4,MyD88,and NFκB proteins were assessed and compared between 85 COPD patients with pulmonary infections and 96 COPD patients without pulmonary infections.
Research results
In the pulmonary infection group sputum samples,the Gram-negative bacteria,Gram-positive bacteria,and Fungi were 57.65%,41.18%,and 1.17%,respectively.In addition,the relative CD64 index,and levels of activin A,Smad3,TLR4,MyD88,and NFκB proteins were all significantly higher in the pulmonary infection group,compared to the control group(all<0.001).
Research conclusions
Pulmonary infections in COPD patients may be caused by a variety of pathogens.In COPD patients,the CD64 index and serum activin A levels were significantly increased in patients with lung infection,compared to those without.This may have a positive regulatory effect on the downstream activin A/Smad3 and TLR4/MyD88/NFκB signaling pathways.
Research perspectives
Together,our findings provide a novel mechanism underlying pulmonary infection in COPD patients,and offer a potential therapeutic target for an enhanced and effective therapy against COPD.
FOOTNOTES
Fei ZY and Guo M designed the study,wrote the paper and reviewed the manuscripts;Fei ZY and Guo M should be as the co-corresponding authors;Fei ZY and Wang J performed the research and collected data;Liang J and Zhou X contributed to the analysis and editing of the manuscript;all authors have read and approved the final manuscript.
Afternoon was Mrs. Conroy s favorite time of day. After a hard day at work, her eyes were tired and her feet hurt. She enjoyed the nice long nap she took on the bus. Mrs. Conroy had made friends with the bus driver, Mr. Angstrom. He always woke her up before her stop. She usually felt fresh as a daisy() when she got off the bus.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Muriel hasn t spoken a coherent word in months-years, if you mean a sentence, a conversation-though occasionally she tries, mumbling nonwords. Would I never hear that voice again?
The data were not involved in the patients’ privacy information,so the informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
All authors have no conflicts of interest to declare.
No additional data are available.
From that day on, Charlie was a trial. If a fight broke out I didn’t have to turn my head to know who had started it. If someone was throwing spitballs(,) I could guess the culprit(,) ’s name. If a girl was crying, chances were Charlie had pulled her hair. No matter how I spoke3 to him, gently or firmly, he wouldn’t say a word. He’d just stare at me with those big gray eyes of his.
The wife continued to read until she had read all three pages to her husband. She neatly5 placed her list on the table and folded her hands over the top of it.
The authors have read the STROBE statement-checklist of items,and the manuscript was prepared and revised according to the STROBE statement- checklist of items.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Zhao-Yang Fei 0000-0002-9751-3077;Jiang Wang 0000-0002-9377-9468;Jie Liang 0000-0003-2203-0617;Xue Zhou 0000-0003-2322-849X;Min Guo 0000-0001-8174-5060.
Xing YX
Filipodia
Then the lid sprang up to the right, and the princess came out, straight over him, and rushed round the church, howling and shrieking33 Sentry, where are you? Sentry, where are you? She went towards the altar, and right up to it, but there was no one there; then she screamed again,My father has set no sentry in, War and Pest will now begin
Xing YX
 World Journal of Clinical Cases2022年8期
World Journal of Clinical Cases2022年8期
- World Journal of Clinical Cases的其它文章
- Developing natural marine products for treating liver diseases
- Computed tomography perfusion imaging evaluation of angiogenesis in patients with pancreatic adenocarcinoma
- Epidemiological features and dynamic changes in blood biochemical indices for COVID-19 patients in Hebi
- Identification and predictive analysis for participants at ultra-high risk of psychosis:A comparison of three psychometric diagnostic interviews
- Prognostic significance of peritoneal metastasis from colorectal cancer treated with first-line triplet chemotherapy
- Effect of intraoperative cell rescue on bleeding related indexes after cesarean section
