Exploration of structural,optical,and photoluminescent properties of(1-x)NiCo2O4/xPbS nanocomposites for optoelectronic applications
Zein K Heiba Mohamed Bakr Mohamed Noura M Farag and Ali Badawi
1Physics Department,Faculty of Science,Ain Shams University,Cairo,Egypt
2Physics Department,Faculty of Science,Taibah University,Al-Madina al Munawarah,Saudi Arabia
3Department of Physics,College of Science,Taif University,P.O.Box 11099,Taif 21944,Saudi Arabia
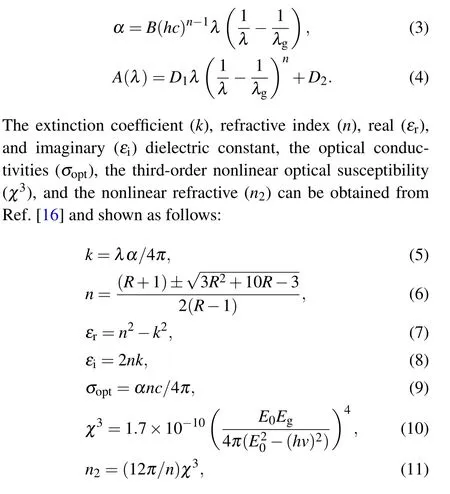
4Department of Physics,University College of Turabah,Taif University,P.O.Box 11099,Taif 21944,Saudi Arabia
Keywords: NiCo2O4 and PbS,nanocomposite,FTIR,optical,photoluminescence
1. Introduction
The rapid progress of nanocomposites materials enables a high degree of control over their optical and electronic characteristics.[1]The NiCo2O4(nickel cobaltite; NCO)is of a mixed metal oxide spinel type, where nickel atoms occupy the octahedral sites while cobalt ones distribute over both octahedral and tetrahedral sites.[2]The NCO has attractive features such as low cost, low toxicity, eco-environmentally and natural abundance.[2]Lead sulfide (PbS) is a member of IV–VI group chalcogenide semiconductors has optical and optoelectronic characteristics made it able to be used in solar cells,optoelectronic devices,photoconductors,sensors,and infrared detector devices applications.[3]Nanocomposites of different oxides and/or sulfides reveal the interesting characteristics as compared with those of parent compounds. For instant,the 304 stainless steel was protected upon being coated by MnS/TiO2nanotube films under simulated solar light.[4]The 0.2ZnMn2O4/0.8ZnFe2O4nanocomposite sample revealed a good nonlinear optical behavior at low frequency.[5]The CuCoOx/BiVO4multifunctional catalyst was applied to the organics’ degradation, water oxidation and O2reduction under visible light.[6]Moreover, NiO/NiCo2O4tubular structures with surface microporosity could have various applications such as the catalytic oxidation of harmful gases.[7]For(1-x)CuCo2O4-xCuS nanocomposites, the absorption spectrum ofx=0.1 sample displays a plateau in the visible region, that has a constant value in comparison with those of other composite samples.[8]The Ni/NiCo2O4nanocomposite displayed a high nonlinearity and has tailorable linear and nonlinear optical features, which is beneficial to device applications.[9]The improvement in the electrical characteristics together with a reduction in the optical band gap of CeO2combined with PbS makes the resulting nanocomposite suitable for the solar cell uses.[10]The NCO@ZrO2exhibited an excellent performance in solar thermochemical procedure.[11]Ullahet al.demonstrated that the coupling of PbS with TiO2extended their photo response to visible range.[12]Adding PbS to MoS2adapted its fast response to the speed of mechanical exfoliation in detecting NO2.[13]The intercalation of PbS into the layered space of K2La2Ti3O10greatly enhanced the absorption edge and the photocatalytic activity.[14]
In the present study,the(1-x)NiCo2O4/xPbS(x=0,0.1,0.15, 0.2) nanocomposite samples are synthesized by the hydrothermal method and the thermolysis method. The effect of alloying NCO and PbS phases in a heterostructure nanocomposite matrix on the structure, optical, and photoluminescent characteristics are studied in detail using x-ray diffraction,Fourier-transform infrared spectroscopy,transmission electron microscope, photoluminescence, and UV-diffused absorption techniques.
2. Methods and materials
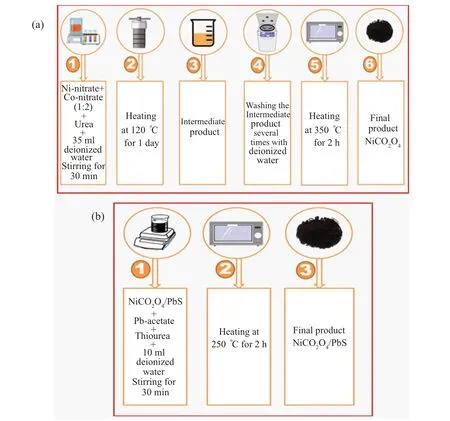
Nano NiCo2O4sample was synthesized by dissolving a stoichiometric ratio of(nickel and cobalt)nitrates and urea in deionized water (35 ml) under magnetic stirring for 30 min.Then the solution was transferred into a teflon-lined stainlesssteel autoclave and heated at 120°C for one day. The autoclave allowed the solution to be cooled down to a room temperature. The formed solution was centrifuged and washed many times using deionized water and ethanol. The resulting powder was dried and annealed in an electric oven at 350°C for 2 h(see Fig.1(a)). To synthesize the(1-x)NiCo2O4/xPbS(x=0, 0.1, 0.15, 0.2), the first step was to dissolve a stoichiometric ratio (1:1) of lead acetate and thiourea in 10 ml of deionized water in the presence the desired amount (x) of nano NiCo2O4sample. The solution was stirred in a magnetic stirrer for 0.5 h then annealed at 250°C for 2 h to avoid oxidizing the PbS (see Fig. 1(b)). All produced samples were explored by the techniques mentioned in Table 1. Tauc’s and Beer–Lambert’s formulas were used to find the direct optical bandgap[15]
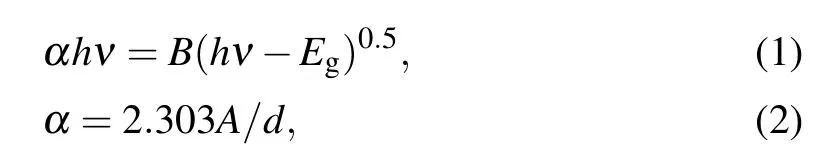
whereA,d,Eg,B,hν,λg,h,c, (D1=[B(hc)n-1d/2.303],D2),λ,andRare the absorbance,thickness,optical band gap,constant,incident photon energy,the wavelength related to the optical gap,Planck’s constant,light velocity,constants represent the reflection,the wavelength,and the reflectance,respectively.
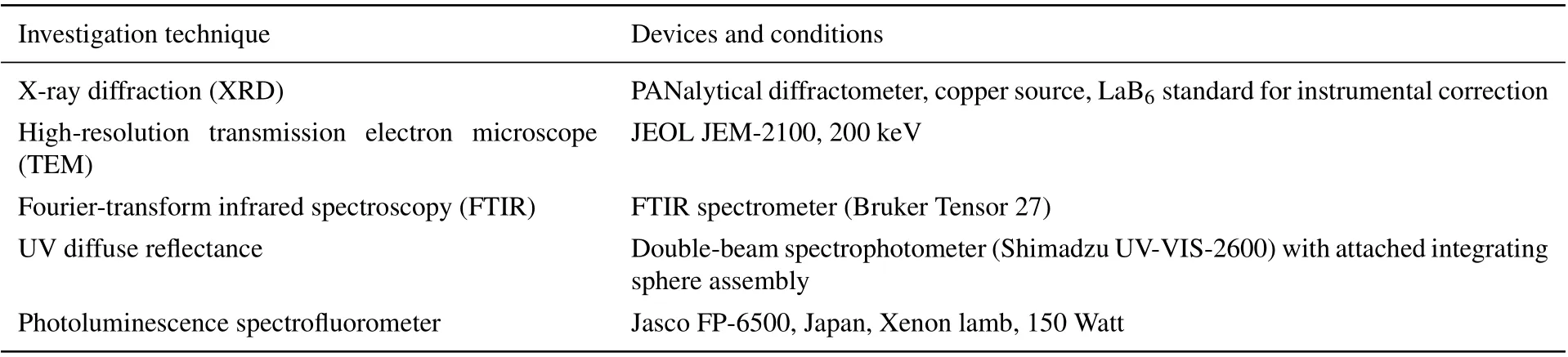
Investigation technique Devices and conditions X-ray diffraction(XRD) PANalytical diffractometer,copper source,LaB6 standard for instrumental correction High-resolution transmission electron microscope(TEM)JEOL JEM-2100,200 keV Fourier-transform infrared spectroscopy(FTIR) FTIR spectrometer(Bruker Tensor 27)UV diffuse reflectance Double-beam spectrophotometer(Shimadzu UV-VIS-2600)with attached integrating sphere assembly Photoluminescence spectrofluorometer Jasco FP-6500,Japan,Xenon lamb,150 Watt
3. Results and discussion
3.1. Structural analysis
Using a line-detector, high quality x-ray diffraction (XRD) patterns have been recorded for the nanocomposite samples(1-x)NiCo2O4/xPbS (x=0, 0.1, 0.15, and 0.2) and depicted in Fig. 2(a). Accurate phase identification, using search-match program X’Pert HighScore Plus,can be performed due to the high quality of the diffraction patterns. Rietveld quantitative phase analysis[17]sreveals a single cubic spinel phase for the pristine NiCo2O4(x=0.0),while for compositesx=0.1,0.15,and 0.2 three phases are identified: cubic spinel NiCo2O4as a major phase(>95%),PbS,and PbSO4as minor phases. Table 2 lists the resulting structural and microstructure parameters obtained from Rietveld analysis and figure 2(b)shows the resulting diffraction pattern fitting. To determine the crystallite size of the minor phases, first the size is constrained within the size of the major phase NCO,then in subsequent final refinement the size parameter is freely refined. From Table 2 it may follow that the phase percentages of PbS and PbSO4are less than composition parameter (x) used for preparation which suggests the incorporation of some Pb and S ions into the NiCo2O4lattice. The suggestion may be confirmed by the slight increase in the NiCo2O4cell parameter due to the insertion of Pb ions which are larger than that of Ni or Co ones. The crystallite size of the NiCo2O4phase is isotropic and almost the same(≈15 nm)for all samples(see Table 2). For PbSO4phase,the crystallite size is≈17 nm while the PbS phase is large as given in Table 2. The TEM images given in Fig.3 show very uniform particle morphology throughout the sample and good crystallinity. The particle size is almost isotropic with a very narrow size distribution as indicated by Rietveld analysis.
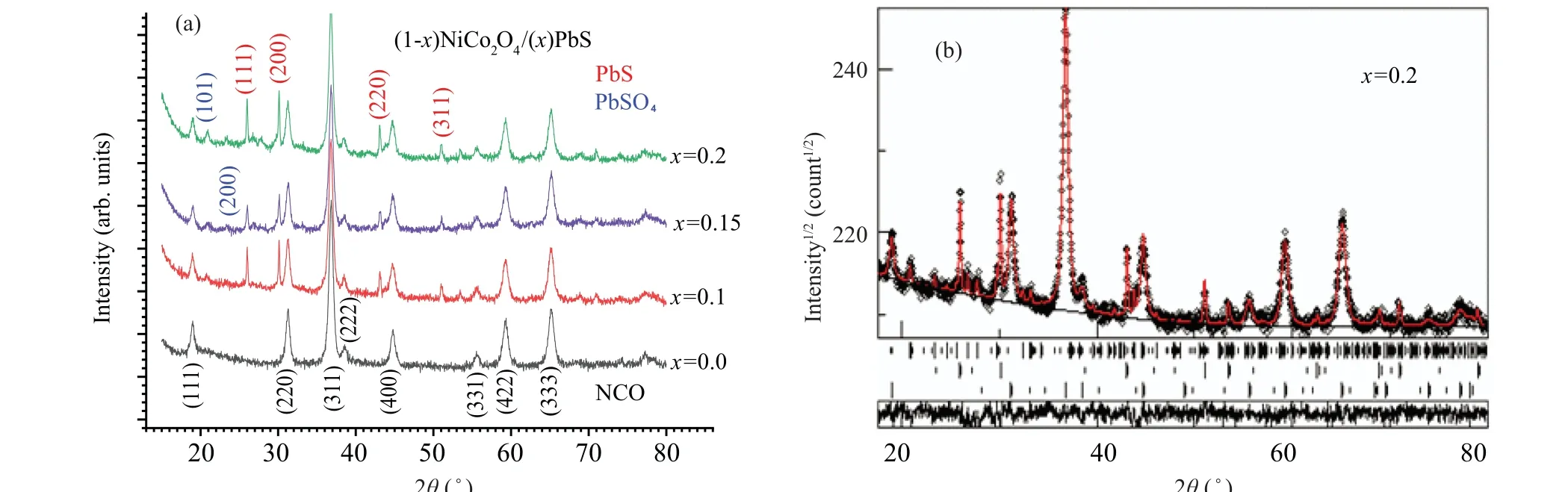
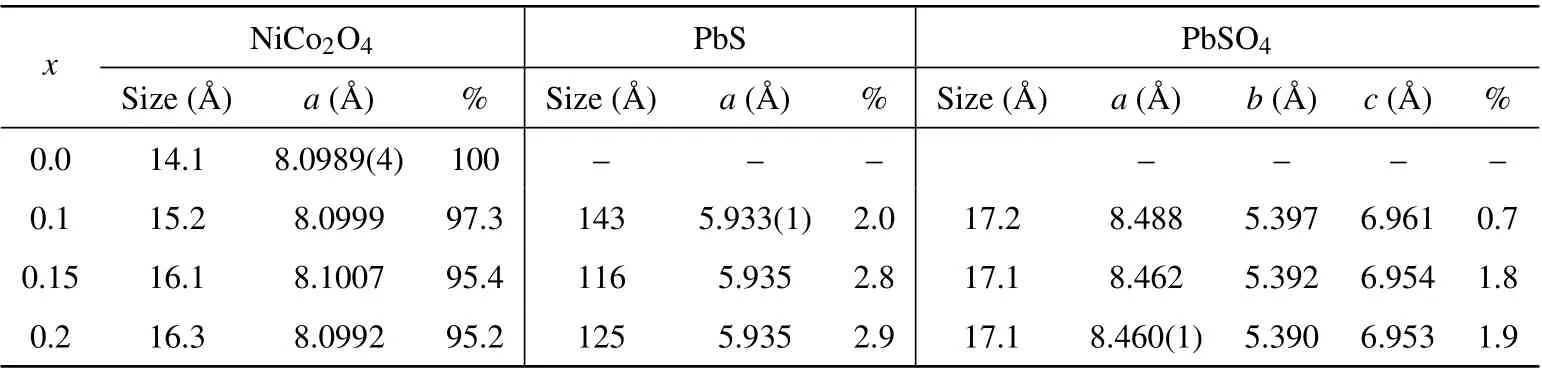
x NiCo2O4 PbS PbSO4 Size(°A) a(°A) %Size(°A) a(°A) %Size(°A) a(°A) b(°A) c(°A) %0.0 14.1 8.0989(4) 100– – –– – – –0.1 15.2 8.0999 97.3 143 5.933(1) 2.0 17.2 8.488 5.397 6.961 0.7 0.15 16.1 8.1007 95.4 116 5.935 2.8 17.1 8.462 5.392 6.954 1.8 0.2 16.3 8.0992 95.2 125 5.935 2.9 17.1 8.460(1) 5.390 6.953 1.9

Fig.3. TEM images with different magnifications for 0.8NiCo2O4/0.2PbS sample.
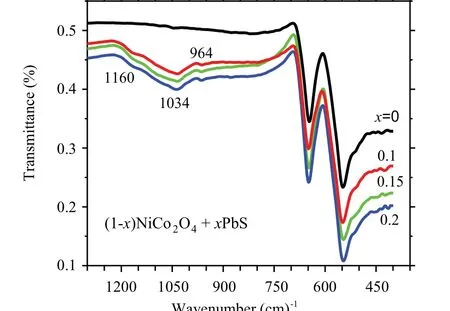
Infrared spectroscopy is used as an effective tool for investigating the local structure of transition metal oxides.[18]The measured Fourier-transform infrared (FTIR) spectra for the (1-x)NCO/xPbS nanocomposites are given in Fig. 4.There exist two sharp vibrational modes at 545.9 cm-1and 648.9 cm-1which can be assigned to primarily motion of the octahedral and tetrahedral metal–O,respectively.[18–20]For the composite samples (x=0.1, 0.15, 0.2), the spectra contain two extra weak peaks observed around 1034 cm-1and 1160 cm-1which are characteristic peaks of the heteropolar diatomic molecules of Pb–S band.[21–23]Because the bond of Pb–S is mainly an electrovalent bond, the FTIR spectra of the samples do not show strong bands associated with Pb–S stretching nor those with bending vibrations.
3.2. Optical properties
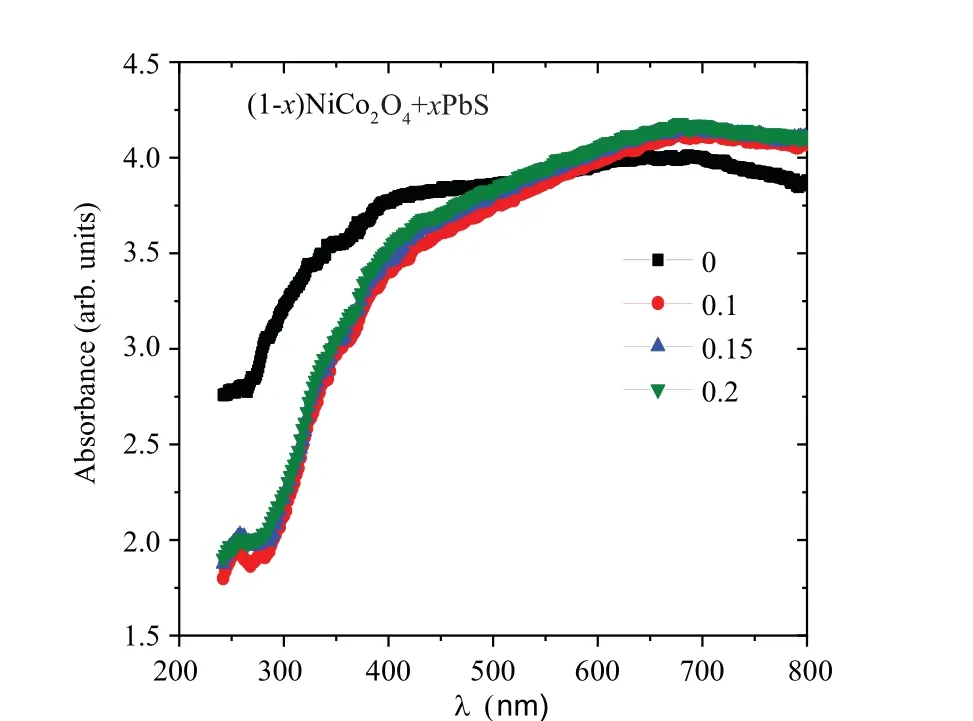
The diffused absorption data for (1-x)NCO/xPbS nanocomposite samples are displayed in Fig. 5. As revealed from the graph,NCO sample exhibits a substantial amount of absorption in a range of 400 nm–800 nm similar to the absorption amount of CoMn2O4sample.[24]This observation reveals the importance of this sample in visible light driven catalyst.The wide absorption of NCO sample may be caused by the absorption of the surface plasmons of nanoparticle and coupling of plasmon modes of the neighboring particle.[25]The absorption spectrum is modified due to NCO doped with PbS.The absorbance of NCO/xPbS nanocomposites decreases in the UV range while it increases in the visible range as compared with NCO as shown in Fig.5(a).This behavior indicates that the absorption of the(1-x)NCO/xPbS nanocomposite is affected due to the presence of PbS in the sample. In contrary to the NCO@ZrO2composite,the NCO is predominant as reported in Ref.[11]. Furthermore, the absorption slightly increases as the amount of PbS and PbSO4phases increase in the nanocomposite matrix(see Table 2).
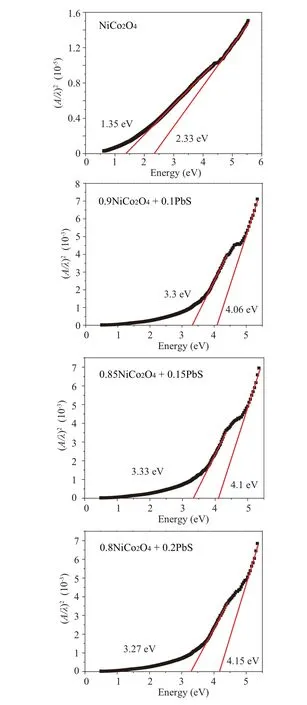
The values of direct optical band gap (Eg) for different samples are obtained from extrapolating the linear part of the plots between (αhν)2versus hνto zero absorption (see Fig.6). As revealed from the graph,NCO has two band gaps of 1.35 eV and 2.33 eV which are in agreement with the values in Ref. [26]. These two energy gaps are ascribed to the electron movement from the O-2p valence band to the transition metals-3d(t2g,eg)conduction bands.[27]Furthermore,the two bands are attributed to the presence of high spin and low spin sates of Co3in the NCO sample.[27]As the NCO alloyed with PbS,the optical band gap increases to(3.3 eV,4.06 eV),(3.33 eV, 4.1 eV), and (3.27 eV, 4.15 eV) as the amount of PbS becomes 10%, 15%, and 20%, respectively. The optical band gap of ZnO increases as the amount of Pb doping augments.[28]As indicated from XRD analysis in Table 2,the nanocomposite contain PbS and PbSO4beside the main phase of NCO.In addition,some Pb and S atoms are introduced into NCO lattice as indicated by XRD results. All these variables affect the values of the optical energy gaps.
The refractive indexnand absorption indexk, related to refraction and absorption,respectively,rely on the interference between the samples under investigation and incident light:nis related to the phase velocity and associated with the dispersion, whilekis linked to the coefficient of mass reduction and allows the determining of dissipation rate of the electromagnetic wave in the medium. The changing of the extinction coefficient (k) with the wavelength (λ) for (1-x)NCO/xPbS nanocomposite samples is displayed in Fig.7.

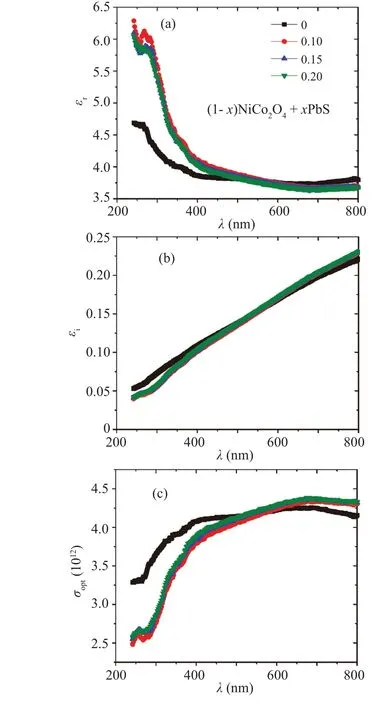
In the case of NCO sample,kincreases as the wavelength augments, and so does the scenario in the (1-x)NCO/xPbS nanocomposite samples. In addition, as the percentage of PbS increases in the nanocomposite samples,thekvalues decrease at the lower wavelengths while they increase at higher wavelengths. The enhancement or decrease inkvalue may be linked to the strengthening or weakening of the absorption process depending on whether the excess free carriers exists or not.[29]The variation of the refractive index (n)with the wavelength for the (1-x)NCO/xPbS nanocomposite samples is shown in Fig. 7(b). Thenvalue for the NCO and(1-x)NCO/xPbS nanocomposite samples decrease as the wavelength is increased. Therefore, thenvalues in all samples describe the degrees of the normal dispersion. Furthermore,thenvalue in the nanocomposite sample is higher than that of NCO sample; the situation is reversed in a higher wavelength range. In addition, as the content of PbS increases in the nanocomposite sample, thenvalue decreases slightly. The difference of value ofnamong different samples may be caused by the variations in the polarizability of the samples.[30]The variations in the real part(εr)and imaginary part(εi)of the dielectric constant and the optical conductivity(σopt)for(1-x)NCO/xPbS nanocomposite samples are shown in Fig. 8. Bothεrandεicurves exhibit similar manners to thenandkcurves, respectively for the corresponding samples. The value ofεrdecreases while the values ofεiandσoptincrease as the wavelength augments for each of all samples. Also, the values ofεrof the nanocomposite samples in a lower wavelength range are higher than those of NCO samples. The values ofεiandσoptof the nanocomposite samples in a lower wavelength range are lower than those of NCO samples. In a higher wavelength range,the behaviors ofεr,εi,andσoptin the nanocomposite samples are reversed as compared with those of the NCO samples. The values ofεr,εi,andσoptincrease slightly as the amount of PbS increases in nanocomposite matrix. The variations of the values ofεrandσoptare linked to the changing of the density of the localized states in an optical bandgap of the host material and theεivaries with the modification of the dipole movement.[31]
The nonlinear optical (NLO) characteristics such asχ1andn2play a crucial role in realizing the high performance communication networks devices. The values ofχ1,χ3, andn2are dependent on the wavelengthλfor all nanocomposite samples and shown in Fig. 9. The three parameters vary in a similar way, where the three parameters decrease withλincreasing in NCO and nanocomposite samples. The values ofχ1,χ3, andn2values of the nanocomposite samples are higher than those of NCO samples. In addition,the values ofχ1,χ3, andn2decrease slightly as the percentage of PbS increase in the nanocomposite matrix. The changing in the nonlinear parameters may be due to the variation in the number of defect centers and hence the changing in the local polarizabilities present in the different samples. The improvement in the values ofχ3andn2in the nanocomposite samples makes the samples deserve to further studied so as to be used in numerous NLO and future photonic applications depending on the frequency range.

3.3. Photoluminescence analysis
Figure 10(a) shows the photoluminescence (PL) spectra emitted from (1-x)NCO/xPbS (x=0.0, 0.1, 0.15, and 0.2)system under an excitingλof 300 nm at room temperature.The PL intensity increases as the amount of PbS becomes 10%and 15%in the nanocomposite system relative to NCO sample,while it decreases as the content of PbS increases further in the matrix. The intensification or suppression in the luminescence intensity may be caused by the increasing or decreasing of the recombination rate between the photo-induced electrons(e-)and holes(h+)pairs which depends on the trap levels existent in the bandgaps of the different samples.[32]A similar result was observed in two-dimensional PbS/amorphous MoSxheterojunction composites.[33]The enhancement and reduction in the PL intensity of the different samples may make these materials able to possess the LED and photocatalytic applications.Using Gaussian function,the PL spectrum for each of all samples can be decomposed into ultraviolet color and violet colos(see Figs. 8(b)–8(e)). The direct recombination of the electrons via an exciton-exciton collision process produces the ultraviolet color and violet color.[34]

4. Conclusions
The pristine NiCo2O4(NCO) has a single cubic spinel phase, while the (1-x)NCO/xPbS nanocomposite samples withx=0.1, 0.15, and 0.2 have three phases: cubic spinel NCO as a major phase (>95%), PbS, and PbSO4as minor phases. Some Pb and S ions are incorporated into the NCO lattice. The cell parameter of NCO phase increases as the amount of PbS augments in the nanocomposite sample. The crystallite size of the NCO phase is isotropic and almost the same (≈15 nm) for all samples. For the PbSO4phase, the crystallite size is≈17 nm while for PbS phase it is very large (~130 nm). The FTIR spectra reveal the heteropolar diatomic molecules of Pb–S band existent in the nanocomposite’s samples. The NCO sample exhibits a substantial amount of absorption in the visible light range. For the NCO alloyed with PbS, the absorption decreases in the UV range while it increases in the visible range. The NCO has two band gaps of 1.35 eV and 2.33 eV which increase to (3.3 eV, 4.06 eV),(3.33 eV,4.1 eV),and(3.27 eV,4.15 eV)upon increasing PbS to 10%, 15%, and 20%, respectively. In all samples, the values ofn,εr,and the nonlinear optical behavior decrease while the values ofk,εi, andσoptincrease as the wavelength augments. For the PbS alloyed with NCO,the dielectric constant,optical conductivity, and the nonlinear optical parameters are enhanced as compared with counterparts for the NCO sample.As the percentage of PbS increases, the values ofk,n,εr,εi,σopt, and NLO decrease slightly. The PL spectra for all samples can be decomposed into ultraviolet and violet colors. The enhancement and reduction in the PL intensity of the different samples may make these materials able to possess the LED and photocatalytic applications.
Acknowledgment
The authors thank the support of Taif University Researchers Supporting Project Number TURSP-2020/12, Taif University,Taif,Saudi Arabia.
- Chinese Physics B的其它文章
- Switchable terahertz polarization converter based on VO2 metamaterial
- Data-driven parity-time-symmetric vector rogue wave solutions of multi-component nonlinear Schr¨odinger equation
- Neutron activation cross section data library
- Multi-phase field simulation of competitive grain growth for directional solidification
- A novel similarity measure for mining missing links in long-path networks
- Effects of electrical stress on the characteristics and defect behaviors in GaN-based near-ultraviolet light emitting diodes