Molecular dynamics simulations of mechanical properties of epoxy-amine: Cross-linker type and degree of conversion effects
Yongqin Zhang(张永钦) Hua Yang(杨华) Yaguang Sun(孙亚光)Xiangrui Zheng(郑香蕊) and Yafang Guo(郭雅芳)
1Department of Mechanics,School of Civil Engineering,Beijing Jiaotong University,Beijing 100044,China
2School of Aeronautics and Astronautics,Zhejiang University,Hangzhou 310027,China
Keywords: polymer,molecular dynamic simulation,mechanical properties,thermodynamics
1. Introduction
Epoxy is a class of thermosetting polymers synthesized chemically by curing molecules containing epoxy groups and hardeners with active hydrogens.[1,2]Each epoxy resin or curing agent monomer typically possesses two or more reactive functionalities. Epoxy-amine, as a common class of epoxy resins, is usually crosslinked to form a network structure by stepwise polymerization. This type of material has the advantage in that it can be cured at room (or low) temperature. Thermosetting epoxy resins form non-fusible, nonsoluble, crosslinked structures after curing. As a result,they exhibit high mechanical strength,strong adhesion,small curing shrinkage, corrosion resistance, electrical insulation,etc., which are desirable properties for a wide range of applications ranging from electronics packaging to structural components in aircraft and aerospace.[3]Besides the above traditional applications, thermosetting polymers can also be used as functional materials, such as covalent adaptable networks (CANs); this type of material adds dynamic chemical bonds to thermoset polymers, which combine the desirable attributes of conventional thermosets with malleability and recyclability.[4,5]
With the fast developments in computer power and advances in physics-based models and efficient algorithms,computational simulation plays an important role in modern science and engineering. Although traditional experimental methods can be used to measure the thermodynamic properties of epoxy-amine systems, the prediction ability of computer simulation will greatly facilitate the final design and development of thermosetting polymers with improved thermodynamic properties. Among computer simulations, fullatomistic molecular dynamics (MD) simulation and coarsegrained molecular dynamics (CGMD) simulations are employed to investigate the structural and mechanical properties of polymeric systems at the atomic scale. Among these studies, the glass transition temperature (Tg) is the most extensively characterized parameter. Understanding and predicting theTgof amorphous polymers is of critical importance due to its crucial role in governing their thermal and mechanical behavior. Experimentally, theTgcan be measured using various techniques,such as dynamical mechanical analysis(DMA),thermodynamic analysis(TMA),and differential scanning calorimetry (DSC).[6]Over the past decade,the MD method has also been used to determine theTgby calculating thermodynamic properties, including density(or specific volume), internal energy, and specific entropy.There has been a great deal of research carried out to characterize the effect of the degree of conversion on the material properties of thermosetting polymers. For example,Natalia and Sharmila[7]used MD and molecular mechanics simulations to investigate the influence of model size,length of epoxy strands, and curing degree on the thermomechanical properties of DGEBA/TETA and DETDA epoxy systems. Liet al.[8]found thatTg, stiffness and yield stress depend strongly on the degree of conversion of a thermosetting polymer composed of epoxy EPON862 and the curing agent DETDA. They also studied the thermo-mechanical response of a thermosetting polymer(diglycidyl ether of bisphenol A with 3,3-diamino-diphenylsulfone) under cyclic loading using MD simulations.[9]Jeyranpouret al.[10]investigated the thermo-mechanical properties of DGEBA/TETA and DGEBA/DETDA epoxy systems, which showed that the DGEBA/TETA epoxy system had a lowerTgand higher mechanical properties. Karuthet al.[11]applied machinelearning-based cheminformatics and CG-MD modeling to investigate the factors that affect theTg.
The thermodynamic properties of thermosetting polymers vary depending on the curing process or conditions (temperature, heating rate, time, the molecular structures of the constituent monomers).[12]For example, Liet al.[13]adopted polymers composed of the epoxy resin DGEBA (EPON825)and a series of cross-linkers with different numbers of active sites and rigidity to quantify the effects of cross-linkers on thermo-mechanical properties. Nobuyuki Odagiriet al.[12]carried out different reactions by changing the amine/epoxy stoichiometric ratio(r),expounding its influence on the crosslinked networks and mechanical properties. Sonieet al.[14]investigated the effect of cross-linker length on the thermal and volumetric properties of epoxy systems. Experiments have also shown that theTgof an epoxy network can be shifted by more than 140 K by synthesizing with different cross-linkers.[15]The effect of the degree of conversion on the material’s properties is essential and worthy of thorough investigation; however, contradictory results are obtained and are still under debate. For example, the studies of Theriaultet al.[16]showed an increase in the Young’s modulus with an increasing degree of conversion. Li and Strachan[8]further reported an almost linear increase in the tensile modulus with the degree of conversion. Marks and Snelgrove[17]even reported a uniform trend of a decreasing tensile modulus with increasing conversion for various amine-cured epoxy thermosets. Gaoet al.[18]used molecular simulation and experimental research to predict and analyze the cross-linking procedure and the microstructure–property relationship in two trifunctional epoxy-amine systems (TDE-85/DDS and AFG-90/DDS). Besides, epoxy resins are generally used for adhesives to attach to the surface of attachments. Yamamotoet al.[19]studied an epoxy-amine system to determine how they move around dynamically and react with each other to form three-dimensional networks at a copper interface. In addition,the molecular diffusivity,density,and molecular orientation at the interface were also studied.
Although these studies greatly advanced our understanding of these materials, the comprehension of the cross-linker type and degree of conversion effects on the structural and mechanical properties of epoxy-amine systems are still limited at the atomic scale. In this paper, several epoxy-amine systems with different types of cross-linkers are built, and the corresponding network properties, such as end-to-end distance,crosslinking density, Young’s modulus and yield stress, are calculated. In addition, the influence of the degree of conversion on thermodynamic properties is also analyzed.
2. Methodology
2.1. Structure of resin and cross linker molecule
The detailed construction of the molecular model of the epoxy-amine system is documented in this section. First, a simulation box consisting of uncrosslinked monomers, i.e.,epoxy resin (EPON862) and the curing agents (1,3-P, DDM and PFE),is built. Second,the detailed crosslinking and equilibrium processes using a large-scale atomic/molecular massively parallel simulator(LAMMPS)are described.[20]
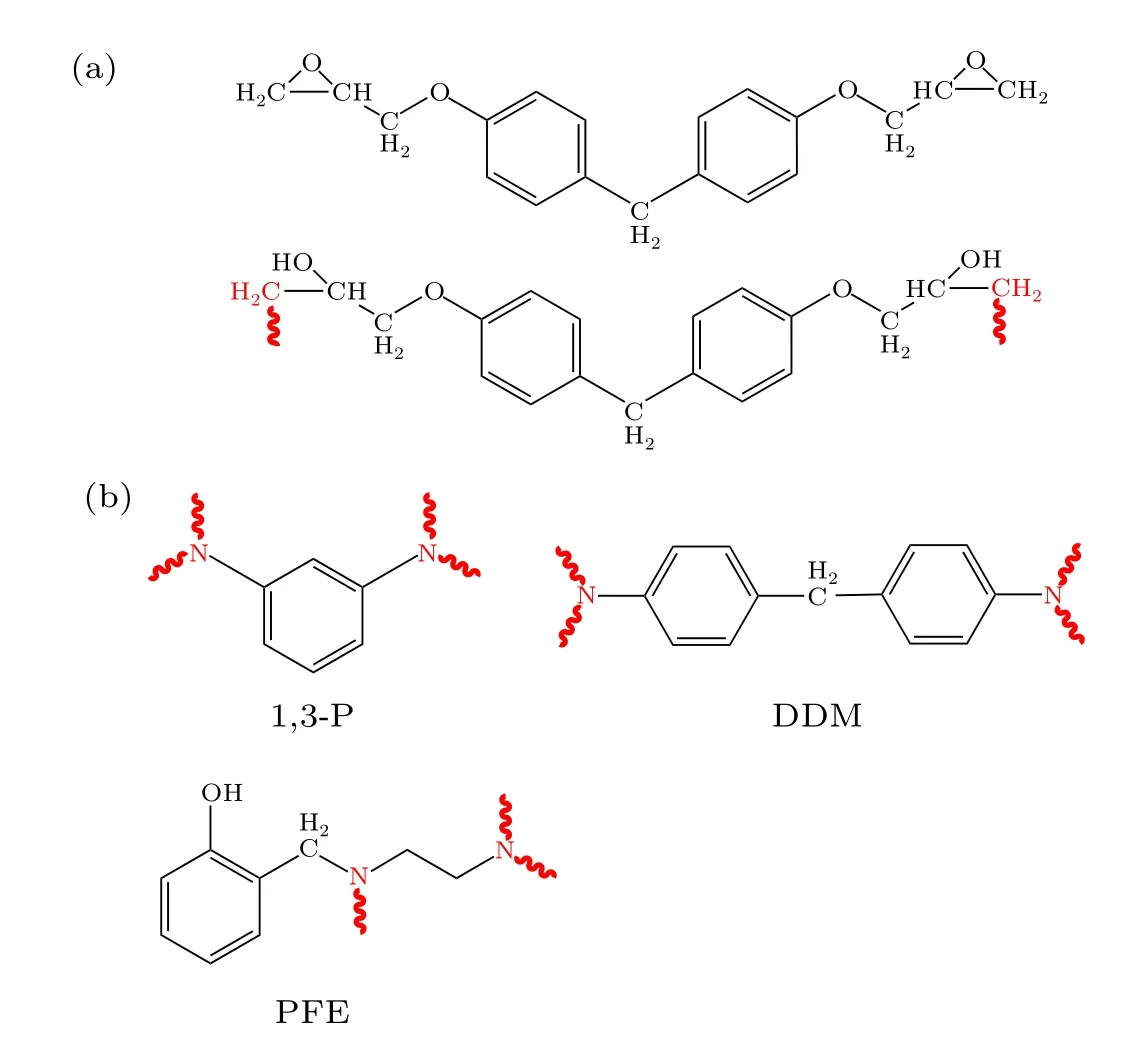
Fig. 1. Molecular structures of epoxy resin and activated cross-linkers: (a)EPON862 and activated EPON862;(b)three types of activated curing agents.
The initial models are built using the Amorphous Cell and Forcite module in Materials Studio 8.0. As listed in Table 1,we establish three types of polymer systems with different crosslinking agents. The stoichiometric ratio of epoxy/amine systems is 1. The degree of conversion is defined as the ratio of the number of bonds created to the maximum possible new bonds, which depend on the type of cross-linker and the total number of cross-linkers in the system. A mixture of the epoxy and curing agent molecules are first packed into a simulation cell with 3D periodic boundary conditions.The molecular structure of EPON862,cross-linkers and their corresponding “activated” structures are shown in Fig. 1, where the reactive sites are highlighted in red. Among the cross-linkers,1,3-P and DDM are common aromatic amines, and both are difunctional cross-linkers. The former contains one benzene ring and two reactive nitrogen atoms,which are meta-nitrogen(m-nitrogen). The latter contains two benzene rings and two reactive nitrogen atoms,which are para-nitrogen(p-nitrogen).PFE is a kind of comprehensive performance phenyl aldehyde amine epoxy curing agent,which has a longer molecular chain length. Due to the presence of secondary amines, it can also increase the relative molecular mass and reduces the proportion of reactive hydrogen in the molecule.
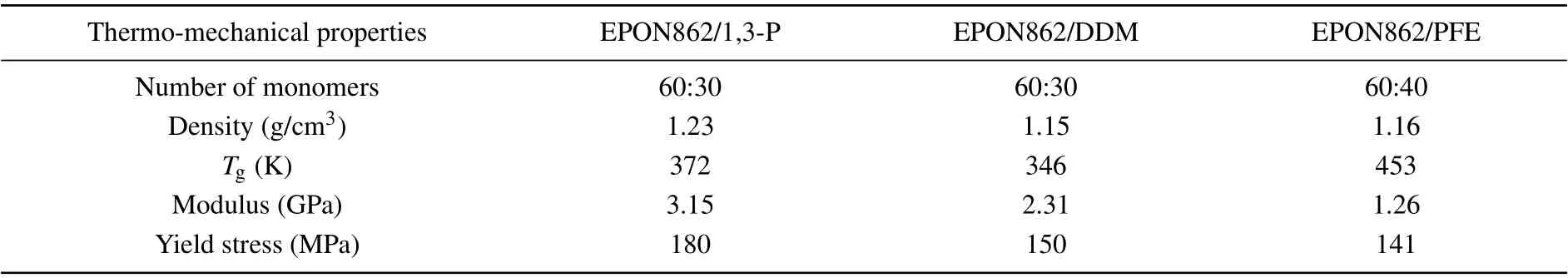
Table 1. Thermo-mechanical properties of epoxy-amine polymers.
2.2. Modeling and cross-linking process
Once the initial polymer systems are constructed, it is important to select appropriate inter-atomic potentials to describe the interactions between atoms. In this paper, simulations are performed using the polymer consistent force field(PCFF), which is parameterized for a large class of organic molecules,allowing it to be applied to synthetic polymers.[21]An energy-minimization procedure is performed using the conjugate gradients method, and then the NVT and NPT ensembles are subsequently used at 500 K and atmospheric pressure to obtain an equilibrated system.A Nose–Hoover thermostat and barostat are used for temperature and pressure control,respectively.[22,23]In this work,we use Li’s[8]method to build thermosetting polymers with different degrees of conversion.A flowchart of the reaction procedure is shown in Fig.2.
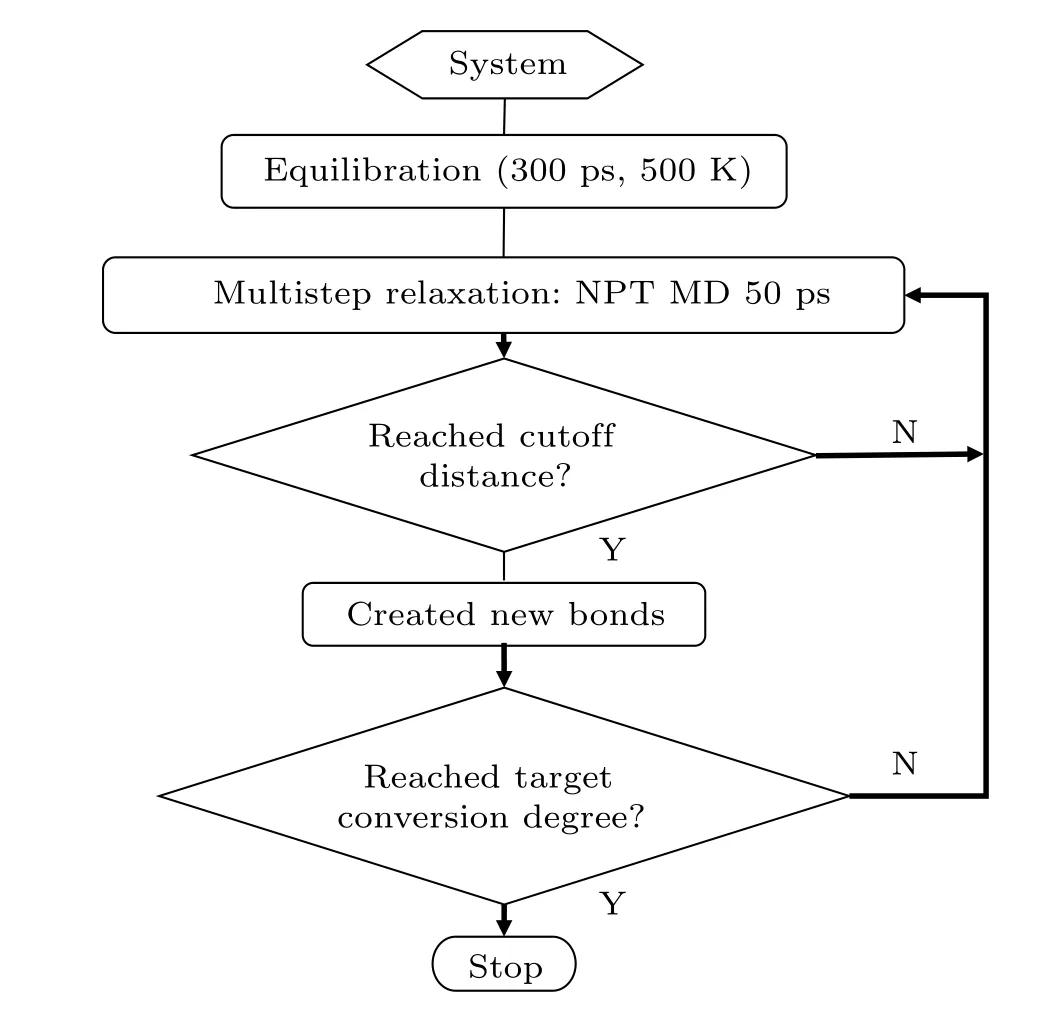
Fig.2. A flowchart of the curing procedure.
During the curing stage, bond creations are simulated in a stepwise manner using a distance-based criterion. If the distance of two active sites is within the cutoff distance we set, the system will automatically create a chemical bond between the active sites. In this work, the ideal degree of conversion is set to be 85%. The cutoff distance of bond creation is set to be 5.64 °A, which is four times the equilibrium N–C bond length (1.41 °A).[24]After new covalent bonds are formed, a 50 ps multistep relaxation procedure is performed to avoid large atomic forces of new bonds.[13,25]As shown in Fig. 3, taking the EPON862/1,3-P system as an example, the potential energy of the system has already become stable after 50 ps NPT relaxation, which means the newly formed bonds are fully relaxed. The MD simulations of polymerization are carried out at 500 K to increase molecular mobility. This procedure is continued until the conversion limit is achieved.

Fig.3. The potential energy changes with time during NPT relaxation.
3. Results
3.1. Glass transition temperature
TheTgis one of the most important parameters in polymers.[26,27]When polymers are cooled down below theTg,neither the molecular chains nor the segments can move,only atoms(or groups)constituting the molecule can vibrate at their equilibrium positions. However, when polymers are heated above theTg, molecular chains and segments begin to move,and the materials exhibit high elasticity. The dramatic change in the dynamics of polymers during the glass-forming process is found to be closely relevant to various thermodynamic and kinetic attributes (e.g., rapid loss in entropy and cooling rate dependence).[28,29]MD simulations are conducted to predict theTgs of three epoxy-amine systems during the transformation procedure. Each system subsequently performs 500 ps NVT relaxation and 1 ns NPT relaxation at 500 K. After the equilibrated structures are obtained, each system is cooled down to 250 K with a cooling rate of 20 K/200 ps. TheTgs of the three systems are listed in Table 1: about 372 K,346 K,and 453 K,respectively.
Here, to investigate the effect of the degree of conversion on theTg,the same high-temperature annealing protocol is used to study the EPON862/1,3-P system with conversions of 0%,33%,45%,66%,and 85%. As shown in Fig.4(a),theTgvalues of the materials can be determined from the intersection of the two straight lines. We can observe an increase in theTgand a decrease in volume as the degree of conversion increases. TheTgs of epoxy-amine systems with different degrees of conversion are plotted in Fig 4(b). Our simulations show that theTgincreases about 40 K when the degree of conversion increases from 0%to 85%.
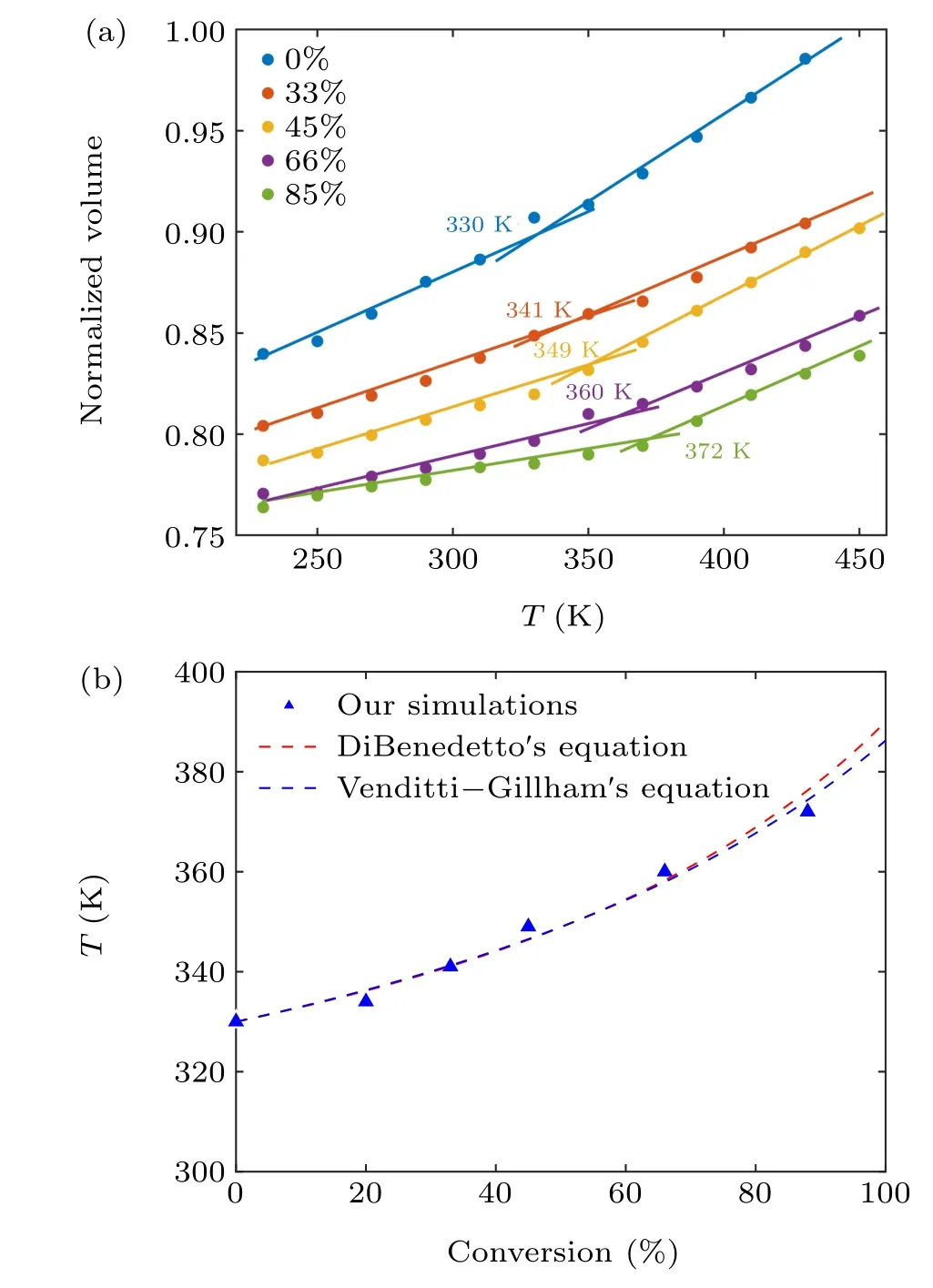
Fig. 4. (a) The normalized volume versus temperature relationship of EPON862/1,3-P systems with different conversions. (b) The Tg as a function of conversions for the EPON862/1,3-P system.
Normally,a degree of conversion higher than 90%is difficult to achieve in experiments and MD simulations.[30]However, theTgclose to the fully cured systems can be estimated by extrapolating theTgprediction of simulations at a lower degree of conversion. The following two equations can be used to describe the relation between theTgand degree of conversion. One is DiBenedetto’s equation,[31]which can be expressed as
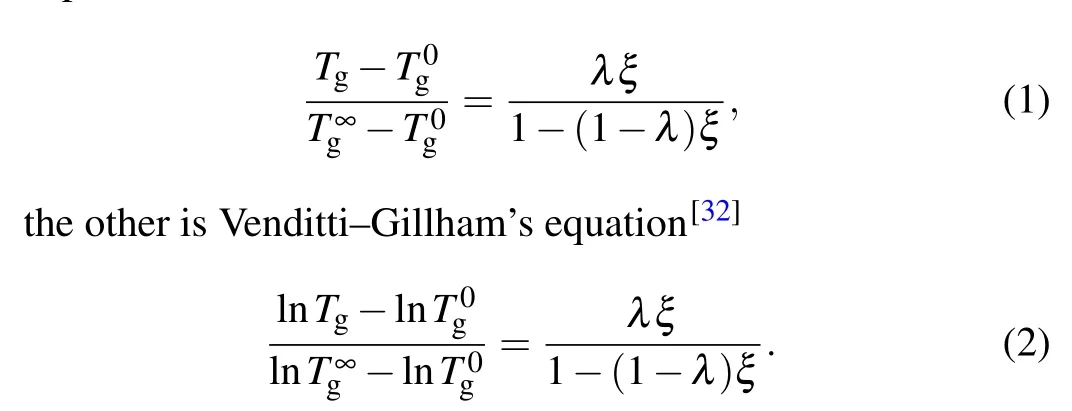
In both equations,λis an adjustable parameter that describes non-linearity in theTgconversion curve,ξstands for the degree of conversion,Tgis the glass transition temperature at the degree of conversionξ, andT0gandT∞gare the glass transition temperatures for degrees of conversion of 0 and 1,respectively. The MD data for the EPON862/1,3-P system with different degrees of conversion are fitted in Fig.4(b)using a polynomial fitting method. The calculated results areT∞g= 390 K andλ= 0.5 for DiBenedetto’s equation, andT∞g=384 K andλ=0.45 for Venditti–Gillham’s equation.Our predicted values ofλboth fall in the range of 0.1–0.6 reported in the literature,[33,34]which indicated that our simulations show good agreement with these two equations.Therefore,it can be predicted for allTgs under high conversions using MD simulations, which are difficult to achieve in experiments.
3.2. Mechanical properties
Mechanical properties are critical for assessing the performance of thermoset polymers and their use in composites,especially the strength and elastic constants. Numerous studies have been conducted on the mechanical properties of thermoset polymers using molecular simulations. Several influencing factors, such as the degree of conversion, strain rate,temperature, and molecular force field accuracy, are studied.In this work, after all the systems reached targeted degrees of conversion, the NVT and NPT MD simulations are conducted for 60 ps under 300 K to equilibrate the systems. MD simulations are performed at 300 K to examine the uniaxial tension behavior of the epoxy network. During the simulation,the length of the simulation box is continuously stretched along thez-axial direction at every MD step with a strain rate of 1×109s-1. Meanwhile, a barostat is used to maintain atmospheric pressure in transverse directions. The stress–strain curves of three systems with 85% conversion degrees are shown in Fig.5(a);the limit of the longitudinal strain is set to be 30%. Young’s moduli of the three systems are calculated by linear fitting the stress–strain curves up to 4% strain, and the yield strengths are defined as the maximum stress attained in the stress–strain curves. The values of Young’s modulus as well as yield strengths for the three systems are listed in Table 1.
As shown in Table 1, the EPON862/1,3-P system exhibits higher strength and modulus than those of the EPON862/DDM system. This may result from the fact that the m-nitrogen atoms in the 1,3-P molecule show greater polarity than p-nitrogen atoms in the DDM molecule,which can form more stable covalent bonds with activated carbon. Compared with the 1,3-P and DDM, the curing agent PFE shows a chain structure, and the binding ability is relatively weaker than aromatic amines,which leads to lower strength and modulus than the other two systems.
Moreover,during the curing process of the thermosetting polymer, the crosslinking between reactive sites will cause a change in the material’s thermodynamic response. The increase in molecular weight leads to an increase in viscosity, glass transition temperature and density. To explore the thermodynamic properties of the epoxy-amine system under different reaction degrees, the tensile processes of the EPON862/1,3-P system with different degrees of conversion in thez-axial direction are also studied. All the simulations are conducted under 300 K.The degree of conversion effect on the stress–strain relationship is shown in Fig. 5(b). The density, strength, and the Young’s modulus values of the epoxy systems cured with 1,3-P at 300 K under different degrees of conversion are summarized in Table 2. It is shown that the strength and modulus increase as the degree of conversion increases. It can be seen from Fig. 5(b) that the strength increases by about 90 MPa when the degree of conversion increases from 30% to 85%. For cross-linked networks, the red molecular chains are displayed in Fig. 6 (only the carbon atoms in the main chain are displayed),the network topology structure under uniaxial stretches for EPON862/1,3-P and EPON862/PFE are observed at the atomistic scale during the tensile processes. It is observed that the EPON862/1,3-P system is highly entangled before it is stretched.With the increase in strain, the molecules start to orient in thez-axial direction,which is shown in Fig. 6(a) (the red molecular chain). However,for the EPON862/PFE system,the molecular chain only undergoes a topological transformation under uniaxial stretching, and no orientation along the stretching direction is observed,which is shown in Fig.6(b)(the red molecular chain).To characterize the orientation of the molecular chain along the stretching direction during the uniaxial tension process,the chain angle is calculated. As shown in Fig.7(a),the chain angle is defined as the angle between the vector formed by the cross-linking point of the molecular chain andz-axis.It can be seen from Fig.7(b)that the chain angle of the EPON862/1,3-P system gradually decreases with the tension, while the chain angle of the EPON862/PFE system does not change significantly during stretching. This can be attributed to the presence of secondary amines in PFE:excessive cross-linking sites restrict the movement of the molecular chain in the stretching direction.
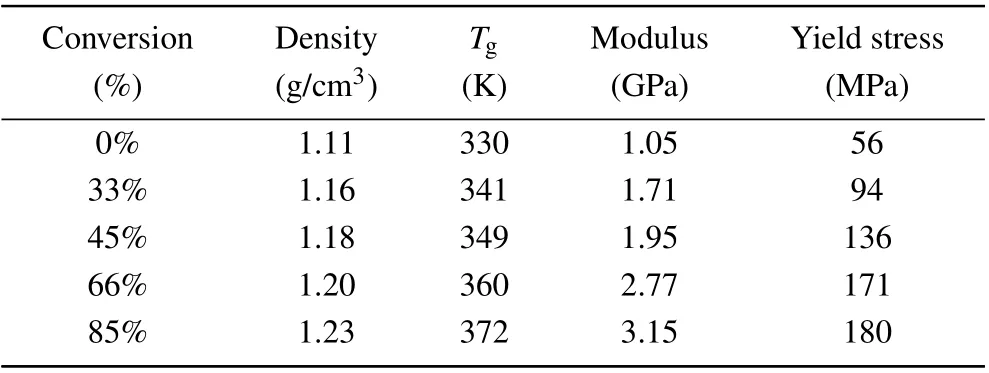
Table 2. Thermo-mechanical properties of the EPON862/1,3-P system with different degrees of conversion.
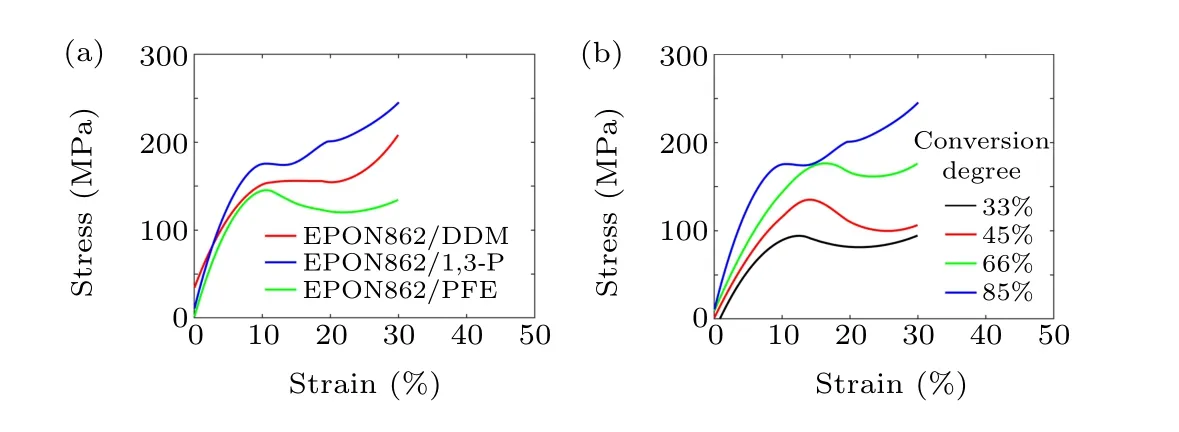
Fig. 5. Stress–strain relationships of (a) different systems with a degree of conversion of 85%,and(b)the EPON862/1,3-P system with different degrees of conversion.
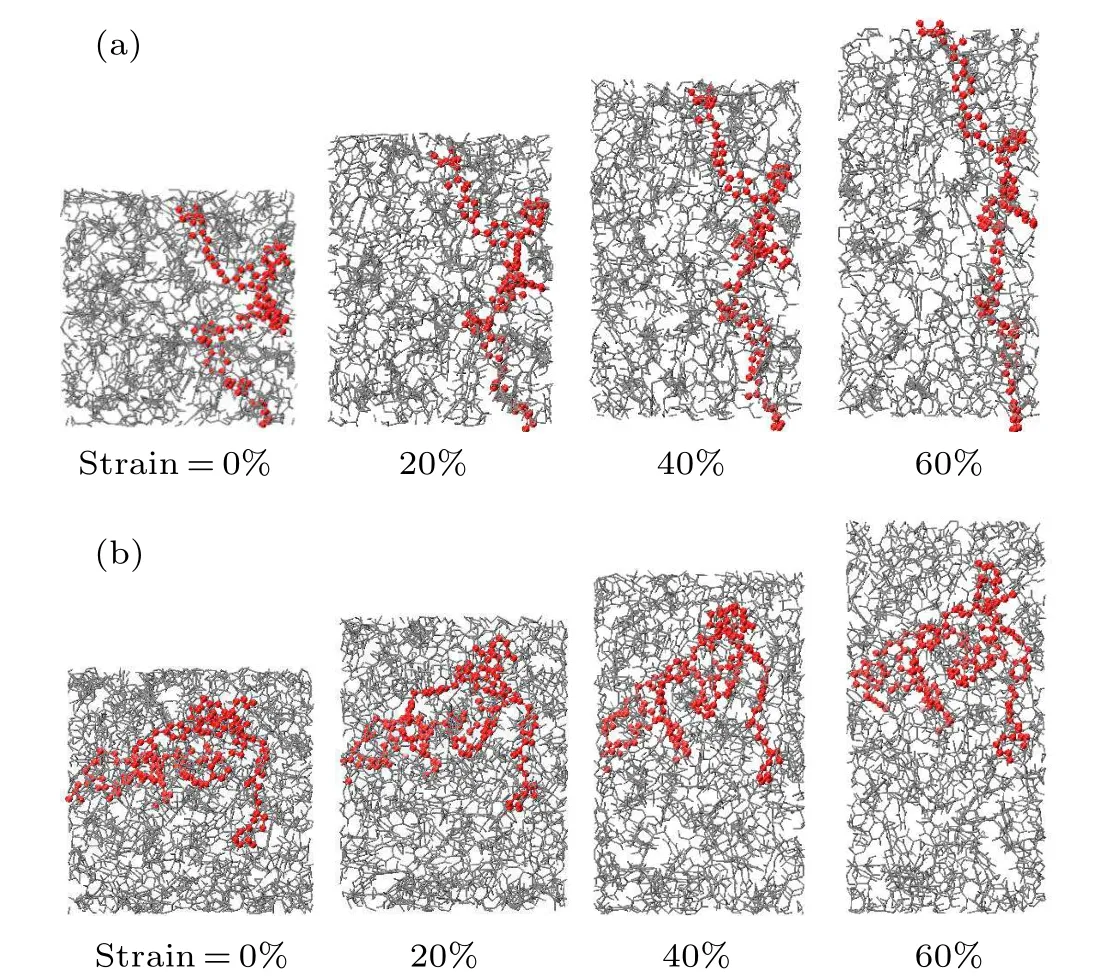
Fig.6. Snapshots of molecular chain topology under uniaxial tension at 300 K (a molecular chain in the network is shown in red): (a) EPON862/1,3-P,(b)EPON862/PFE.
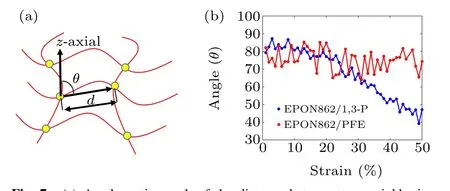
Fig. 7. (a) A schematic graph of the distance between two neighboring crosslinking sites and the chain angle. (b)Evolutions of the chain angle as a function of strain.
The degree of conversion not only has a significant effect on theTg, but also has a significant effect on the mechanical properties of the material. As shown in Fig. 5(b), a distinct increase in strength is observed when the conversion reached about 66%.This is due to the fact that more covalent bonds are created between reactive sites,leading to a decrease in mobility of polymer chains. Meanwhile,the largest molecule percolates throughout the entire box,which marks the gel point for the system.[35]
4. Discussion
4.1. End-to-end distance
Generally, higher conversion results in higher density of covalent bonds and stiffer networks.To explore the changes in the network topology during the cross-linking process,the distribution of reaction sites in the network was studied.Here,the end-to-end distance is used to describe the topological properties of the network,which are defined as the distance between two carbon atoms at the neighboring crosslinking sites.[36,37]Figure 8 shows the normalized end-to-end distance of systems with different degrees of conversion. It can be seen that the end-to-end distance undergoes no significant change at the initial stage of the crosslinking process. When the degree of conversion reaches about 50%,the end-to-end distance undergoes a significant decrease. Besides,the EPON862/1,3-P and EPON862/DDM systems show shorter end-to-end distances than that of the EPON862/PFE system. This can be attributed to the fact that the 1,3-P and DDM have symmetrical structures,which reduce the steric hindrance of the polymer chain,while the structural heterogeneity of PFE greatly increases the steric hindrance of the polymer chain in crosslinked networks.The smaller end-to-end distance leads to a denser network,and the corresponding strength and elastic modulus will be higher.
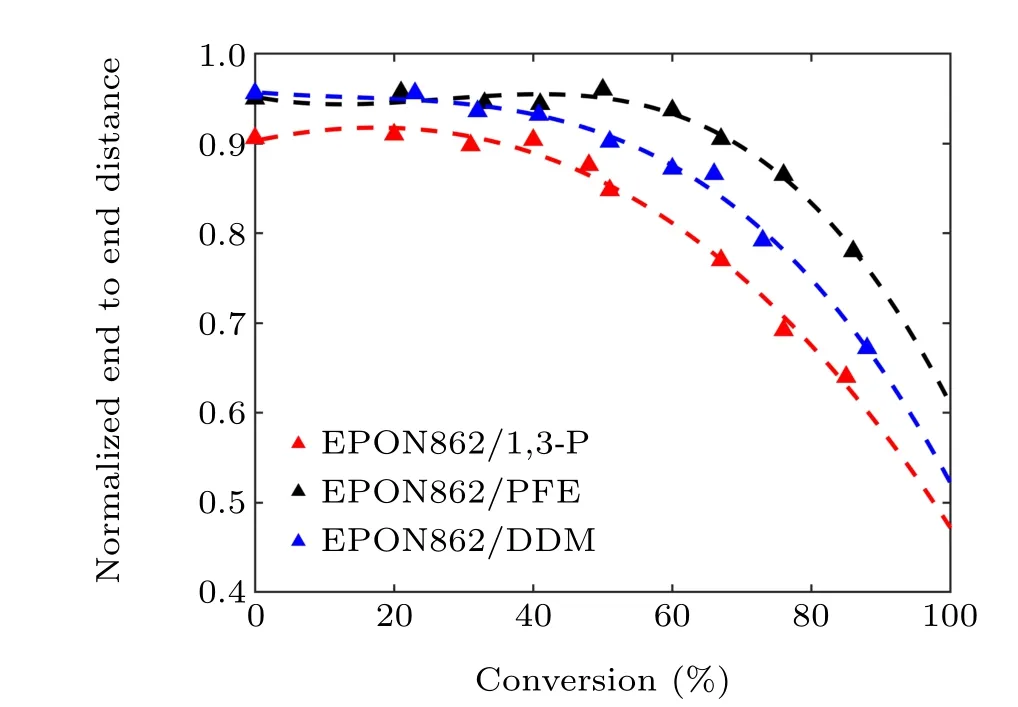
Fig.8. Evolutions of the normalized end-to-end distance as a function of the degree of conversion.
4.2. Effect of crosslinking density
Crosslinking density(CD)is an important parameter used to understand the network properties of the polymers during the curing process. In the past, several experimental techniques have been developed to quantify the CD, such as swelling experiments, infrared spectroscopy (FTIR) and nuclear magnetic resonance.[38]As shown in Fig. 5(b), the strength and modulus increase with the increasing degree of conversion. This is closely related to the change in the CD of the network during the curing process. In this paper, the CD can be expressed as follows:
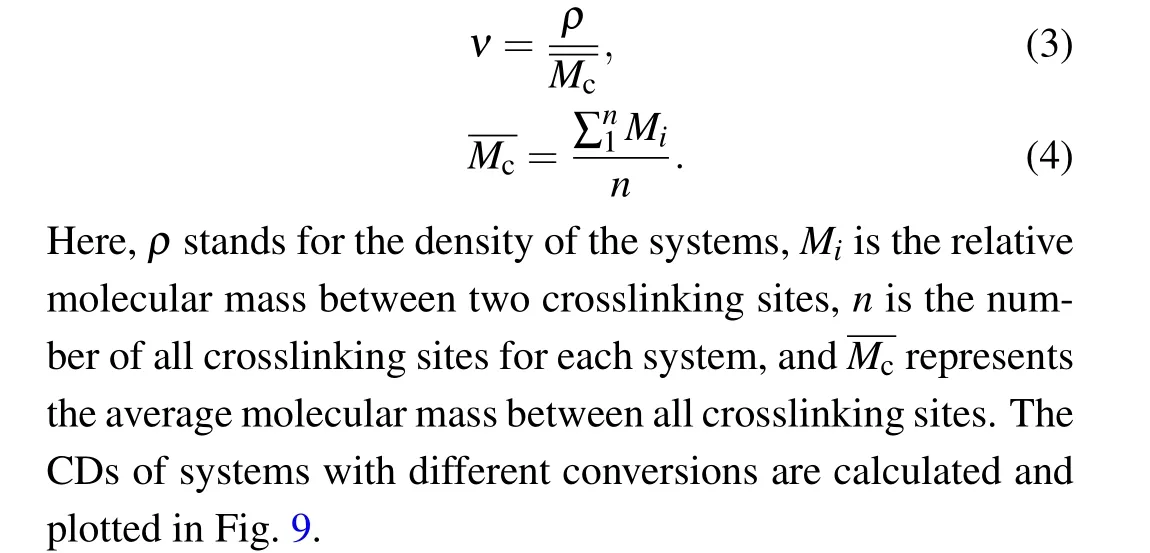
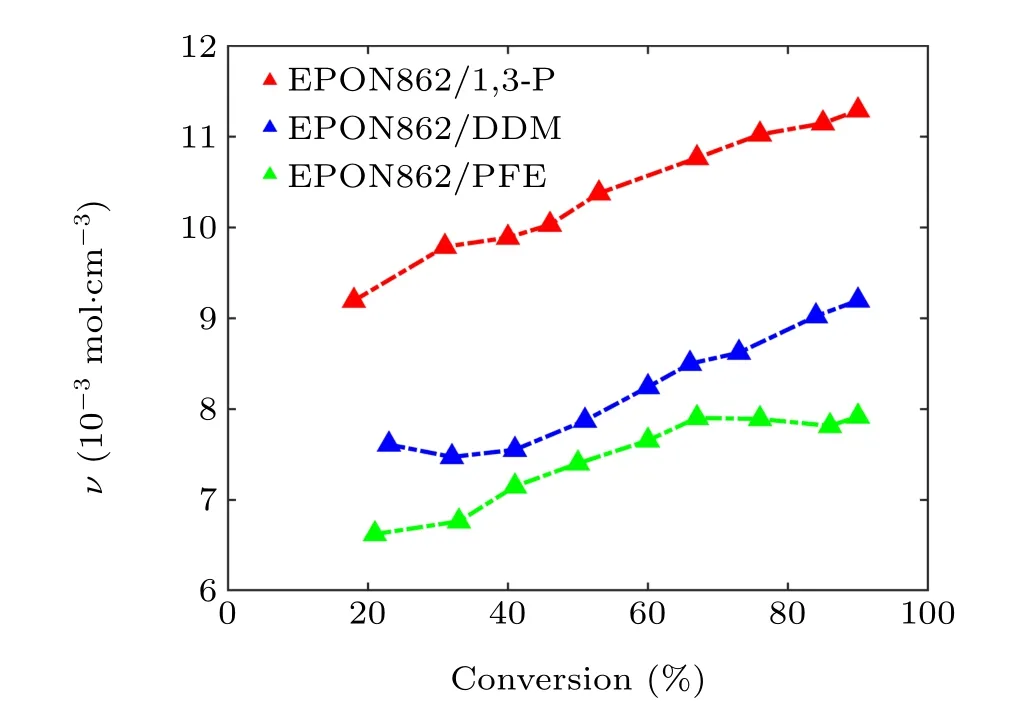
Fig.9. Crosslinking density versus the conversion relationship for three systems.
It can be seen from Fig. 9, as increasing numbers of active sites participate in the cross-linking reaction, the CD increases with the increase in the degree of conversion. Obviously,the system cured with 1,3-P shows the the highest CD,which physically explains the reason why the EPON862/1,3-P system has higher strength and modulus than the other systems. In contrast to the system cured with 1,3-P and DDM,although the system cured with PFE owns the maximum number of crosslinking sites, the CD is the lowest. Correspondingly,the system cured with PFE shows lower strength and modulus due to its smaller CD.
5. Conclusion
In this paper, molecular dynamics (MD) simulations are conducted to study the thermo-mechanical properties of epoxy-amine systems composed of the epoxy EPON862 and different curing agents (1,3-P, DDM, PFE). Firstly,Tgvalues of three systems are obtained. Our simulation results indicate that theTg,yield strength and Young’s modulus increase with the increasing degree of conversion. The simulation results also show good agreement with the existing theoretical predictions. In addition, the mechanical properties of three different cross-linking systems are investigated. The evolution of network topology and elastic properties are analyzed. It is shown that the system cured with the chain structure exhibits lower strength and elastic modulus due to its longer end-toend distance and lower crosslinking density. The results imply that the properties of thermosetting polymers are strongly dependent on the detailed molecular structures of cross-linker molecules and the degree of conversion.
Acknowledgment
Project supported by the National Natural Science Foundation of China(Grant No.11772043).
- Chinese Physics B的其它文章
- Switchable terahertz polarization converter based on VO2 metamaterial
- Data-driven parity-time-symmetric vector rogue wave solutions of multi-component nonlinear Schr¨odinger equation
- Neutron activation cross section data library
- Multi-phase field simulation of competitive grain growth for directional solidification
- A novel similarity measure for mining missing links in long-path networks
- Effects of electrical stress on the characteristics and defect behaviors in GaN-based near-ultraviolet light emitting diodes