Emerging evidence of neuronal cells-targeting SARS-CoV-2 causing anosmia associated with COVID-19
Abdul Mannan Baig
The anosmia and hypogeusia that accompany the syndromic manifestations of coronavirus disease 2019 (COVID-19) have become an increasing area of research during the current pandemic. These deficits are experienced as a major concern and are found to compromise the lifestyle of patients during and after acute phase of COVID-19. The key issue is the prevalence of smell and taste disorders that have been reported in some patients after partial recovery from COVID-19. Very little is known about the exact mechanism underlying anosmia or parosmia in COVID-19. The olfactory mucosa (OM) (Figure 1A
), which is situated deep in the upper part of the nose and adjoining areas below the cribriform plate in the skull, is composed of diverse neuronal and nonneuronal cells (Figure 1A2
) that act in concert to enable olfaction. In humans, the nose is a known site of the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Figure 1A
), in addition to the mouth and the eyes. After acquisition of SARS-CoV-2, the virus from the nose and mouth reaches the respiratory passages to cause pulmonary infection (Figure 1A6
). During the period, the virus stays in the noise and a few days to weeks before its spread to the lungs, anosmia commences (Figure 1A1
) and continues for a variable period during and after the acute phase of COVID-19. Baig et al. (2020) reported that the occurences of anosmia, hypogeusia and convulsions reported during the early days of SARS-CoV-2 outbreak suggest neurotroposm (Figure 1A6
), which provides altert information to clinicians and healthcare professions.OM:
The olfactory epithelium (OE) of OM (Figure 1A3
) is a specific collection of diverse cell types within the nasal cavity that is involved in the perception and transmission of smell. In microsmatic mammals like humans, OM measures ~9 cmand lies on the roof of the nasal cavity about 6–7 cm superior and posterior to the nostrils (Figure 1A
).Cell types in the OM:
The OE is mainly composed of primary olfactory sensory neurons (OSNs), neural stem cells, sustentacular (supporting) cells, Bowman’s glands, and microvillous cells. On the apical side of OM, the dendrites of OSNs project into the nasal cavity (Figure 1A2
-bottom), while on the basal side, the axons of OSNs merge into fila, which protrude superiorly through the cribriform plate directly into the olfactory bulb (Figure 1A2
-top) thereby being also in contact with cerebrospinal fluid (Figure 1A3
-black tubule). OSNs are bipolar cells. The horizontal and globose basal cells constitute the range of neural/neuronal cells within the OM.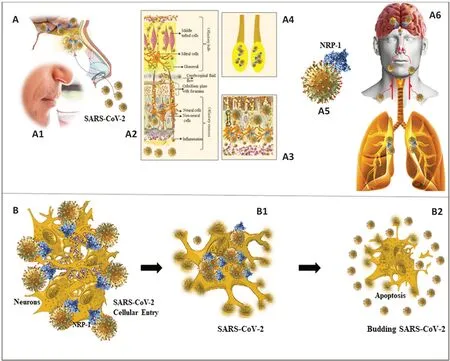
Figure 1|Anosmia in COVID-19.Schematic illustration showing SARS-CoV-2 viral loads in the nasal cavity (A) that causes anosmia (A1). The olfactory mucosa (OM; A2) harbors the virus that has been reported to be present beneath the cribriform plate (A3) and olfactory bulb (OB; A4) where SARS-CoV-2 RNA has been detected in fatal cases of COVID-19. Inflammatory congestion in the OM (A3-bottom), and NRP-1 (A5) dependent host cell uptake by the olfactory sensory neurons (A), as well as ACE2 medicated entry into the non-neuronal olfactory mucosal cells (A2) cause anosmia in COVID-19. Neuronal cell entry (B) causes cell death with DNA fragmentation (B1 and B2), a feature of apoptotic cell death. COVID-19: Coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Current evidence of neural cells in the OM as a target of SARS-CoV-2:
A generalized inflammatory response that is elicited against SARS-CoV-2 in the OM is understandably expected to get scattered over the mucosal epithelium without a predilection of any particular cell type. In addition to targeting the OM in COVID-19, SARS-CoV-2 has been also reported to affect the OB where mRNA of SARS-CoV-2 has been found in autopsies of patients who have died from COVID-19 (Meinhardt et al., 2021). An earlier variant SARS-CoV-1 is also known to affect the OM. The fact that anosmia continues even after the acute exudative phase of infection and has been reported in patients with long-COVID raises the suspicion of particular cells targeted by SARS-CoV-2. The SARS-CoV-2 loads in the nasal cavity which contains the OE make it a susceptible target of the virus (Cantuti-Castelvetri et al., 2020; Meinhardt et al., 2021). The ACE2 expression, used by SARS-CoV-2 for cell invasion, was reported in cells of the OM (Meinhardt et al., 2021). Determining intracelluar entry neuropilin-1 (NRP-1) dependent SARS-CoV-2 and its expression in the apical olfactory epithelial cells as detected by immunostaining for spike protein in autopsies of patients who have died from COVID-19 has help clarify the role of direct SARS-CoV-2 mediated cellular damage in the mechanism underlying anosmia in COVID-19 (Cantuti-Castelvetri et al., 2020). Localization of the spike protein (S) in neuronal cells in OM along with anti-SARS-CoV S protein antibodies in the olfactory sensory neurons in COVID-19 samples further reinforces the neurotropic potential to play role in anosmia associated with COVID-19.Possible pathogenic events involved in anosmia in COVID-19:
Given the sensitivity of OSNs and other cells mentioned above in the OE to injury, it can be deduced that the cytokines and diverse inflammatory mediators can cause reversible or irreversible damage leading to anosmia. Also, masking OM with the mucus and exudate resulting from inflammation can cause anosmia by preventing the odorant molecules to reach the receptor binding sites on OSNs in the OE. The fact that anosmia persists even after the acute phase of COVID-19 as in long-COVID raises the interesting possibility of direct neuronal damage involving the OSNs or neural stem cells in the OM. Ramani et al. (2020) reported the possibility of neuronal apoptosis in the 3D human brain organoid. The finding of fragmented DNA and other features of apoptosis in dead cells (Figure 1B
,B1
, andB2
) could provide a preliminary step towards further investigations into exploring the mechanism underlying direct cellular injury incuded by SARS-CoV in mature human neurons. The finding of damage in the olfactory nerve (Bulfamante et al., 2020) and OM (Meinhardt et al., 2021) and investigating the traces of SARSCoV around the cribriform plate, OB, and the overlying inferior surface of the frontal lobe were considered to possibly support the hypothesis of transcribrial access of SAR-CoV-2 to the brain (Baig et al., 2020). The finding of loads of SARSCoV-2 viral RNA within the OM sampled directly beneath the cribriform plate, OB (Meinhardt et al., 2021) and the frontal lobe of the brain hints towards the “transcribrial route” as one of the natural pathway opted by SARS-CoV-2 towards the brain (Baig et al., 2020). The finding of virus-like particles in the neuronal cytosol may explain not only the potential of direct SARS-CoV-2 damage but also the loss of cognitive function reported in acute and chronic phases of COVID-19 aflecting the frontal lobe of the brain.Discussion and conclusion:
Though SARS-CoV is discussed here within the context of its possibility to cause anosmia, there is clear evidence that it targets different regions of the brain, resulting in neurologic deficits (Baig, 2022). SARS-CoV has been detected in the medulla oblongata, midbrain, and cerebellum during autopsies (Meinhardt et al., 2021). SARS-CoV has been shown to directly damage neurons and also damage neurons through inflammation and cytokines (Baig, 2022). In many patients, anosmia is resolved after the acute phase of COVID-19, which can be explained by differential viral loads, clearance of inflammatory reaction in and around the OM, replenishing of OSNs by basal cells, and immune system-mediated combat and clearance of SARSCoV-2 from the OM. It remains a real possibility for the virus to reach the CNS, in particular the brain, during and after the acute phase of COVID-19 via the bloodstream by causing endothelial damage in the cerebral microcirculation and a breached blood-brain barrier. Anosmia is a troubling symptom of COVID-19, not only because it adversely affects the lifestyle of the patient but it also at times endangers patient’s safety. The neurotropic threat imposed by SARS-CoV-2 is not a spurious concept, as evidenced by the findings of SARSCoV-2 RNA loads beneath the cribriform plate, its traces in the overlying OB, and SARS-CoV-2 like viral particles in the neurons of the frontal lobe of the brain (Meinhardt et al., 2021). With debates on the variable expression of ACE2 receptor in the neurons, the finding of NRP-1 receptor expressed in OSNs and neurons of the CNS, which facilitates SARS-CoV-2 entry to the cells, and the potential of neuroinvasiveness associated with SARS-CoV-2 are becoming unblemished. The exact mechanism by which SARS-CoV-2 directly causes neuronal damage needs to be elucidated in the future, but the finding of DNA fragmentation caused by SARSCoV-2 hints towards an apoptotic cell death.Abdul Mannan Baig
Department of Biological and Biomedical Sciences, Aga Khan University, Karachi, Pakistan
Correspondence to:
Abdul Mannan Baig, MBBS, PhD, abdul.mannan@aku.edu.https://orcid.org/0000-0003-0626-216X(Abdul Mannan Baig)
Date of submission:
November 23, 2021Date of decision:
December 22, 2021Date of acceptance:
January 25, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.344843
How to cite this article:
Baig AM (2023) Emerging evidence of neuronal cells-targeting SARS-CoV-2 causing anosmia associated with COVID-19. Neural Regen Res 18(2):463-464.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

