Identification of injury type using somatosensory and motor evoked potentials in a rat spinal cord injury model
Rong Li , Han-Lei Li, Hong-Yan Cui, Yong-Can Huang, Yong Hu
Abstract The spinal cord is at risk of injury during spinal surgery. If intraoperative spinal cord injury is identified early, irreversible impairment or loss of neurological function can be prevented. Different types of spinal cord injury result in damage to different spinal cord regions, which may cause different somatosensory and motor evoked potential signal responses. In this study, we examined electrophysiological and histopathological changes between contusion, distraction, and dislocation spinal cord injuries in a rat model. We found that contusion led to the most severe dorsal white matter injury and caused considerable attenuation of both somatosensory and motor evoked potentials. Dislocation resulted in loss of myelinated axons in the lateral region of the injured spinal cord along the rostrocaudal axis. The amplitude of attenuation in motor evoked potential responses caused by dislocation was greater than that caused by contusion. After distraction injury, extracellular spaces were slightly but not significantly enlarged; somatosensory evoked potential responses slightly decreased and motor evoked potential responses were lost. Correlation analysis showed that histological and electrophysiological findings were significantly correlated and related to injury type. Intraoperative monitoring of both somatosensory and motor evoked potentials has the potential to identify iatrogenic spinal cord injury type during surgery.
Key Words: contusion injury; dislocation injury; distraction injury; electrophysiology; heterogeneity; histopathology; injury mechanism; motor evoked potential; somatosensory evoked potential; spinal cord injury 1Department of Orthopedics and Traumatology, The University of Hong Kong -Shenzhen Hospital, Shenzhen, Guangdong Provinve, China; 2Department of Neurosurgery, Neuroscience Center, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong Provinve, China; 3Institute of Biomedical Engineering, Chinese
Introduction 422 Methods 422 Results 424 Discussion 426
Graphical Abstract
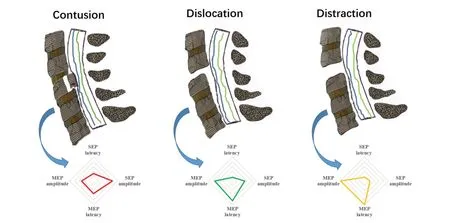
The change patterns of SEPs and MEPs can reflect SCI induced by different mechanical mechanisms
Introduction
Spinal cord injury (SCI) is heterogeneous in terms of injury location, injury type, and extent of neurologic impairment at the time of presentation (Tator, 2006; Choi et al., 2021; Ghasem-Zadeh et al., 2021). Rescue measures differ for different types of SCI. Choo et al. (2007) compared histological changes in the acute injury phase between three types of SCI and found that dislocation and contusion resulted in extensive hemorrhage and membrane compromise, whereas distraction injury was characterized by longer longitudinal membrane disruption without detectable hemorrhage. In a rat SCI model, contusion, dislocation, and distraction injuries exhibited distinct histopathological changes and behavioral recovery (Chen et al., 2016; Tian et al., 2021; Chen and Li, 2022). Moreover, the pattern of secondary spinal cord degeneration varies according to injury type, indicating that treatment for different although clinically-related injuries may ultimately require targeted neuroprotective strategies (Choo et al., 2008). Identification of SCI type is important because SCI type has significant treatment implications.
Spinal surgery carries the risk of iatrogenic SCI. Althoughin vitro
histological and functional analyses have revealed important SCI pattern details, the various patterns are difficult to distinguish and apply to diagnosis since they do not yield real-time information. Integrated monitoring of somatosensoryevoked potentials (SEPs) and motor-evoked potentials (MEPs) has been advocated as an essential tool to assess functional integrity of the spinal cord during spine surgery (MacDonald et al., 2019; Nuwer and Schrader, 2019). A previous report suggested abnormal changes in SEPs and MEPs can be detected early in ischemic SCI (Kakinohana et al., 2007). Other studies have shown that both are lost rapidly in contusion injuries and gradually after compression injury (Morris et al., 2017; Huang et al., 2018). Dislocation injury mainly affects SEP amplitude rather than latency (Mattucci et al., 2019). A comprehensive analysis of SEP and MEP signal characteristics may allow differentiation of SCI type. In this study, we aimed to examine and compare electrophysiological and histopathological changes between contusion, distraction, and dislocation injuries in a rat model. Furthermore, we evaluated evoked potentials as a predictor of injury type. We hypothesized that different SCI injury types have distinct patterns of evoked potential signal change.Methods
Animals
All procedures were conducted in accordance with the Care and Use of Laboratory Animals guidelines (Institute of Laboratory Animal Resources, National Research Council, 1996). The study was approved and supervised by the Research Ethics Committee at Peking University Shenzhen Hospital (approval No. 2017-004) on August 26, 2017. Thirty-nine male Sprague-Dawley rats (specific-pathogen-free level, aged 7 to 8 weeks, weight 280 to 320 g) were purchased from Guangdong Medical Laboratory Animal Center (license No. SCXK (Yue) 2018-0002) and randomly assigned to contusion (n
= 10), dislocation (n
= 10), and distraction (n
= 10) injury groups and three sham groups (n
= 3, respectively).SCI models
Animals were anesthetized for SCI, evoked potential testing, and sacrificed using intraperitoneally injected pentobarbital sodium (60 mg/kg; Sigma, St. Louis, MO, USA) and xylazine (10 mg/kg; Sigma). The injury level was located between T12 and T13. Using standard aseptic principles and techniques, the thoracolumbar spine was surgically exposed. Customized vertebral clamps were used to rigidly hold the transverse processes of T11, T12, T13, and L1. Spinal cord function was continuously monitored using SEPs and MEPs to ensure no accidental damage occurred prior to injury. The rats were placed on a thermostatic pad at 37°C to receive a subcutaneous injection of 5 mL physiological saline solution to prevent dehydration. Contusion, distraction, and dislocation injuries were performed between T12 and T13 (Figure 1
).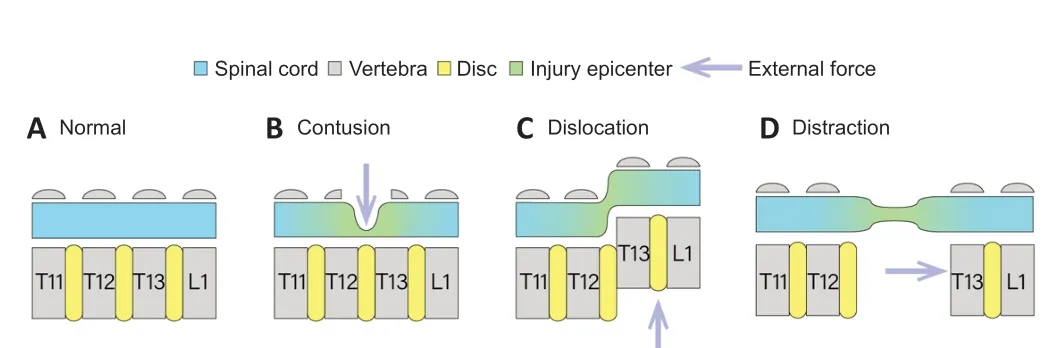
Figure 1|Schema of normal spinal cord and contusion, dislocation, and distraction spinal cord injury mechanisms. (A) Normal spinal cord. (B) To generate contusion, a laminectomy was performed and the dorsal spinal cord was impacted vertically between T12 and T13 using a flat, 2 mm diameter tip. (C) For dislocation injury, T12–T13 facetectomy was performed and dislocation was performed using an actuator coupled with vertebral clamps to apply an external dorsal displacement of the T12 vertebral body relative to T13. (D) For distraction injury, the vertebral clamps were used to apply distraction between T12 and T13 after the facetectomy.
Contusion injury was created by spinal cord displacement using the NYUMASCIS impact system (Rutgers, New Brunswick, NJ, USA) as previously reported (Wang et al., 2015). Briefly, a small circular laminectomy was performed to expose the spinal cord dura between T12 and T13 (Figure 1B
). Stabilization clamps were used to immobilize the T12 and T13 vertebrae during impact. The injury was produced by dropping a 10 g rod from a height of 20 mm onto the exposed dural surface. For dislocation injury, dorsal ligament resection and facet arthrotomy were performed at the T12–T13 interspace. The T11 and T12 vertebrae were fixed using customized vertebral clamps, while another clamp was tightly connected to the T13 and L1 vertebrae. T13 and L1 were dorsally dislocated by 2 mm and then returned to the initial position (Figure 1C
). To model distraction injury, the T12–T13 facets were removed and clamps coupled to a distraction apparatus were placed on T11/T12 and T13/L1. The respective clamps were distracted rostrally and caudally to produce a displacement of 3 mm and held for 1 second before being returned to their initial position (Figure 1D
). The sham groups received identical surgical procedures except for injury induction.Neuroelectrophysiological assessment
Immediately after SCI, electrophysiological evaluation (YRKJ-G2008; Yirui Technology Co., Ltd., Zhuhai, China) was conducted as described in our previous study (Hu et al., 2011). SEP latency was defined as the time from stimulation to the maximum negative deflection. Amplitude was measured as the maximum voltage between the peak of the positive and negative deflections.
Tibial SEPs were evoked from stimulation proximal to the ankle via a pair of needle electrodes (NE-S-1500/13/0.4; Friendship Medical Electronics Co., Ltd., Xi’an, China) using the following parameters: 0.1 ms duration, 5.3 Hz frequency, and 3–5 mA intensity (to elicit mild toe twitching). Recordings were collected using two scalp needle electrodes subcutaneously inserted over the primary somatosensory cortex and a frontal midline reference electrode. Signals were averaged over 500 responses and filtered between 30 and 3000 Hz.
For MEPs, constant current stimulation was applied to the motor cortex using two needle electrodes (0.1 ms duration, 300 Hz frequency, 30–50 mA intensity). MEPs were recorded from the gastrocnemius muscle using needle electrodes. The signals were band-pass filtered at a frequency from 30 to 3000 Hz.
Latency measures the time to onset of SEP waveform in response to stimulation. Amplitude measures the peak-to-peak intensity of SEP waveform. Latency extension is presented as (L1 – L0)/L0 where L0 indicates baseline latency and L1 indicates postoperative latency. Amplitude reduction is presented as (A0 – A1)/A0 where A0 indicates baseline latency and A1 indicates postoperative latency.
Histological analysis
The influence of different primary injury types on spinal cord histopathology was assessed immediately after electrophysiological assessment. Transcardial perfusion was performed approximately 20 minutes after SCI in all animals with 200 mL 0.01M phosphate-buffered saline followed by 300 mL of 4% paraformaldehyde. Spinal cord samples 10 mm in length containing the lesion epicenter were collected, placed overnight in 4% paraformaldehyde, and cryoprotected in graded concentrations of sucrose (12%, 18%, 24% in phosphate-buffered saline). Samples were sequentially cross sectioned at a thickness of 20 µm for histology and divided equally into 10 sets of sections at 200 µm intervals.
One set of spinal cord slides was stained with hematoxylin and eosin for analysis of overall injury distribution and hemorrhage (Huang et al., 2018). Briefly, slides were rinsed in distilled HO, stained with hematoxylin, differentiated in 1% aqueous HCl, and then counterstained with eosin. Sections were dehydrated in ethanol and xylene and coverslipped with mounting medium.
Luxol fast blue (LFB) staining was performed on another set of slides to examine white matter myelination after SCI (Walker et al., 2016). Briefly, slides were dehydrated with graded ethanol. Each section was then placed in LFB solution at 65°C for 2 to 3 hours and differentiated using 0.05% lithium carbonate. Then, sections were washed with distilled water until the desired staining level was achieved. Finally, sections were dehydrated and coverslipped in xylene-based mounting medium.
Immunofluorescence preparation was performed as previously described (Schumacher et al., 2000). Sections were blocked in normal donkey serum (1:10, Jackson ImmunoResearch Laboratories, Baltimore, MD, USA, Cat# 017-000-121, RRID: AB_2337258) for 30 minutes at room temperature and double-stained with purified neurofilament marker against SMI312 (mouse, 1:1000, Biolegend, San Diego, CA, USA, Cat# 837904, RRID: AB_2566782) and myelin basic protein (rabbit, 1:500, Abcam, Cambridge, UK, Cat# ab40390, RRID: AB_1141521) overnight at room temperature in combination with donkey anti-mouse IgG conjugated with DyLight 594 (1:200, Abcam, Cat# 150108, RRID: AB_2732073) and donkey anti-rabbit IgG conjugated with DyLight 488 (1:200, Abcam, Cat# 150073, RRID: AB_2636877) for 2 hours at room temperature. Slides were subsequently washed in phosphate-buffered saline and mounted with Fluorescent Mounting Medium (Cat# ab104135, Abcam).
Hematoxylin and eosin- and LFB-stained tissues were imaged (4× objective lens) under a light microscope (Axioplan 2, Carl Zeiss, Oberkochen, Germany) equipped with a monochrome red, green, and blue wavelength camera. SMI312/myelin basic protein-stained tissues were examined under a fluorescence microscope at 400× magnification (DM4000, Leica, Wetzlar, Germany). Brightness and contrast remained constant for all images in each group section. In each group, select slices at a specific distance from the injury epicenter were used for analysis.
Data processing
Hemorrhage volume was calculated using the Cavalieri method (Howell et al., 2002) with a 50 µm × 50 µm spacing of point probes on sections (15 to 17 per animal) spaced 200 µm apart. Cross-sectional area of the spare tissue and density of myelinated axons were measured in the region of interest (Figure 2
) using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012). Electrophysiological data processing was conducted using MATLAB 2016a (MathWorks, Natick, MA, USA).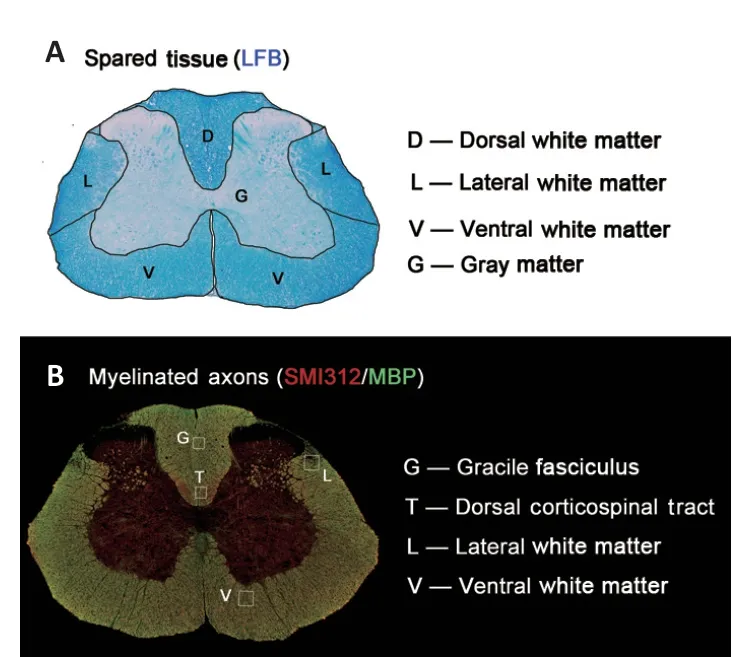
Figure 2|Histological regions of interest. (A) The lateral, dorsal, and ventral white matter and gray matter were segmented to analyze the residual spinal cord area. (B) Four boxes (100 µm × 100 µm) were placed to compare density of myelinated axons in sections stained with myelin basic protein (MBP) and SMI312. Box locations were middle-right region of the dorsal corticospinal tract, center of the gracile fasciculus, right lateral white matter around the dorsal horn, and right ventral white matter at the edge of the ventral nerve root. LFB: Luxol fast blue.
Statistical analysis
Means were compared between groups using a one-way analysis of variance. With sample sizes of 10 in the three study groups (a total of 30 subjects), the power to detect differences among the means was greater than 95% using anF
test with 0.05 significance level. No animals or data points were excluded from the analysis. Data of all animals was double-blinded and statistically analyzed using SPSS software version 22.0 (IBM, Armonk, NY, USA). Intergroup differences in the histopathologic data were compared using two-way repeated-measures analysis of variance followed by Tukey’s honestly significant difference testing. One-way analysis of variance with Bonferronipost hoc
testing was used to compare the latency and amplitude of SEP and MEP. Pearson’s correlation testing was performed between histological and electrophysiological outcomes. Data are presented as means ± standard deviation.P
< 0.05 was considered significant.Results
Electrophysiological differences among different SCI types
Evoked potential waveforms differed between contusion, dislocation, and distraction injuries (Figure 3
). Different SCI types showed distinct changes in evoked potential patterns (Figure 4A–D
). Contusion injury resulted in significant latency extension and amplitude reduction in both SEPs and MEPs (Figure 4B
). Dislocation injury was mainly characterized by MEP deterioration, particularly amplitude attenuation (Figure 4C
). In distraction injury, SEP amplitude only showed a slight decrease but MEPs disappeared (Figure 4D
).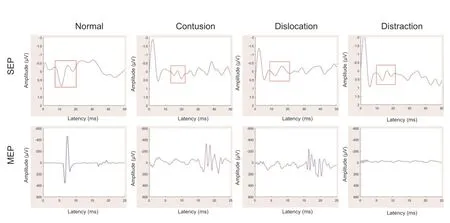
Figure 3|Representative graphs of evoked potential waveform changes observed with contusion, dislocation, and distraction spinal cord injuries. SEPs deteriorated in both dislocation and contusion injuries and were slightly reduced in distraction injury. MEPs mainly showed prolonged latency in contusion and dislocation injuries but were abolished after distraction injury. MEP: Motor evoked potential; SEP: somatosensory evoked potential.
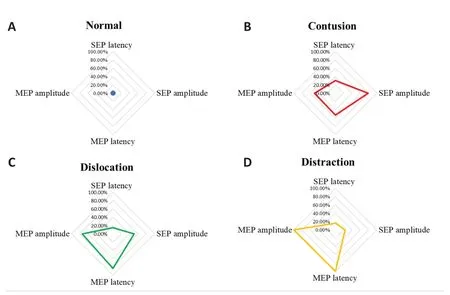
Figure 4|Time-domain parameters distribution of SEP and MEP amplitude and latency according to type of spinal cord injury. (A) No significant changes were observed after sham injury. (B) Contusion injury resulted in significant latency extension and amplitude reduction in both SEPs and MEPs. (C) Dislocation injury was mainly characterized by MEP attenuation, particularly decreased amplitude. (D) Distraction injury resulted in a slight reduction in SEP amplitude that was accompanied by loss of MEPs. MEP: Motor evoked potential; SEP: somatosensory evoked potential.
SEP latency was significantly longer in rats with contusion injury (16.22 ± 2.33 ms) than in those with distraction (13.26 ± 1.48 ms,P
= 0.003) and dislocation (13.67 ± 1.25 ms,P
= 0.002) injuries. SEP latency was similar in the distraction and dislocation injury groups (P
= 0.459) and did not significantly differ from SEP latency in the sham group (12.21 ± 0.47 ms,P
= 0.872 for distraction;P
= 0.378 for dislocation). Similarly, SEP amplitude was significantly lower in rats with contusion injury (0.41 ± 0.15 µV) than in those with distraction (0.81 ± 0.18 µV,P
= 0.031) and dislocation (0.89 ± 0.24 µV,P
= 0.001) injuries. SEP amplitude in the distraction and dislocation injury groups was similar (P
= 0.603) and significantly lower than SEP amplitude in the sham group (1.65 ± 0.54 µV,P
= 0.025 for distraction;P
= 0.013 for dislocation).Compared with the sham group, MEP onset latency was delayed or absent in the three injury groups (Figure 3
). Nine of ten rats in the contusion group and eight of ten in the dislocation group exhibited reduced MEP amplitude and prolonged latency; MEP responses were absent in the remaining rats. In all rats of the distraction injury group, MEP responses were absent. Although MEP amplitude significantly differed between the contusion and dislocation injury groups (482.02 ± 94.13 µVvs
. 245.38 ± 69.18 µV,P
= 0.002), MEP latency did not (17.12 ± 0.84 msvs
. 16.87 ± 0.13 ms,P
= 0.674). Additional electrophysiological signal change data are reported inAdditional file 1
.Histological differences among different SCI types
Examination of hematoxylin and eosin-stained slides showed extensive primary hemorrhage following contusion or dislocation injuries and slight focal hemorrhage after distraction injury (Figure 5
). The hemorrhage was concentrated in the gray matter in contusion injury, whereas dislocation injury resulted in hemorrhage in both gray and white matter. Hemorrhage volume in the dislocation (1.04 ± 0.33 mm) and contusion (0.98 ± 0.21mm) injury groups was similar and significantly larger than hemorrhage volume in the distraction injury group (0.21 ± 0.08 mm;Figure 6
;P
= 0.023 for dislocation,P
= 0.014 for contusion).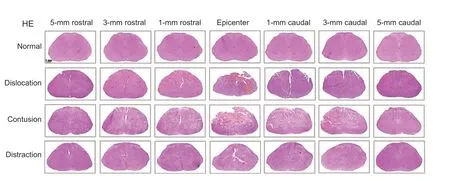
Figure 5|Representative images showing the asymmetrical rostrocaudal extent of primary hemorrhage after dislocation, contusion, and distraction spinal cord injuries. Contusion injuries produced concentrated hemorrhage in the gray matter, whereas appreciable hemorrhage was detected in both gray and white matter after dislocation injury. Distraction injury produced minor focal hemorrhage in the white matter. Arrows indicate hemorrhage. Scale bar: 1 mm. HE: Hematoxylin and eosin.
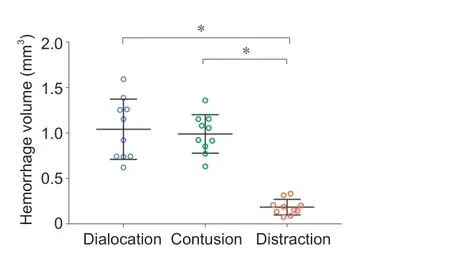
Figure 6|Primary hemorrhage volume according to type of spinal cord injury. Hemorrhage volume was similar in contusion and dislocation injuries and significantly lower in distraction injury. Data shown are means ± standard deviation (n = 10 in each group). *P < 0.05 (two-way repeated-measures analysis of variance followed by Tukey’s honestly significant difference testing).
Examination of Luxol fast blue-stained slides showed that the rostrocaudal extent of spinal cord disruption was limited to within 3 mm of the injury epicenter in distraction and contusion injuries. For dislocation injuries, laceration was identified 5 mm from the epicenter in some animals (Figure 7
). Geometrical change was observed in the spinal cord after contusion and dislocation injuries (Figure 7
). Total cross-sectional area of the spinal cord at the epicenter was smaller in the contusion (5.28 ± 0.37 mm,P
= 0.003) and dislocation injury groups (4.87 ± 0.59 mm,P
= 0.001) than in the sham group (7.12 ± 0.14 mm,Figure 8A
). Compared with the contusion injury group, spinal cords in the dislocation injury group showed greater tissue damage caudal to the lesion site. In contrast, spinal cord cross-sectional area was larger in the distraction injury group (7.06 ± 0.32 mm) than in the dislocation and contusion injury groups; however, the area in the distraction injury and sham groups did not significantly differ (Figure 8A
).Cross-sectional area of total spared white matter was smaller in the contusion and dislocation injury groups than in the distraction injury and sham groups at the following locations relative to the epicenter of injury (Figure 8B
): 2.0 mm rostral, 0.6 mm rostral and caudal, 0 mm, and 3.2 mm caudal. The total crosssectional area of spared white matter was smaller in the dislocation injury group than in the contusion injury group, which was mainly because of a large difference in the dorsal and lateral columns (Figure 8C
andD
). In the ventral spinal cord, cross-sectional area of spared white matter surrounding the epicenter did not differ between the sham group and any of the three injury types (Figure 8E
). Compared with the sham group, the area of spared gray matter around the epicenter of injury was smaller in both the dislocation and contusion injury groups; the area was smallest in the dislocation injury group (Figure 8F
). Area of spared gray matter was not significantly different between the distraction injury and sham groups.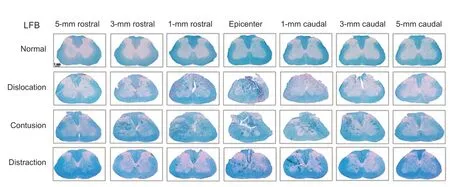
Figure 7|Representative photomicrographs of Luxol fast blue (LFB)-stained spinal cord sections. Tissue loss and atrophy as well as fissure injuries were noted on histopathological examination. The lesion extent was limited to 3 mm from the epicenter of injury in distraction and contusion injuries; however, in dislocation injuries, laceration was observed 5 mm rostral and caudal to the epicenter. Arrows indicate tissue loss. Scale bar: 1 mm.
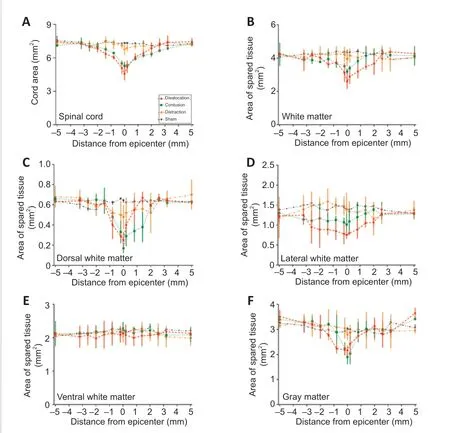
Figure 8|Cross-sectional area of total spinal cord and spared tissue on Luxol fast blue-stained spinal cord sections. The spinal cord was analyzed relative to the injury epicenter from −5 mm (rostral) to +5 mm (caudal). (A, B) Cross-sectional areas of the total spinal cord and total white matter were smaller in the contusion and dislocation groups than in the sham group. Tissue damage was greater in the dislocation group. (C) Compared with the control group, dorsal white matter area was smaller in the dislocation and contusion groups; area was particularly small in the contusion group caudal to the lesion site. (D) Lateral white matter area was lower around the epicenter in the dislocation and contusion groups than in the control group; area was particularly low in the dislocation group. (E) Ventral area of spared white matter did not differ between the sham group and any of the three spinal cord injury types. (F) Gray matter cross-sectional area was greater in the distraction injury group than in the dislocation and contusion injury groups; however, gray matter cross sectional area did not differ between the distraction injury and sham groups. Data shown are means ± standard deviation (n = 10 in each group) and were analyzed by twoway repeated-measures analysis of variance followed by Tukey’s honestly significant difference testing.
SMI312/myelin basic protein immunofluorescence staining showed that density of myelinated axons qualitatively differed among the three injury types and varied according to location. At the epicenter of injury in the gracile fasciculus of the contusion injury group, myelinated axon density was low and a cavity filled with myelin debris was observed; damage extended 5 mm caudally (Figure 9A
). In the dislocation injury group, the degree of damage and cavitation was slightly lower. Although most axons at the epicenter were variably destroyed, most ascending sensory axons were preserved 3 and 5 mm rostral and caudal to the epicenter; partial sparing was observed as close as 1 mm from the epicenter (Figure 9A
). The extent and distribution of damage differed in the distraction injury group, which showed a relative reduction in packing density of myelinated axons because of an increase in extracellular spaces (Figure 9A
).Similar patterns of myelinated axon injury were observed in the dorsal corticospinal tract. In the contusion and dislocation injury groups, approximately 10% to 50% of myelinated corticospinal axons were spared 3 mm proximal to the injury; few were spared at the epicenter, where myelin debris, tract interruption and cavitation were observed (Figure 9B
). Many corticospinal axons were preserved at all locations in the distraction injury group. Both groups showed extensive degeneration of lateral white matter axons, a large amount of myelin debris, and many demyelinated axons (Figure 10A
). Axon damage in the lateral white matter was limited to within 1 mm from the epicenter in the contusion and dislocation injury groups. Interestingly, ventral white matter damage was not observed in any of the three injury types (Figure 10B
).In the gracile fasciculus, few myelinated axons survived at the epicenter after contusion and dislocation injuries, particularly so for the dislocation injury group (Figure 11A
andB
). In the lateral white matter, axon damage was limited to within 1 mm of the epicenter in the contusion and dislocation injury groups; myelinated axon density was lower in the contusion group (Figure 11C
). The distraction injury group did not show signs of lateral white matter damage. Furthermore, no differences were found in the ventral white matter between the three injury mechanisms (Figure 11D
).Correlation between evoked potentials and histological findings
Scatterplots of electrophysiological and histological findings are shown inFigure 12
. SEP latency and amplitude significantly correlated with number of myelinated axons in the gracile fasciculus (r
= –0.51 and 0.60, respectively;P
= 0.0042 and 0.0031, respectively;Figure 12A
). MEP latency significantly correlated with number of myelinated axons in the lateral (r
= –0.46,P
= 0.0302), dorsal (r
= –0.62,P
= 0.0335), and ventral (r
= –0.45,P
= 0.0062) white matter. MEP amplitude significantly correlated with number of myelinated axons in the lateral white matter (r
= 0.66,P
= 0.0191), but not in the corticospinal tract and ventral white matter (Figure 12B
).
Figure 9|Immunofluorescence micrographs of myelinated axons in the gracile fasciculus (A) and dorsal corticospinal tract (B) (CST). Representative immunofluorescence imaging of regions of interest for quantitative analysis of white matter damage showing axons (SMI312+, red, stained by DyLight 594) and myelin sheaths (MBP+, green, stained by DyLight 488). Normal tracts show dense small myelinated axons in the gracile fasciculus (GF) and dorsal CST, typically with a green myelin ring surrounding a red axon in the middle. In contusion and dislocation injuries, both the gracile fasciculus and CST were destroyed and replaced by a cavity with extensive myelin fragments. At 3 and 5 mm from the epicenter, the proximal part of the ascending sensory axon mostly remained after dislocation and was partially spared 1 mm from the epicenter. After contusion injury, the injury extended up to 5 mm caudal to the epicenter. In contrast, the density of myelinated axons did not significantly change after distraction injury and many axons consistent with typical small corticospinal axons remained at all positions. Scale bar: 20 µm. MBP: Myelin basic protein.
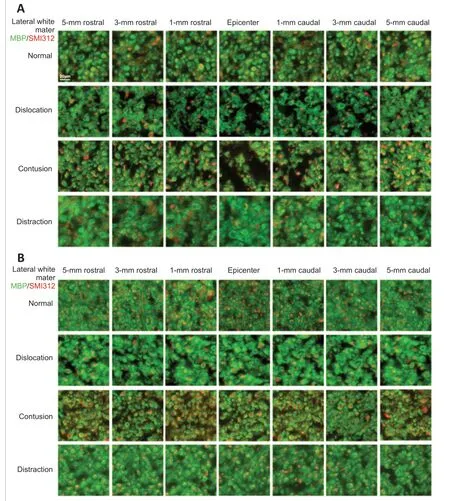
Figure 10| Immunofluorescence micrographs of myelinated axons in the lateral white matter (A) and ventral white matter (B). Representative immunofluorescence imaging of regions of interest for quantitative analysis of white matter damage showing axons (SMI312+, red, stained by DyLight 594) and myelin sheaths (MBP+, green, stained by DyLight 488). Normal tracts show dense small myelinated axons in the lateral and ventral white matter, typically with a green myelin ring surrounding a red axon in the middle. Lateral and ventral white matter show a red axon in the middle surrounded by a dense typical green myelin sheath. Contusion and dislocation injuries produced pronounced damage at the injury epicenter and further distally, while no significant damage was observed in the lateral and ventral white matter after distraction injury. Scale bar: 20 µm. MBP: Myelin basic protein.
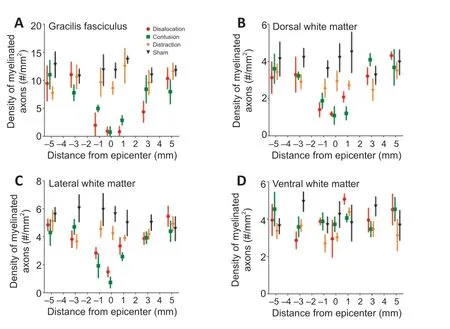
Figure 11|Density of myelinated axons according to type of spinal cord injury. (A) Density in the gracile fasciculus (i) differences from control: dislocation –1, to +3 mm; contusion –3 to +5 mm; distraction none (ii) differences between contusion and dislocation: –3, +3 mm. (B) Density in the dorsal corticospinal tract: (i) differences from control: contusion –3 to +1 mm; dislocation –5, –1 to +1 mm; distraction: none; (ii) differences between dislocation and contusion: –1 mm. (C) Density in the lateral white matter: (i) differences from control: dislocation -3 to +3 mm; contusion –5 to +3 mm; distraction none; (ii) differences between dislocation and contusion: –1, 0 mm. (D) No differences were found between the three injury types in the ventral white matter. Data shown are means ± standard deviation (n = 10 in each group). Data were analyzed using two-way repeated-measures analysis of variance followed by Tukey’s honestly significant difference testing.
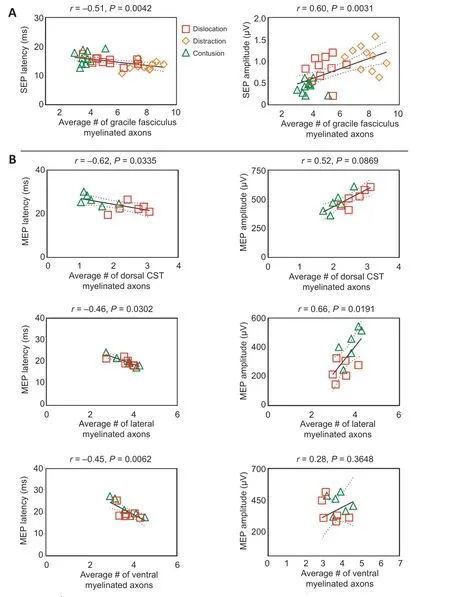
Figure 12|Scatterplots showing the relationship between electrophysiological and immunofluorescence outcomes using Pearson’s correlation analysis. The linear regression line of the best fit is represented by a solid line. The 95% confidence interval is represented by a dotted line.
Discussion
SCI during spinal surgery occurs insidiously and has various causes. Early and accurate neurophysiological identification of injury type is challenging but important (Guo et al., 2021). Our study is based on the rationale that different modes of SCI will lead to different pathological changes in the spinal cord and induce distinct sensory and motor signal responses via separate neural pathways. Moreover, identification of these signal response changes is the basis for identifying different SCI types. We compared electrophysiological and histological findings between dislocation, contusion, and distraction types of SCI and demonstrated distinct differences in SEPs, MEPs, and primary histological changes. Contusion caused the greatest rostrocaudal extent of tissue injury and was associated with significantly attenuated SEP and MEP responses. Dislocation resulted in the greatest overall loss of white matter tissue, particularly in the lateral white matter, as well as a greater reduction in MEP responses than contusion. Although enlarged extracellular spaces were observed without substantial structural alteration following distraction injury, SEP responses slightly decreased and MEP responses were lost. Furthermore, histological and electrophysiological findings were correlated. These results demonstrate the complex and varied electrophysiologic and histopathologic effects of SCI and reveal the potential value of SEPs and MEPs in diagnosing and identifying intraoperative SCI type.
Spinal deformities often require manual correction, which carries a risk of iatrogenic SCI. The type of injury varies according to cause. Spinal cord dislocation often occurs in osteoporotic patients with kyphotic deformity, who are at risk of postoperative fracture and dislocation (Ruf et al., 2006). Contusion may occur due to errant bone screw placement (Safain et al., 2014). Distraction injury may result from overstretching during scoliosis correction (Sawyer et al., 2016). Therefore, early diagnosis and necessary measures are essential before neurological deficit occurs. The three SCI types examined in our study exhibited a series of deformation patterns in the spinal cord that were related to electrophysiological differences. Contused animals showed the greatest deterioration in SEP and MEP responses, with prolonged latency and reduced amplitude; furthermore, compared with animals with dislocation or distraction injuries, contused animals showed the least sparing of myelinated axons in the dorsal white matter. Dislocation injury caused the greatest loss in number of myelinated axons, particularly in the lateral white matter. Also in this type, signal changes were larger in MEP responses than SEP responses: SEP amplitude was only partially reduced, whereas MEP latency was prolonged and amplitude was reduced. Distraction injury caused virtually no spinal cord tissue damage, although SEP responses were attenuated and MEP responses abolished. These findings are similar to those reported in a study of electrophysiological monitoring during spinal scoliosis surgery. In that study, after mechanical distraction was applied, MEP response threshold voltage significantly increased followed by a complete loss of motor responses; however, SEP responses did not change (Lyon et al., 2004). The discrepancy between histologic and electrophysiologic findings may be attributed to transient ischemia induced by spinal microcirculation block without structural axonal disruption (Kawahara et al., 2005; Kato et al., 2008). This emphasizes the predictive value of electrophysiological assessment, in which MEPs are more sensitive to distraction SCI than SEPs. This, along with our results, indicate that SCI type affects both spinal cord histopathology and electrophysiology.
In theory, contusion and dislocation injuries produce localized compressive and high lateral tensile strain on the spinal cord, while the strain caused by distraction is more uniformly distributed and has a lower peak (Li and Dai, 2009; Russell et al., 2012; Mihara et al., 2018; Ma et al., 2019; Guo et al., 2020; Huang et al., 2021). In our study, both dislocation and contusion injuries caused tissue damage and loss along the rostrocaudal axis of the injured spinal cord and contusion injuries resulted in maximal loss of myelinated axons in the dorsal white matter. Although we observed no obvious acute white matter tissue damage or degenerative changes after distraction injury, a previous study reported detection of substantial spinal cord damage 8 weeks after injury that was characterized by a large degree of central destruction and far-ranging demyelination throughout all levels of the spinal cord (Chen et al., 2016). Therefore, long-term follow-up examination after experimental injury is vital to fully evaluate the effect of various injury types.
Hemorrhage is often used as an indicator of primary injury because it plays an important role in the initiation of subsequent neuropathological events (Losey et al., 2014; Okada, 2016; Ellingson et al., 2019). In a rat SCI model, Choo et al. found that contusion and dislocation injuries caused similar central damage to the gray matter vascular system and similarly localized increased membrane permeability 5 minutes after injury; however no obvious hemorrhage was observed after distraction. Furthermore, both injury-related ischemia and inflammatory cell infiltration contribute to free radical generation and oxidative stress, which exacerbates cellular damage (Kwon et al., 2010; Jiang et al., 2016; Li et al., 2019; Huang et al., 2020). The time window for treatment of distraction injury may be longer because it causes less hemorrhage. In agreement, our results showed that contusion and dislocation injuries caused hemorrhage in the gray matter and distraction injuries caused only minor focal hemorrhage. Hemorrhage and hemorrhagic necrosis increase with injury severity (Lau et al., 2013; Mondello et al., 2015). However, our study focused on the association of electrophysiological testing with injury type rather than severity. Because SEPs and MEPs disappear in several injury types, severity of injury induced in this study was set to mild-tomoderate based on previous models (Dabney et al., 2004; Fiford et al., 2004; Jin et al., 2014).
Because animals were sacrificed soon after SCI owing to ethical considerations, progression of secondary degeneration and convergence/divergence of injury patterns was not assessed. However, biomechanical injury was the variable prioritized and it was necessary to sacrifice animals early to assess spatial distribution of injury and electrophysiological changes without confounding secondary events. Furthermore, early sacrifice precluded longterm assessment of spinal cord damage. A better experimental design with restricted injury severity control and post-injury examination should be investigated in a future study.
This study showed that spinal cord tissue damage differs between contusion, dislocation, and distraction types of SCI. These injury types exhibit distinct SEP and MEP changes that significantly correlate with their different histologic findings. Patterns of electrophysiologic changes in combination with SEPs and MEPs have the potential to indicate the type of SCI during surgery, which can guide appropriate treatment.
Author contributions:
Study conception and design, manuscript review and edit: YH; experimental implementation: RL, HLL, HYC; data analysis/interpretation: YCH; manuscript writing: RL. All authors contributed to this study and approved the final manuscript.
Conflicts of interest:
The authors declare that they have no conflict of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Electrophysiological signal changes in animals of the three groups.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

