Treatment of radiation-induced brain injury with bisdemethoxycurcumin
Yun-Qian Chang , Gui-Juan Zhou , Hong-Mei Wen, Duan-Qun He , Chen-Lin Xu , Ya-Rui Chen Yi-Hui Li Shuang-Xi Chen , Zi-Jian Xiao , Ming Xie
Abstract Radiation therapy is considered the most effective non-surgical treatment for brain tumors. However, there are no available treatments for radiation-induced brain injury. Bisdemethoxycurcumin (BDMC) is a demethoxy derivative of curcumin that has anti-proliferative, anti-inflammatory, and anti-oxidant properties. To determine whether BDMC has the potential to treat radiation-induced brain injury, in this study, we established a rat model of radiation-induced brain injury by administering a single 30-Gy vertical dose of irradiation to the whole brain, followed by intraperitoneal injection of 500 µL of a 100 mg/kg BDMC solution every day for 5 successive weeks. Our results showed that BDMC increased the body weight of rats with radiation-induced brain injury, improved learning and memory, attenuated brain edema, inhibited astrocyte activation, and reduced oxidative stress. These findings suggest that BDMC protects against radiationinduced brain injury.
Key Words: astrocytes; bisdemethoxycurcumin; brain edema; brain tumor; curcumin; learning and memory; neuronal injury; oxidative stress; radiation therapy; radiation-induced brain injury

Introduction 416 Methods 417 Results 418 Discussion 419
Graphical Abstract
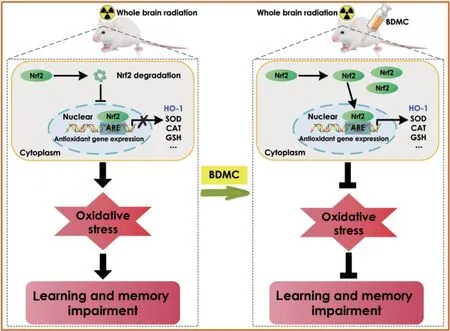
Effects of BDMC on learning and memory impairments following radiation-induced brain injury
Introduction
Radiation-induced brain injury (RBI), also known as radiation encephalopathy, is an extremely acute complication that is common in tumor patients after partial or whole-brain radiotherapy, and occasionally occurs in ionizing radiation accidents (Balentova and Adamkov, 2015; Kale et al., 2018; Tang et al., 2019). In recent years, the development and application of multiple new radiotherapy technologies has led to an increase in the incidence of RBI (Lee et al., 2012; Ma et al., 2019; Zuo et al., 2021). Overall, 50–90% of RBI survivors show moderate to severe cognitive dysfunction (Meyers and Brown, 2006). Severe RBI greatly affects patients’ quality of life and can even be fatal (Xu et al., 2017b; Yang et al., 2017; Andrews et al., 2018). Treatment options for RBI is currently limited, and the treatments that do exist have poor clinical efficacy; furthermore, the underlying mechanisms of these treatment options are not well understood (Zhou et al., 2011). Therefore, it is very important to find a safe and available drug to treat RBI (Greene-Schloesser et al., 2012; Tong et al., 2016).
RBI is considered a dynamic, complex, and cascading process, and multiple theories have been proposed to explain its pathophysiology, including direct lesions caused by radiation (Oh et al., 2013), immunoinflammatory responses (Bostrӧm et al., 2018), oxidative stress (Liao et al., 2017), and damage to the cerebrovascular system (Kamiryo et al., 2001). A recent study reported that molecules with anti-inflammatory and anti-oxidative stress properties can prevent or ameliorate RBI (Liu et al., 2021), and research efforts are currently underway to identify additional agents that can alleviate the damage caused by RBI through inhibiting inflammation and reducing oxidative stress. One of these candidates is bisdemethoxycurcumin (BDMC), a demethoxy derivative of curcumin that is the most stable and potent known curcuminoid (Fiala et al., 2007; Sandur et al., 2007; Cashman et al., 2008). Curcumin, a well-known phenolic compound derived from Curcuma longa, has a multiple of beneficial features, including anti-oxidant (Uğuz et al., 2016), anti-inflammatory (Derosa et al., 2016), and neuroprotective properties (Begum et al., 2008). BDMC, which is based on the curcumin matrix and generated by removing the methoxy group at the three position on the bilateral benzene ring while retaining the four-position hydroxyl group (Zhang et al., 2019), exhibits greater nuclear uptake, stability, hydrophilicity, polarity, and water solubility than curcumin (Giguère et al., 2018). As a consequence, BDMC has more potential pharmacological properties than curcumin (Ramezani et al., 2018). In addition, the anti-proliferative, anti-inflammatory, and anti-oxidant properties of BDMC in the nervous system have been well documented (Sandur et al., 2007; Basile et al., 2009; He et al., 2020). BDMC can antagonize Alzheimer’s disease by modulating adenosine 5′-monophosphate-activated protein kinase (Xu et al., 2020a) and up-regulating sirtuin 1 to reduce oxidative stress (Xu et al., 2020b). In addition, curcumin and its derivatives can protect against the induction of brain lesions by 6-hydroxydopamine hydrobromide in rats (Agrawal et al., 2012). Thus, accumulating evidence indicates that BDMC may be a useful drug for the treatment of RBI. Given the key roles of BDMC in the diseased nervous system (Xu et al., 2020a, b), we hypothesized that BDMC may ameliorate the learning and memory impairments that occur after RBI in rats. Therefore, the aim of this study was to investigate the effects of BDMC on RBI and the mechanisms by which it exerts its beneficial effects.
Methods
Animals
Because hormones may influence drug effects, only male rats were used in this study. Specific pathogen-free male Sprague-Dawley rats (n
= 60, 7 weeks old) with an average body weight of 200–220 g were obtained from the Hunan Medical Laboratory Animal Center (Changsha, Hunan Province, China; license No. SYXK (Xiang) 2015-0001) and maintained at 22°C on a 12/12-hour light/dark cycle, with ad libitum food and water. The Laboratory Animal Ethics Committee of The First Affiliated Hospital of University of South China approved the study on October 12, 2019 (approval No. 20191012003). All experiments were designed and reported according to the Animal Research: Reporting ofIn Vivo
Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).RBI model
The rats were anesthetized prior to irradiation via intraperitoneal injection with 42 mg/kg magnesium sulfate, 85 mg/kg trichloroacetaldehyde monohydrate, and 17 mg/kg sodium pentobarbital (MilliporeSigma, Burlington, MA, USA; Merck KGaA, Darmstadt, Germany). Irradiation was carried out using a medical linear accelerator (Siemens, Berlin, Germany). The rats in the group received a single 30-Gy vertical dose of irradiation to the whole brain. The absorbed dose rate was 300 cGy/min, and the distance between the source and the skin was 100 cm. Each rat was placed in a special 25-cm × 25-cm lead mold with 10 2.5-cm-diameter holes in the head region. The front boundary of the irradiation field was located at approximately the posterior canthus of both eyes, the back boundary was located approximately behind the ears, and the remainder of the rat’s body was shielded by the lead block. Rats in the Control and BDMC-only groups were anesthetized, placed in the lead mold, and placed in the linear accelerator, but were not irradiated.
Preparation of BDMC solution
BDMC (consisting of more than 80% curcumin and more than 94% curcuminoid content) (Cat# 33171-05-0, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in phosphate-buffered saline (PBS) containing 0.5% dimethyl sulfoxide, as described in a previous study (Parlar and Arslan, 2019). Mass spectrometry and nuclear magnetic resonance spectroscopy were used to analyze the solution, as shown inAdditional Table 1
.Treatment groups
Sixty rats were randomly allocated to one of two groups: the RBI group and the non-RBI group (n
= 30 for each group). After irradiation, the 30 rats in the RBI group were randomly allocated equally to one of two groups: the RBI-only group and the RBI + BDMC group (n
= 15 for each group). The 30 non-RBI rats were randomly allocated to the control (n
= 15) or BDMC-only group (n
= 15). As described above, these rats were anesthetized and placed in the irradiation environment, but were not irradiated. The RBI + BDMC and BDMC-only groups received 500 µL of a 100-mg/kg BDMC solution via intraperitoneal injection daily for 5 weeks after injury. The control group received 500 µL of PBS containing 0.5% dimethyl sulfoxide via intraperitoneal injection daily for 4 weeks. The RBI group received 500 µL of PBS containing 0.5% dimethyl sulfoxide via intraperitoneal injection daily for 4 weeks. None of the animals died during the experimental perfio (Figure 1
).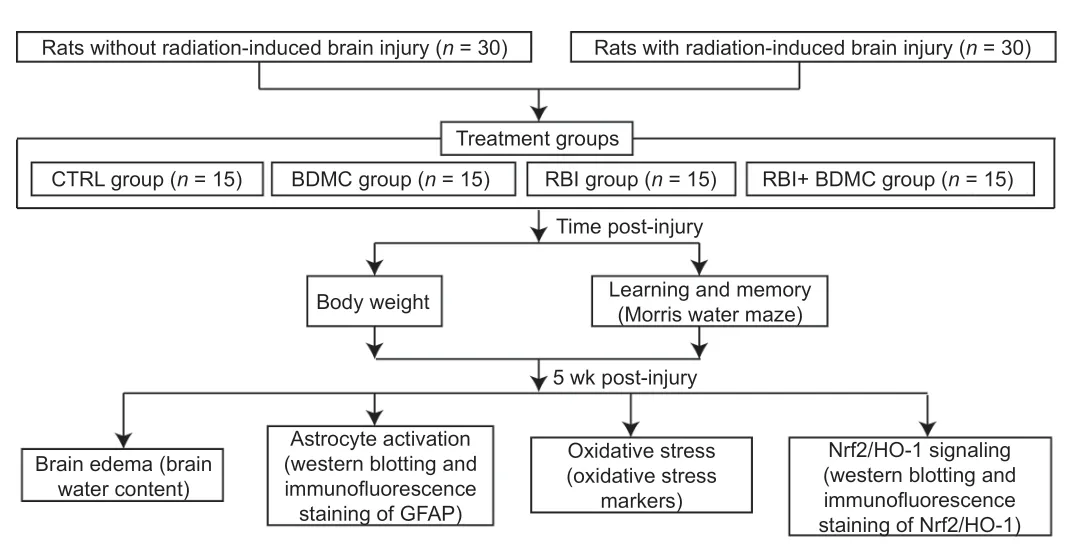
Figure 1|Study design.BDMC: Bisdemethoxycurcumin; CTRL: control; GFAP: glial fibrillary acidic protein; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid-2-related factor 2; RBI: radiationinduced brain injury.
Body weight
To evaluate the effect of BDMC on the body weight of rats with RBI, body weight was measured weekly post-injury using an electronic balance (Lizhong Environmental Technology Co., Ltd., Shenyang, China).
Morris water maze
To evaluate the effect of BDMC on the spatial learning and memory capacity of rats after RBI, the Morris water maze test was performed as described previously (Vorhees and Williams, 2006; Xu et al., 2020b) every week postinjury. Briefly, the maze (Cat# XR-XM101, Shanghai Xinruan Information, Technology, Co., Ltd., Shanghai, China), a plastic, circular pool 1.2 m in diameter and filled with water at 22°C to a depth of 31 cm, was divided into four quadrants (A, B, C, and D). A transparent platform was submerged 1 cm under the surface of the water in the C quadrant.
This test comprised three phases: the acquisition trial, the probe trial, and the visible platform test. During the acquisition trial, rats were placed in the water in one of the four quadrants and allowed to swim freely for up to 120 seconds to find the platform. The escape latency was defined as the time needed for the rat to find the transparent platform. If the rat did not find the platform within 120 seconds, the escape latency was recorded as 120 seconds, and the investigator then guided the rat to the platform, where it was allowed to remain for 20 seconds. This trial was performed once a day for 5 consecutive days.
On the sixth day, the transparent platform was removed. The rats were placed in the water in the opposite quadrant and allowed to swim freely for 120 seconds. The degree of memory consolidation was indicated by the number of times that the rat crossed the area were the platform had been and the time spent in the target quadrant.
On the seventh day, a visible platform was installed in the pool to test the visual and motor function of each rat and exclude the effect of the possible deficits in vision or motor processing in the prior experiment. The platform was located 2 cm above the surface of the water in the target quadrant. The time needed to reach the platform and the average swimming speed were recorded.
Brain water content
The water content of the brain was evaluated 5 weeks post-injury using a wet-dry method, as previously described (Wang et al., 2020). Rats were euthanized by inhalation of 0.5% isoflurane (Cat#26675-46-7, Sigma-Aldrich) and sacrificed, and the brains were collected and immediately weighed to determine the wet weight. Then, the brains were dried at 80°C for 72 hours and weighed to determine the dry weight. The brain water content was calculated as follows: (wet weight – dry weight)/wet weight) × 100%.
Tissue preparation
To measure oxidative stress markers and perform histological staining, rats were sacrificed 5 weeks post-injury via inhalation anesthesia using 0.5% isoflurane. Then, tissue preparation was performed as described previously (Chen et al., 2019, 2020a, b).
To measure oxidative stress markers, the hippocampi were homogenized in PBS, following by centrifugation at 12,000 ×g
for 20 minutes at 4°C.For western blotting, the hippocampi were homogenized in 100 µL of radioimmunoprecipitation assay buffer (Cat#R0010, Solarbio, Beijing, China) containing 1% phenylmethanesulfonyl fluoride (Cat#329-98-6, Solarbio). The homogenates were then centrifuged at 14,000 ×g
at 4°C for 15 minutes, and the supernatants were collected.For histological staining, the rats were subjected to transcardial perfusion with saline, followed by perfusion with 4% paraformaldehyde diluted in PBS via the left cardiac ventricle. The brains were then harvested, incubated at 4°C in 15% and 30% sucrose solutions diluted in PBS for 24 hours each, and postfixed in 4% paraformaldehyde for 24 hours at 4°C. Finally, the fixed brains were cut into 10-µm-thick sections.
Measurement of oxidative stress markers
The malondialdehyde (MDA), catalase, superoxide dismutase, and glutathione levels were determined using corresponding assay kits (Cat# A003-1-2, A007-1-2, A001-3-2, A005-1-2, Jiancheng Biotech Ltd., Nanjing, China) according to the manufacturer’s instructions and previously described methods (He et al., 2020; Xu et al., 2020a).
Western blot assay
To detect the protein levels of markers of astrocyte activation, as well as nuclear factor erythroid-2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling, 5 weeks after RBI, western blotting was performed as we described previously (Chen et al., 2015; Jiang et al., 2016; Xu et al., 2017a; Yi et al., 2021). Briefly, 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to separate the protein samples at 90 V, followed by electroblotting onto polyvinylidene difluoride membranes at 300 mA for 3 hours. Then, the membranes were blocked with 5% bovine serum albumin (Cat# 9048-46-8, Solarbio) diluted in Tris-HCl saline buffer supplemented with 0.1% Tween-20 (TBST; pH 7.4), followed by incubation with mouse anti-glial fibrillary acidic protein (GFAP) (1:1000, CST, Boston, MA, USA; Cat# 3670s, RRID: AB_561049), mouse anti-Nrf2 (1:500, Abcam, Cambridge, UK; Cat# ab89443, RRID: AB_2041334), mouse anti-HO-1 (1:1000, Proteintech, Chicago, IL, USA; Cat# 66743-1-Ig, RRID: AB_2882091), or mouse anti-βactin (1:10,000, Proteintech; Cat# 60008-1-Ig, RRID: AB_2289225) at 4°C overnight. Membranes were washed three times for 5 minutes each with TBST. Then, the membranes were incubated with a horseradish peroxidase–conjugated goat anti-mouse secondary antibody (1:5000, Proteintech; Cat# SA00001-1, RRID: AB_2722565) diluted in TBST at room temperature for 1 hour. Membranes were washed three times for 5 minutes each using TBST. Next, the immunoreactive signals were visualized using an enhanced chemiluminescence solution (Bio-Rad, Hercules, CA, USA). Finally, the band intensity was quantified using ImageJ 1.52a (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Histochemical and immunofluorescence staining
To detect changes in GFAP (for evaluating astrocyte activation) and Nrf2 expression at 5 weeks after RBI, histochemical and immunofluorescence staining were carried out as we described previously (Chu et al., 2017; Chen et al., 2020c; Tan et al., 2021).
For hematoxylin and eosin staining, briefly, the nuclei were stained using Harris hematoxylin for 8 minutes, followed by several-second destaining with a 0.3% acid alcohol. Then, the cytoplasm was stained using eosin for nearly 2 minutes, followed by dehydration in a graduated ethanol series: 95%, 95%, and 100% for 2 dips each, 100% for 2 minutes, and 100% for 12 minutes. Images used for observation were digitalized by light microscopy (YS-100, MBF Nikon Microscope, Tokyo, Japan).
For immunofluorescence staining, the hippocampus sections were blocked with 10% normal donkey serum (Cat# 017-000-001, RRID: AB_2337254, Jackson ImmunoResearch Labs, West Grove, PA, USA) diluted in PBS for 1 hour at room temperature, followed by overnight incubation at 4°C with a mixture of mouse anti-GFAP (1:200, CST; Cat# 3670s, RRID: AB_561049) and mouse anti-Nrf2 (1:200, Abcam; Cat# ab89443, RRID: AB_2041334). After washing with PBS, the samples were incubated with Alexa Fluor 488 fluorescent secondary antibody (1:1000, Invitrogen, Carlsbad, CA, USA, Cat# A-21121, RRID: AB_2535764) or Alexa Fluor 546 fluorescent secondary antibody (1:1000, Cat# A-21123, RRID: AB_141592, Invitrogen). After washing with PBS, the slides were mounted and imaged using an AxioObserver A1 microscope with AxioVision 4.6 software (Carl Zeiss, Oberkochen, Baden-Württemberg, Germany).
Statistical analys
isData in this study are shown as mean ± standard deviation (SD). The statistical analyses were carried out using GraphPad Prism 6.00 (GraphPad Software, San Diego, CA, USA, www.graphpad.com). The data were analyzed by oneway analysis of variance followed by Bonferronipost hoc
test. Statistical significance was defined asP
< 0.05.Results
Treatment with BDMC results in increased body weight in rats with RBI
To investigate the effects of BDMC on body weight in rats with RBI, body weight was assessed post-injury. Compared with the control group, the BDMC-only group exhibited similar body weight throughout the experimental period, and the RBI-only group exhibited lower body weight from 2 to 5 weeks post-injury. Compared with the RBI-only group, the RBI + BDMC group exhibited significantly increased body weight from 3 to 5 weeks post-injury (P
< 0.001;Figure 2
).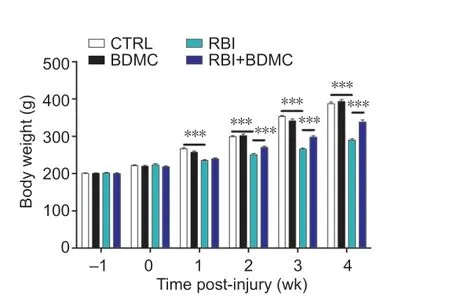
Figure 2|Effect of BDMC on the body weight of rats with RBI. The average body weight of rats with RBI at each time point (0 (immediately after injury), 1, 2, 3, 4, and 5 weeks) increased following treatment with BDMC. Data are expressed as mean ± SD (n = 5). ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). BDMC: Bisdemethoxycurcumin; CTRL: control; RBI: radiation-induced brain injury.
BDMC improves learning and memory in rats with RBI
To investigate the effects of BDMC on learning and memory in rats with radiation-induced brain injury, the Morris water maze trial was performed. In the acquisition trial period, there was no difference in escape latency between the control group and the BDMC treatment group. Compared with the control group, the escape latency was increased in the RBI group and decreased in the RBI + BDMC group (P
< 0.001;Figure 3A
andB
). Increased platform-crossing times and time spent in the target quadrant were observed in the RBI + BDMC group compared with the control group (bothP
< 0.01;Figure 3C
andD
). No change in escape latency and swimming speed during in the visible platform test were observed among the groups (Figure 3E
andF
).Treatment with BDMC reduces brain edema in rats with RBI
To investigate the effects of BDMC on brain edema in rats with radiationinduced brain injury, the brain water content was evaluated, and hematoxylin and eosin staining were performed. Compared with the control group, the BDMC group exhibited no change in brain water content, and the RBI group exhibited higher brain water content. Compared with the RBI group, the BDMC + RBI group exhibited significantly decreased brain water content (P
< 0.05;Figure 4A
).Hematoxylin and eosin staining showed that the neural cells and vascular endothelial cells in the control and BDMC groups had complete structures, normal morphology, and no interstitial edema, and were neatly arranged. In contrast, tissue samples from the RBI group exhibited swelling of nerve cells in the hippocampus, swelling of the cytoplasm of vascular endothelial cells, congestion and expansion of capillaries, and obvious interstitial edema. Compared with the RBI group, the swelling of nerve cells and vascular endothelial cells in the hippocampus of rats from the RBI + BDMC group was significantly improved, and the capillary congestion and dilatation and interstitial edema were improved (Figure 4B
).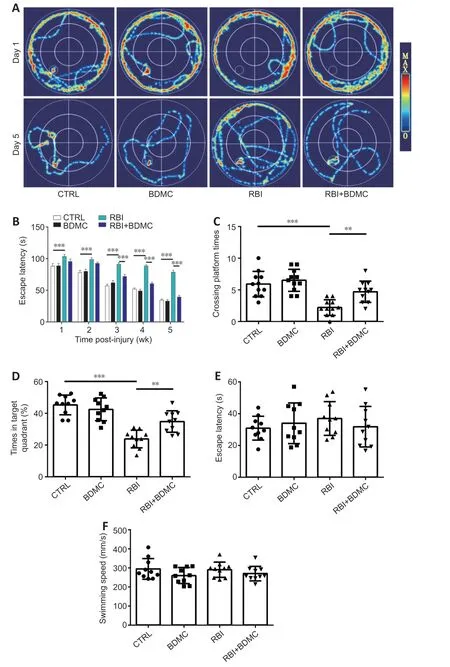
Figure 3|Effect of BDMC on learning and memory in rats with RBI. (A) Representative images of the swimming paths of rats with RBI searching for the underwater platform rats in the Morris water maze test on the first and fifth days of training. Treatment with BDMC simplified the swimming paths of rats with RBI. (B–D) Escape latency when searching for the underwater platform (B), number of times crossing the platform (C), and time spent in the target quadrant (D) during the 5 days of the spatial navigation probe experiment. (E, F) Escape latency (E) and swimming speed (F) during the visible platform test,. Data are expressed as mean ± SD (n = 10). **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). BDMC: Bisdemethoxycurcumin; CTRL: control; RBI: radiation-induced brain injury.
BDMC inhibits astrocyte activation in rat hippocampi after RBI
To investigate the effects of BDMC on astrocyte activation in the hippocampi of rats with radiation-induced brain injury, western blot and inmmunofluorescence staining were performed to evaluate changes in GFAP expression. Compared with the control group, the BDMC group exhibited similar GFAP levels, and the RBI group exhibited higher GFAP levels. Compared with the RBI group, the BDMC + RBI group exhibited significantly decreased GFAP levels (P
< 0.001;Figure 5A
andB
). A similar pattern was observed for GFAP-positive cells in the four groups (Figure 5C
).BDMC inhibits oxidative stress rat hippocampi after RBI
To investigate the effects of BDMC on oxidative stress in the hippocampi of rats with radiation-induced brain injury, the activity of MDA, CAT, SOD, and GSH activities was measured. Compared with the control group, the BDMC group exhibited similar MDA level, and the RBI group exhibited higher MDA levels (P
< 0.001). Compared with the RBI group, the BDMC + RBI group exhibited significantly decreased MDA levels (P
< 0.01;Figure 6A
). The reverse patterns for catalase, superoxide dismutase, and glutathione levels were observed (Figure 6B–D
).
Figure 4|Effect of BDMC on brain edema in rats with RBI. (A) The brain water content of rats with RBI decreased following treatment of BDMC. Data are expressed as mean ± SD (n = 3). *P < 0.05 (one-way analysis of variance followed by Bonferroni post hoc test). (B) Representative images of H&E staining showing brain edema. Treatment with BDMC improved pathological morphology in the hippocampi of rats with RBI. Arrows indicate nerve cells in the hippocampus, the cytoplasm of vascular endothelial cells, capillaries, and interstitial edema. Scale bar: 20 µm. BDMC: Bisdemethoxycurcumin; CTRL: control; H&E staining: hematoxylin-eosin staining; RBI: radiation-induced brain injury.
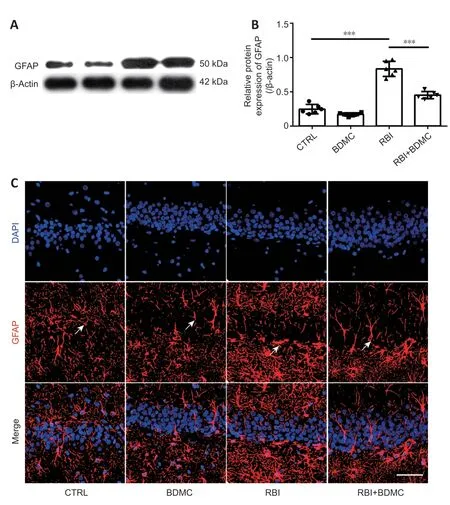
Figure 5|Effect of BDMC on astrocyte activation in the hippocampi of rats with RBI. (A, B) GFAP expression levels were down-regulated in the hippocampi of rats with RBI following treatment of BDMC (western blotting). Data are expressed as mean ± SD (n = 6). ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). (C) Representative images showing GFAP-positive cells (arrows; red, stained with Alexa Fluor 546) in the hippocampus. The number of GFAP-positive cells in the hippocampi of rats with RBI decreased following treatment with BDMC. Scale bar: 100 µm. BDMC: Bisdemethoxycurcumin; CTRL: control; GFAP: glial fibrillary acidic protein; RBI: radiationinduced brain injury.
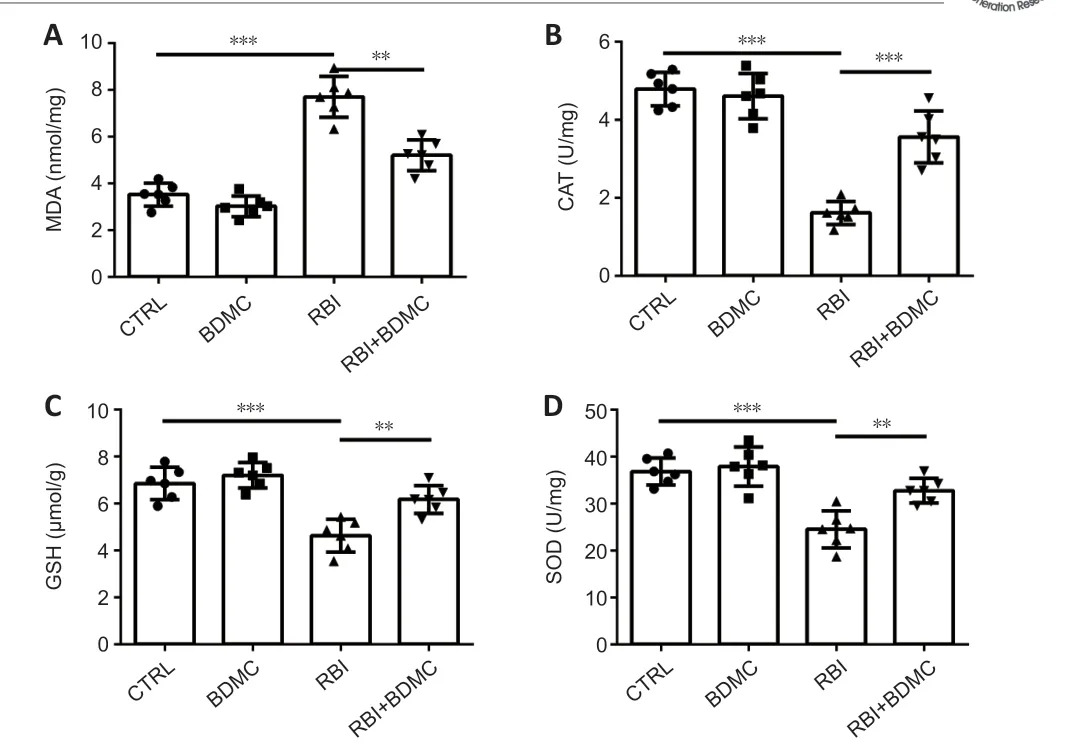
Figure 6|Effect of BDMC on oxidative stress in the hippocampi of rats with RBI.(A) MDA levels decreased, and (B) CAT, (C) GSH, (D), and SOD levels increased in the hippocampi of rats with RBI following treatment with BDMC. Data are expressed as mean ± SD (n = 6). **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). BDMC: Bisdemethoxycurcumin; CAT: catalase; CTRL: control; GSH: glutathione; MDA: malondialdehyde; RBI: radiation-induced brain injury; SOD: superoxide dismutase.
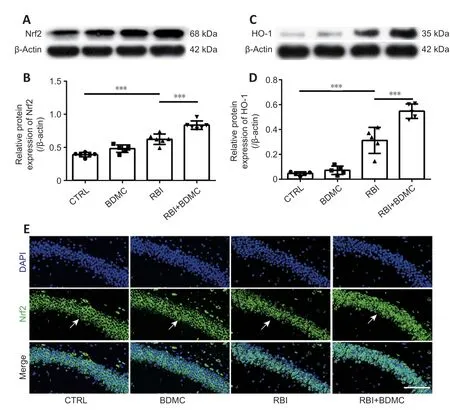
Figure 7|Effect of BDMC on Nrf2/HO-1 signaling in the hippocampi of rats with RBI. (A–D) Nrf2 (A, B), and HO-1 (C, D) levels were increased in the hippocampi of rats with RBI following treatment with BDMC (western blotting). Data are expressed as mean ± SD (n = 5). ***P < 0.001 (one-way analysis of variance followed by Bonferroni post hoc test). (E) Representative images showing Nrf2-positive cells (arrows; green, stained with Alexa Fluor 488) in the hippocampus. The number of Nrf2-positive cells was increased in the hippocampi of rats with RBI following treatment with BDMC. Scale bar: 100 µm. BDMC: Bisdemethoxycurcumin; CTRL: control; HO-1: heme oxygenase-1; Nrf2: nuclear factor erythroid-2-related factor 2; RBI: radiation-induced brain injury.
BDMC up-regulates Nrf2/HO-1 signaling in rat hippocampi after RBI
To investigate the effects of BDMC on Nrf2/HO-1 signaling in the hippocampi of rats with radiation-induced brain injury, western blotting and inmmunofluorescence staining were performed to evaluate changes in Nrf2 and HO-1 levels. Compared with the control group, the BDMC group exhibited similar Nrf2 levels, and the RBI group exhibited higher Nrf2 levels. Compared with the RBI group, the BDMC + RBI group exhibited significantly increased Nrf2 levels (P
< 0.001;Figure 7A
andB
). A similar pattern was observed for HO-1 levels by both western blotting and immunofluorescence staining (P
< 0.001;Figure 7C
andD
). The western blotting results for Nrf2 levels were confirmed by inmmunofluorescence staining (Figure 7E
).Discussion
In previous studies, we demonstrated the neuroprotective roles of BDMC in neurodegenerative diseases, including AD and PD (He et al., 2020; Xu et al., 2020a, b). Here, we show that BDMC can improve learning and memory, inhibit brain edema, inhibit astrocyte activation, and inhibit oxidative stress after RBI in rats. It has been reported that more than 50–90% adult patients with brain tumors who surviving for more than half a year following irradiation suffer from radiation-induced cognitive impairment (Crossen et al., 1994; Johannesen et al., 2003; Meyers and Brown, 2006), which is characterized by a decreased ability to solve the novel problems, spatial memory, verbal memory, and attention (Hochberg and Slotnick, 1980; Twijnstra et al., 1987; Roman and Sperduto, 1995). In the current study, we found that BDMC is able to improve learning and memory in rats after RBI.
It is widely acknowledged that interventions that reduce chronic oxidative stress can prevent or ameliorate late symptoms of RBI, including cognitive impairment (Greene-Schloesser et al., 2012). Many studies have shown that there is clear oxidative stress in the context of radiation-induced brain damage (Ramanan et al., 2010; Alkis et al., 2019; Sisakht et al., 2020). Generally, chronic oxidative stress is considered to be a consequence of the inflammatory response. Immune cells and activated microglia can increase oxidative stress levels. In addition, irradiated microglial cells can induce astrogliosis, contributing to radiation-induced edema (Hwang et al., 2006). In the current study, we found that BDMC can inhibit brain edema in rats after RBI.Juxtacrine signaling between endothelial cells and astrocytes is essential for generating and maintaining the blood-brain barrier, which is a functional vascular structure that prevents blood-derived elements from entering the brain (Janzer and Raff, 1987). Accumulating evidence shows that astrocytes can protect neurons and endothelial cells from oxidative injury (Wilson, 1997). After injury, astrocytes display hypertrophic cell bodies/nuclei, undergo proliferation, and exhibit up-regulated GFAP expression (Seifert et al., 2006; Yazlovitskaya et al., 2006; Zhou et al., 2011). In the current study, we found that BDMC can inhibit GFAP expression in rats after RBI.
Ionizing RBI can be reduced by anti-oxidants via neutralizing reactive oxygen species (ROS) (Kondziolka et al., 1997; Erol et al., 2004; Sezen et al., 2008). ROS production is a leading cause of tissue damage induced by radiation (Steen et al., 2001; Panagiotakos et al., 2007; Ishisaka et al., 2011; Durak et al., 2017). MDA, a product of lipid oxidation, is considered to be a hallmark of cell membrane lesions (Halliwell and Chirico, 1993), while superoxide dismutase, glutathione, and catalase are essential anti-oxidant enzymes that inhibit the overproduction of ROS (Zhang et al., 2018). In the current study, we found that BDMC can inhibit oxidative stress after RBI.
Nrf2 can be activated by ROS, and, combined with the anti-oxidant response element located in the promoter region of its target gene, is considered to be an anti-oxidant (Miura et al., 2019). In the context of oxidation, Nrf2 translocates to the nucleus, where it promotes HO-1 transcription, thereby exerting its anti-oxidant effects (Xuan and Hou, 2014). Nrf2/HO-1 signaling can also be activated by various compounds associated with the immune response after injury (Li et al., 2018). In the current study, we found that BDMC can activate Nrf2/HO-1 signaling after RBI.
Taken together, our findings show that BDMC improves learning and memory in a rat model of RBI by inhibiting oxidative stress. These results suggest that BDMC, a natural and pure demethoxy derivative of curcumin, could be novel candidate for treating RBI.
Although our results are promising, the study had some limitations. First, we did not investigate the effect of BDMC on neurons in the hippocampus after RBI. Second, we did not inhibit Nrf2/HO-1 signaling to determine whether BDMC exerts its effects via Nrf2/HO-1. Thus, further studies are needed to evaluate the neuroprotective effects of BMDC on neurons in rats with RBI and the underlying mechanism related to Nrf2/HO-1 signaling.
Author contributions:
Study design: MX, ZJX, SXC; experiment implementation: YQC, GJZ, DQH, CLX, YRC, YHL; primary samples and analysis and data analysis: YQC, GJZ; manuscript writing: YQC, GJZ, SXC; manuscript revision: ZJX, SXC. All authors approved the final version of this paper.
Conflicts of interest:
All authors declare no competing interests.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Komal Kalani, Florida International University, USA.
Additional files:
The Mass spectrometry and nuclear magnetic resonance spectroscopy of BDMC.
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

