β-Estradiol 17-acetate enhances the in vitro vitality of endothelial cells isolated from the brain of patients subjected to neurosurgery
Sonia Guzzo , Pasquale De Bonis, Barbara Pavan , , Luciano Fadiga,
Abstract In the current landscape of endothelial cell isolation for building in vitro models of the blood-brain barrier, our work moves towards reproducing the features of the neurovascular unit to achieve glial compliance through an innovative biomimetic coating technology for brain chronic implants. We hypothesized that the autologous origin of human brain microvascular endothelial cells (hBMECs) is the first requirement for the suitable coating to prevent the glial inflammatory response triggered by foreign neuroprosthetics. Therefore, this study established a new procedure to preserve the in vitro viability of hBMECs isolated from gray and white matter specimens taken from neurosurgery patients. Culturing adult hBMECs is generally considered a challenging task due to the difficult survival ex vivo and progressive reduction in proliferation of these cells. The addition of 10 nM β-estradiol 17-acetate to the hBMEC culture medium was found to be an essential and discriminating factor promoting adhesion and proliferation both after isolation and thawing, supporting the well-known protective role played by estrogens on microvessels. In particular, β-estradiol 17-acetate was critical for both freshly isolated and thawed female-derived hBMECs, while it was not necessary for freshly isolated male-derived hBMECs; however, it did counteract the decay in the viability of the latter after thawing. The tumor-free hBMECs were thus cultured for up to 2 months and their growth efficiency was assessed before and after two periods of cryopreservation. Despite the thermal stress, the hBMECs remained viable and suitable for re-freezing and storage for several months. This approach increasing in vitro viability of hBMECs opens new perspectives for the use of cryopreserved autologous hBMECs as biomimetic therapeutic tools, offering the potential to avoid additional surgical sampling for each patient.
Key Words: β-estradiol 17-acetate; 17β-estradiol; cryopreservation; gender-specific; gray matter; human brain microvascular endothelial cells; surgical resections; vascular protection; white matter

Introduction 389 Methods 390 Results 391 Discussion 393
Graphical Abstract
β-Estradiol 17-acetate-sustained human cerebral microvascular endothelial cells
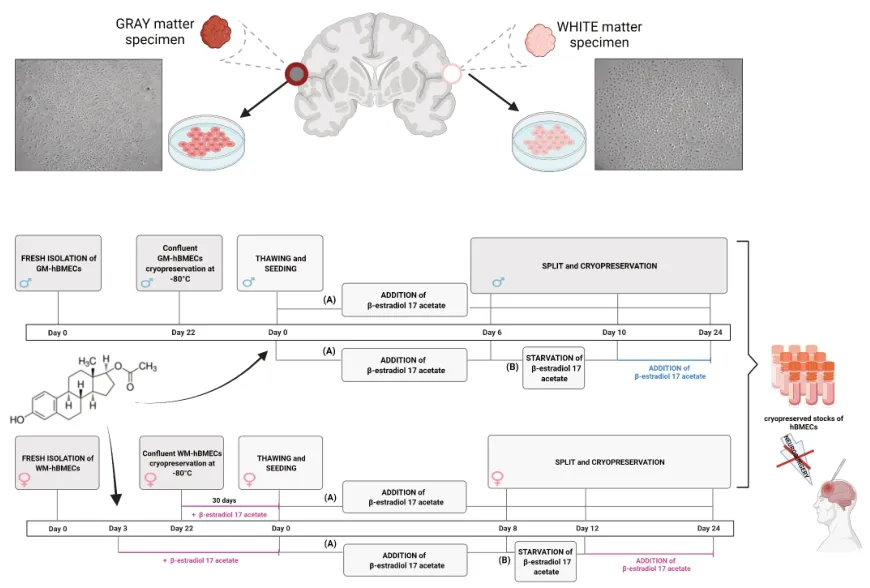
Introduction
Cultures of human brain microvascular endothelial cells (hBMECs) provide a uniquein vitro
system allowing their role in neuroinflammatory responses of the central nervous system to be explored (Weber and Clyne, 2021). In addition, replicative cultures of autologous hBMECs can be useful as a new therapeutic tool in biomimetic coating technology for brain implants to prevent the formation of the glial scar around chronic implants, such as in th recording or stimulating systems (Bouafsoun et al., 2007; Vitale et al., 2018; Gulino et al., 2019). In general, activation of astrocytes and microglia leads to an inflammatory response and ultimately to device encapsulation and neuron degeneration. Despite their role in the inflammatory response, astrocytes also serve as sources of growth factors and nutrients and remove toxins from the extracellular compartment. Close contact between astrocytes and neurons is essential for long-term neuronal survival. One strategy to obtain benefits from astrocytes and, at the same time, potentially obviate their deleterious effects is to develop a method for the selective adhesion of neurons and glia to specific compartments of a neural prosthesis (Vitale et al., 2018; Gulino et al., 2019). Anatomically, the adhesion between the perivascular endings of astrocytes and the endothelial wall of cerebral capillaries constitutes the so-called blood-brain barrier (BBB), which selectively restricts the transport of substances from the blood to the cerebral spinal fluid and to the nervous tissue, acting as a “biological filter” (Gulino et al., 2019). Besides, the BBB controls the intrathecal passage of macrophages, T, and B lymphocytes as well as immunoglobulins or antibodies, acting as an “immunological filter” (Gulino et al., 2019). Therefore, coating of neural probes by hBMECs from cortical gray matter (GM) or subcortical white matter (WM) derived microvessels would make it possible to reproduce the features of specific neurovascular units, achieving glial compliance and consequently acting as a biomimetic interface between neural devices and the complex cellular topography of the brain (Bouafsoun et al., 2007; Vitale et al., 2018; Gulino et al., 2019). In fact, to improve the long-term patency of synthetic vascular prostheses in arteries, the concept of endothelial cell seeding has already been put forward and the composite structure created by combining biologically active cells with prosthetic materials provides more biocompatible vascular substitutes (Bordenave et al., 2005). “To achieve endothelialization of synthetic grafts, previous efforts aimed at “one-stage” procedure in the 1980s seemed clinically feasible but results of reported clinical trials were controversial and mostly disappointing. An alternative method is anin vitro
complete and preformed endothelial coating at the time of implantation: the “two-stage” procedure which implies harvest and culture of autologous endothelial cells” (Bordenave et al., 2005).A great challenge when cultivating hBMECs isolated from brain specimens of adult patients is to achieve viability and reproducible growth. Commercial batches of primary hBMECs are commonly derived from fetal or postmortem adult human brain cortex, the former is still immature and the latter is characterized by very limited proliferative capability negatively correlated with donor age. Therefore, on one hand, cell viability decreases with longer postmortem cell isolation intervals caused by the death of autoptic material and donor age, while on the other hand, surgical samples from adult and living patients are typically very small, adhesion to the substrate and/or lifespan of hBMECs often represent a difficult goal (Weber and Clyne, 2021). Furthermore, hBMECs show unique features compared to peripheral endothelial cells in achieving a specific permeability (Chen et al., 2020), thus acting as transducers that receive signals from circulating molecules with particular sensitivity to their blood concentration and then transmitting these signals in the brain (Wyss-Coray, 2016). Development of methods to isolate hBMECs from human brain cortical GM has been previously reported (Weksler et al., 2005; Navone et al., 2013; Hartz et al., 2018; Spaethling et al., 2018; Luo et al., 2020; Weber and Clyne, 2021; Park et al., 2022). To our knowledge, only a few studies reported the isolation of endothelial cells from poorly vascularized subcortical WM, deriving from rat, bovine, or pig brains (Philips et al., 1979; Nyúl-Tóth et al., 2016).In our study, we assessed the possibility of efficiently culturing hBMECs obtained from neurosurgical resections of GM and WM of aged patients as well. As foreseen, preliminary experience in our lab showed that early drawbacks were due to non-adhesion and/or low growth rate, poorer performance associated with patient age, in particular as regards adult or older female patients. Awaring that a decline in cerebral and peripheral vasculature is a plausible condition in post-menopausal women (Wong et al., 2016), we decided to test whether 17β-estradiol, the most active form of estrogen hormone, might play a role in this decline (Simpson, 2003; Razmara et al., 2008). This hormone is known as a vascular protective factor in replacement therapy for post-menopausal females (Razmara et al., 2008; Gersh and Lavie, 2020) and as an enhancer for adhesion of human umbilical vein endothelial cells to different culture substrates (Farhat et al., 1996). Furthermore, the brain is a steroidogenic organ, in which the rate of synthesis of neurosteroids with potent neuromodulatory actions is affected by fluctuations in peripheric plasma estrogen levels (Zárate et al., 2017). Consequently, 17β-estradiol could be a good candidate to improve the viability of cultured adult hBMECs.
Based on this background, we developed a culturing system of primary hBMECs isolated from peritumoral resections of subcortical WM of adult or older female patients to assess the renewal effect of 17β-estradiol in addition to specific growth factors in the culture medium. In comparison, hBMECs isolated from cortical GM and subcortical WM of male patients were also treated with this estrogen to assess the gender specificity of the potential responsiveness of hBMECs to 17β-estradiol.
Methods
Neurosurgery harvest, isolation, and experimental design of hBMEC culture
The hBMECs were isolated from cortical GM and subcortical WM biopsies obtained during scheduled brain tumor resection surgery from five Caucasian male patients (M1-M2-M3-M4-M5) aged 55–72 years and five Caucasian female patients (F1-F2-F3-F4-F5) aged 65–75 years (Table 1
), diagnosed with grade II or III glioma and/or meningioma. The protocol was approved by the local Ethical Committee, Comitato Etico di Area Vasta Emilia Centro (approval code number CE-AVEC 640/2018/Sper/UniFe) on October 18, 2018. Peritumoral neurosurgical resections were collected at the Azienda Ospedaliero-Universitaria of Ferrara (Italy), using standard operating procedures for enrollment and consent of only adult patients, as well as for tissue collection, handling, and de-identification of patient clinical data. Postmenopausal condition was inferred by the age of female patients. It has to be specified that the neurosurgical intervention itself was not the subject of this study. Resected tissues were immediately transferred to sterile conical tubes containing ice-cold Hibernate A medium (Thermo Fisher Scientific, Milan, Italy) supplemented with 50X B27 Plus and 50 mM GlutaMAX-I supplements (Thermo Fisher Scientific) and delivered to the laboratory of translational neurophysiology of the University of Ferrara. The hBMECs were isolated from GM and/or WM resections as previously described (Perrière et al., 2005) with slight modifications. Briefly, a gentle mechanical dissociation of both GM and WM specimens weighing 300–400 mg was performed with a glass potter followed by the first step of digestion using a mixture of collagenase/dispase (270 IU collagenase/mL, 0.1% w/v dispase) and DNAse (10 IU/mL) in Advanced DMEM serum-free medium (Thermo Fisher Scientific) for 60 minutes at 37°C in a thermostatic orbital shaker. The cell pellets were separated and demyelinized by 20% w/v bovine serum albumin (BSA)/DMEM by centrifugation (1000 ×g
for 15 minutes, at 4°C), followed by second digestion of the pellet in the collagenase/dispase mixture for 45 minutes at 37°C. According to previously reported methods of primary cultures of human brain microvascular endothelial cells by Weksler et al. (2005) and Park et al. (2022), capillary endothelial cells were retained on a 10-µm nylon mesh cell strainer, removed from the filter with MCDB 131 medium (Thermo Fisher Scientific), an endothelial cell basal medium for the culture of human microvascular endothelial cells, supplemented with 20% v/v bovine plasmaderived serum (PDS; First Link Ltd., Birmingham, UK) and antibiotics (penicillin, 100 IU/mL; streptomycin, 100 µg/mL), seeded on 30-mm Petri dishes and then flasks coated with 150 µg/mL rat tail collagen type I (Thermo Fisher Scientific).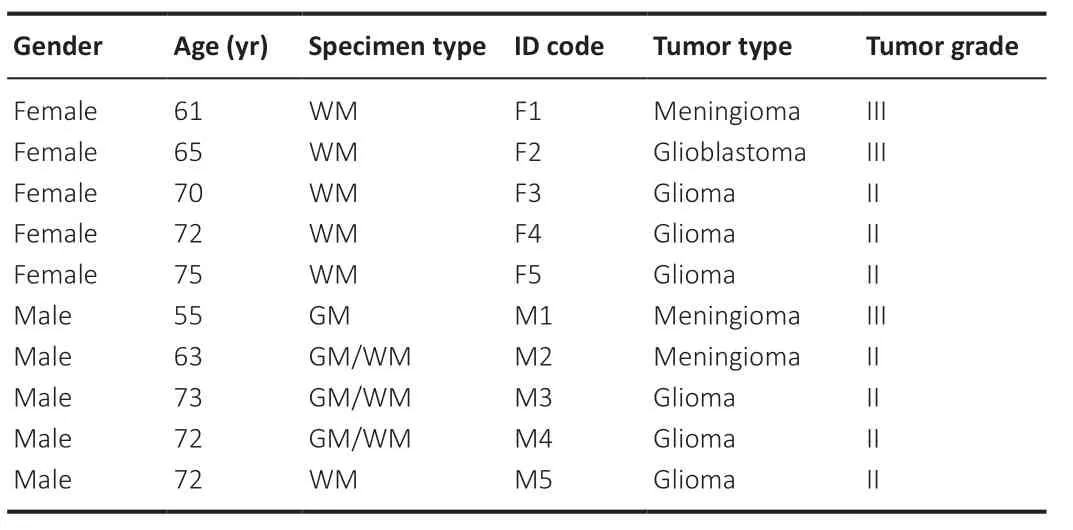
Table 1 |Patient recruitment and classification and tumor type and grade
During the first 2 days of culture, the hBMEC medium contained 4 µg/mL puromycin to eliminate P-glycoprotein negative contaminating cell types (Perrière et al., 2005). Vascular endothelial growth factor (VEGF; 1 µg/mL) was then added to the medium. Following this procedure, only BMECs expressing high levels of P-glycoprotein selectively survived over other cell types (Perrière et al., 2005). This culture medium was then removed and replaced by the same MCDB 131 medium supplemented with 20% bovine plasma-derived serum, but containing a mixture of 2 ng/mL basic fibroblast growth factor, 500 ng/mL hydrocortisone, 1 µg/mL VEGF. This latter culture medium was further spiked with 10 nM β-estradiol 17-acetate, dissolved in dimethyl sulfoxide (DMSO), which reached a 0.0001% final concentration of vehicle in the medium, used to grow WM-derived hBMECs freshly isolated from female patients F4–F5 immediately after thawing and splitting; otherwise used to grow GM-derived hBMECs isolated from patients M1-M2-M3-M4 exclusively to allow their growth immediately after thawing and splitting. All the above hormones and growth factors were purchased from Merck, Milan, Italy.
All hBMEC cultures were incubated at 37°C, 95% humidity, and 5% COuntil cells reached approximately 80% confluence when cells were passaged using trypsin/EDTA (Thermo Fisher Scientific). All the hBMECs growing in culture were cryopreserved at confluence in freezing medium consisting of 90% FBS plus 10% DMSO and stored at –80°C.
Cell counting on phase contrast images of hBMECs two-dimensional
monolayers
Cell morphology was examined by phase contrast microscopy imaging in a Motic microscope (Seneco S.r.l., Milan, Italy) using phase-contrast optics and related images were acquired with a Moticam digital camera (Seneco S.r.l.). hBMECs were routinely photographed under a light phase-contrast microscope, the related images acquired and counted by ImageJ 1.46r software (Image Processing and Analysis in Java; available at https://imagej.nih.gov/ij/index.html) to estimate cell number and density. Each image was initially converted to grayscale before adjusting the threshold to highlight cells. Later, to split up the merged cells, the watershed tool was used. After applying these functions, a binary image was created for cell counting. Data for growth curves were then plotted as graphs and analyzed with GraphPad Prism 8.43 (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
Experimental design
Experiments were planned to improve the adhesion and proliferation rate of hBMECs, both after fresh isolation and after freezing and thawing of the primary cultures. From the preliminary experiments in our laboratory the early disadvantages due to non-adhesion and/or low growth rate of endothelial cells in culture emerged with regard to the first three adult or older adult female patients (F1–F3). Therefore, the final experimental procedure was set by adding β-estradiol 17-acetate to the growth medium containing nonfrozen, cryopreserved and thawed F4–F5 WM-derived hBMECs, followed by the withdrawal of the hormone (estrogenic starvation) from the medium for 4 days after the first split post-thawing and re-addition to test cell recovery. Conversely, growth of M1-M2-M3-M4-GM- and M2-M3-M4-WM-derived hBMECs was sustained by β-estradiol 17-acetate only after thawing, starved of the hormone for 4 days after the first split post-thawing (estrogenic starvation) before switching again to the hormone to test their recovery. In particular, similarly to the male GM-derived hBMECs, the freshly isolated M5-WM-derived hBMECs were also grown in normal medium and β-estradiol 17-acetate was added after the first split to simply evaluate whether male WM-derived hBMECs could be estrogen sensitive as well as WM-derived female hBMECs. An outline of the procedure is illustrated inFigure 1
.
Figure 1|Experimental design. Sequences of fresh isolation and culture of hBMECs in the presence and absence of 10 nM β-estradiol 17-acetate were evaluated. Cell recovery following cryopreservation, thawing, and culture in the presence and after deprivation and re-addition of 10 nM β-estradiol 17-acetate was subsequently tested. Cell culture splits are marked with vertical lines. hBMECs: Human cerebral microvascular endothelial cells.
Viability of hBMECs
Cell viability of male and female GM- and WM-derived hBMECs cultured on 30-mm Petri dishes was determined by trypan blue exclusion test following the procedure of Hansen et al. (2021). Confluent cells were harvested in 5 mL of growth medium. About 1 mL aliquot of cell suspension was centrifuged for 5 minutes at 100 ×g
. Cell pellets were resuspended in 1 mL of PBS. Approximately 100 µL of this suspension was mixed with 100 µL of 0.4% Trypan blue solution (Thermo Fisher Scientific). Cells excluding Trypan blue (viable cells) and dead cells were counted under the microscope using a Bürker hemocytometer (Brand GmbH, Wertheim, Germany).The percentage of viable cells was calculated as follows:
Viable cells (%) = total number of viable cells per mL cell suspension/total number of cells per mL cell suspension× 100. The resulting data were plotted as graphs and analyzed with GraphPad Prism 8.43 (GraphPad Software).
Immunofluorescence staining
The primary antibodies mouse anti-human CD62E (Cat# MCA1969, RRID: AB_2186827), mouse anti-human CD31 (Cat# MCA1738, RRID: AB_322710) and mouse anti-human CD54 (Cat# MCA532, RRID: AB_321784) were purchase from Bio-Rad Laboratories S.r.l, Milan, Italy, and were used at the company’s recommended working dilution for immunofluorescence. CD62E antibody is the endothelial leukocyte adhesion molecule-1 marker that mediates the initial interaction of leukocytes and platelets with endothelial cells, and it was directed towards the binding site of leukocytes to human E-selectin. Epitope recognized by CD31 antibody also known as platelet endothelial cell adhesion molecule 1 (PECAM-1) is an essential marker for transendothelial migration under most inflammatory conditions. It has been mapped to be within residues 228 and 234 of cluster of differentiation 3. The epitope of CD54 antibody, also known as inter cellular adhesion molecule 1 (ICAM-1), is the intracellular adhesion molecule-1 that participates in cellcell adhesion between leukocytes and endothelial cells, facilitating leukocyte recruitment and transmigration at the sites of inflammation. It has been mapped to the first domain of the ICAM-1 molecule. Secondary antibodies fluorochrome-conjugated goat anti-mouse Alexa Fluor 488 (Cat# A-21121, RRID: AB_2535764), goat anti-mouse Alexa Fluor 568 (Cat# A-21124, RRID: AB_2535766), and goat anti-mouse Alexa Fluor 633 (Cat# A-21146, RRID: AB_2535782) were purchased from Thermo Fisher Scientific, and were used at the manufacturer’s recommended working dilution.
The hBMECs were seeded on the glass bottom coated with 150 µg/mL collagen type I of CELLVIEWculture dishes (Greiner Bio-One, VWR International, Milan, Italy) and grown until confluence. The cells were then washed three times with the Hank’s buffer salt solution (HBSS), fixed in 3% v/v paraformaldehyde for 15 minutes at room temperature (RT) and incubated with 5% w/v BSA/HBSS blocking solution for 60 minutes at room temperature on an orbital shaker. Mouse anti-human CD62 monoclonal antibody, mouse anti-human CD31 monoclonal antibody and mouse anti-human CD54 monoclonal antibody were used with a diluition of 1:50, and incubated for 4 hours at room temperature. Then, secondary antibodies fluorochromeconjugated goat anti-mouse Alexa Fluor 488, goat anti-mouse Alexa Fluor 568 and goat anti-mouse Alexa Fluor 633 were used at a diluition of 1:400, and incubated for 1 hour at room temperature in the dark. Total nuclei were stained with 15 µg/mL Hoechst 33342 blue dye solution (Cat# B2261, Merck). Immunofluorescence staining of all markers was merged with the nuclear Hoechst dye. The cells were washed three times with HBSS and embedded with glycerol/PBS 1:1, and finally stored at 4°C until images were acquired by an Olympus BX51 fluorescence microscope using Neurolucida software (MBF Bioscience, Williston, VT, USA). Cell fluorescence intensity was measured by ImageJ 1.46r software (Image Processing and Analysis in Java; available at https://imagej.nih.gov/ij/index.html). The corrected total cell fluorescence (CTCF) was calculated using the following formula: CTCF = integrated density - (area of selected cell × mean fluorescence of background readings). Data were then plotted as graphs and analyzed with GraphPad Prism 8.43 (GraphPad Software).
Positive control for primary antibody staining
hCMEC/D3 human cerebral microvascular endothelial immortalized cell line (Cat# CLU512, RRID: CVCL_U985; Weksler et al., 2005) was employed as positive control fitting for all primary antibodies. hCMEC/D3 cells were activated by human tumor necrosis factor-alpha (TNF-α; Thermo Fisher Scientific) as previously reported (Weksler et al., 2005; Jassam et al., 2016;
Dunst et al., 2017). hCMEC/D3 cells were purchased from TebuBio (Magenta, Milan, Italy) and cultured under similar conditions for primary hBMECs. Subsequently, hCMEC/D3 cells were grown to confluence on 150 µg/mL rat tail type I collagen coated glass coverslips in the 30-mm Petri dishes, washed with fresh MCDB 131 medium, and activated by 24-hour stimulation with 10 ng/mL TNF-alpha in the same medium. Finally, cells were washed and labeled with antibodies CD62-E, CD54 and CD31, and processed for the visualization and quantification of cells immunofluorescence intensity as described above.
Statistical analysis
Results are presented as mean values from individual cell preparations obtained from each donor, with error bars representing the standard error of the mean (SEM) value. Graphs and data were plotted and analyzed with GraphPad Prism 8.43 (GraphPad Software). Statistical analysis was performed by one-way analysis of variance followed by Bonferroni’s multiple comparisons test or Dunnet’spost hoc
test and two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. Significance was set atP
< 0.05.Results
Primary endothelial cell cultures from neurosurgical resections of GM and WM of adult or older adult patients
Samples after recruitment consisted of cortical GM and subcortical WM specimens collected from five female patients (F1-F2-F3-F4-F5) and five male patients (M1-M2-M3-M4-M5), all aged 55–75 years old. For clinical reasons, resected peritumoral specimens of both WM and GM could not always be removed in useful sizes from each patient, therefore both WM and GM were resected from M2 and M4, while only GM specimens were taken from M1, and only WM specimens were removed from all female patients and the male M5 patient (Table 1
). Upon first plating, GM-derived hBMECs isolated from male patients M1 to M4 spontaneously adhered to the substrate and showed rounded cell clusters associated with the residues of capillary fragments and scattered single cells and debris. These endothelial cells displayed morphological characteristics with typical swirling patterns and whorls and no evident vacuoles, highlighting their overall health. No giant cells or spreading and non-contrasted cells (sign of degeneration) appeared during any growth steps after isolation (see the phase contrast images inFigure 2A
). After 1–3 days of culture, cells sprouted from clusters and elongated; after 20 days, confluent colonies of hBMECs isolated from GM of all four male patients were observed. Cultures of WM-derived hBMECs from male patients M2-M adhered and proliferated, but showed lower cell density at confluence compared with GM-derived hBMECs (P
< 0.001), even if belonging to the same patient, as reported by the growth curves ofFigure 2C
.In contrast, resections of WM from female patients F1, F2 and F3 did not provide adherent or growing cultures, independently of their age, because the addition of β-estradiol 17-acetate to the culture medium of the deriving hBMECs had not yet been considered. In fact, when 10 nM β-estradiol 17 acetate was further added to the growth medium after 2 days in culture, WM-derived hBMECs from patients F4 and F5 interestingly showed strong adhesion and growth capacity significantly exceeding that of GM-derived male hBMECs (P
< 0.001) after 10 days in culture (Figure 2C
). Therefore, following the addition of β-estradiol 17-acetate, F4- and F5-derived WMhBMECs displayed a swirling pattern similar to the GM-hBMECs isolated from male patients (see the phase contrast images inFigure 2B
), achieving a significant increase in cell number 13 days after isolation (P
< 0.001) and confluent colonies after 22 days (Figure 2B
). Notably, subcultures of F4- and F5-derived WM-hBMECs treated with the same concentration of DMSO as a vehicle (0.0001%) or with hormone at the concentrations lower than 10 nM failed to adhere or lose their growth behavior compared with 10 nM estrogen-treated endothelial cells. Consequently, these cells were discarded as waste. Therefore, 10 nM has shown the lowest effective concentration of β-estradiol 17-acetate that can stimulate endothelial cell growthin vitro
, as also observed in our previous study on dose-dependent release of PGE2 induced by estrogen in human amnion-derived WISH cells (Pavan et al., 2001). Consequently, a 10 nM concentration of β-estradiol 17-acetate was used to maintain the viability of both male and female WM- and GM-derived hBMECs in culture.The addition of 10 nM β-estradiol 17-acetate to the growth medium of hBMECs after fresh isolation from WM of male patients M4 and M5 induced a significant increase in cell density after 20 days (P
< 0.05) compared with the untreated male WM-hBMECs (Figure 2A
andC
), and clearly never reached the responsiveness to the hormone exhibited by the female WM-hBMECs nor by the male GM-hBMECs after thawing (see below).Finally, it is noteworthy that in all the hBMEC cultures described above, once confluence was reached, contact inhibition of their proliferation without formation of post-confluent multilayer foci was observed, as a fundamental behavior of normal endothelial cells.
Effects of cryopreservation on WM-derived hBMECs isolated from female patients and exposed to β-estradiol 17-acetate
F4–F5-WM-hBMECs, treated with β-estradiol 17-acetate, were cryopreserved at –80°C for 1 month. Then they were thawed and seeded in growth medium spiked again with 10 nM β-estradiol 17-acetate. β-Estradiol 17-acetate induced a complete recovery of viability of F4–F5-WM-hBMECs and sustained their proliferation until the thirty-third day of culture with routine splitting every 8 days (Figure 3A
). Then F4–F5-WM-hBMECs were immediately harvested for subsequent freezing stages. In particular, withdrawal of β-estradiol 17-acetate from the growth medium in separate dishes of F4–F5-WM-hBMECs at the first split 12 days post-thawing decreased their proliferation rate and adhesion to the substrate (Figure 3C
). No recovery was induced by a new addition of the hormone after 4 days of estrogenic starvation (Figure 3C
inserted panel).
Figure 2|Effects of donor’s gender, tissue source and 10 nM β-estradiol 17-acetate on hBMECs recovery and growth after fresh isolation from neurosurgical specimens. (A) Representative phase contrast images of morphology and density of female WMderived hBMECs spiked with 10 nM β-estradiol 17-acetate, after 4 and 22 days in culture from isolation. Scale bars: 20 µm. (B) Representative phase contrast images of male GM-derived hBMECs in the absence of 10 nM β-estradiol 17-acetate, and male WMderived hBMECs in the absence and presence of 10 nM β-estradiol 17-acetate, after 4 and 22 days in culture from isolation. Scale bar: 20 µm. (C) Comparison between growth curves of GM-derived hBMECs freshly isolated from male patients M1-M4 in the absence of 10 nM β-estradiol 17-acetate (blue squares) and WM-derived hBMECs freshly isolated from male patients M2-M5 in the absence (green circles) and presence of 10 nM β-estradiol 17-acetate (violet triangles) and WM-derived hBMECs isolated from female patients F4 and F5 and spiked with 10 nM β-estradiol 17-acetate (dark pink triangles). Each independent culture was related to GM-derived hBMECs (n = 4) and to WM-derived hBMECs (n = 4) obtained from male patients, and to WM-derived hBMECs (n = 2) obtained from female patients. Each point represents the mean ± SEM of four experiments from individual cell preparations obtained from each donor. Effects of days of culture, gender and/or estrogen treatment on growth curves were analyzed by two-way analysis of variance followed by the Tukey’s multiple comparisons test. *P = 0.001 for male GM-hBMECs vs. male WM-hBMECs; °P = 0.05 for male WM-hBMECs vs. male β-estradiol 17-acetate-treated WM-hBMECs; §P = 0.001 for female β-estradiol 17-acetate-treated WM-hBMECs vs. male GM-hBMECs; £P = 0.01 for female β-estradiol 17-acetate-treated WM-hBMECs vs. male β-estradiol 17-acetate-treated WM-hBMECs. GM: Gray matter; hBMECs: Human cerebral microvascular endothelial cells; WM: white matter.
β-Estradiol 17-acetate restores viability and proliferation of male GMderived hBMECs after thawing
Unlike female WM-derived hBMECs, which adhered and grew to the confluence only in the presence of β-estradiol 17-acetate, cultures of patients M1–M4 GM-derived hBMEC never exposed to the estrogen hormone after fresh isolation grew sufficiently and were thus cryopreserved in freezing medium. However, after thawing and seeding, all the hBMECs deriving from the corresponding patients M1 to M4 showed rapid decreases in adhesion to the substrate and proliferation rate with progressive weakening and loss in culture. Therefore, in order to verify a possible estrogen sensitivity of male hBMECs as far as recovery from freezing is concerned, frozen aliquots of patients M1–M4 GM-derived hBMECs were thawed and seeded in a growth medium added with 10 nM β-estradiol 17-acetate during 32 days of routine subculture. Interestingly, a huge response to estrogens by male GM-derived hBEMCs in terms of viability, cell density at confluence and proliferation rate (Figure 3B
) were observed, with a complete recovery occurring even faster than the one observed in hBMECs from female patients, although not statistically different (P
> 0.05). Withdrawal of β-estradiol 17-acetate from the growth medium in separate dishes of M1-M4-GM-derived hBMECs at the first split 4 days post-thawing induced a fully reversible slow viability reduction (Figure 3C
) when hormone was added back to the culture medium after 8 days of estrogenic starvation.Recovery in endothelial cell viability
As shown inFigure 4
, after thawing and splitting, the yield from male GMderived hBMECs was 8 to 9 × 10live cells/mL and 5 to 6 × 10dead cells/mL resulting in a viability of 93 ± 4% by the trypan blue dye exclusion assay. The yield from male WM-derived hBMECs was 6 to 7 × 10live cells/mL and 1 to 1.2 × 10dead cells/mL, resulting in a viability of 84 ± 2% . The yield of female WM-derived hBMECs was 6 to 7 × 10live cells/mL and 0.8 to 1 × 10dead cells/mL with a viability of 88 ± 3%. There were no statistically significant differences in the number of live cells yielded by WM-versus
GM-derived hBMECs (P
= 0.1035).
Figure 3|Effect of donor’s gender, tissue source and 10 nM β-estradiol 17-acetate on hBMECs recovery and growth after thawing from cryopreservation and splitting. (A) Representative phase contrast images of morphology and density of male GMderived hBMECs and of female WM-derived hBMECs subcultured for 6 days and 12 days, respectively, after thawing from cryopreservation both in the presence and in the absence of 10 nM β-estradiol 17-acetate. Scale bars: 20 µm. (B) Comparison between growth curves related to GM-derived hBMECs isolated from male patients M1-M4, thawed and subcultured in the absence (blue triangles) and in the presence (violet squares) of 10 nM β-estradiol 17-acetate, and growth curves related to WM-derived hBMECs isolated from female patients F4–F5, thawed and subcultured in the presence (pink triangles) and in the absence (red circles) of 10 nM β-estradiol 17-acetate. (C) Effects of withdrawal (blue circles) and new addition (violet triangles) of 10 nM β-estradiol 17-acetate on recovery and growth after thawing of GM-derived hBMECs isolated from male patients M1-M5 and effects of withdrawal of 10 nM β-estradiol 17-acetate on recovery and growth after thawing of WM-derived hBMECs isolated from female patients F4–F5, each subcultured after thawing. The inserted graph represents the magnification of the failed recovery after withdrawal and re-addition of 10 nM β-estradiol 17-acetate in WM-derived hBMECs from female patients F4–F5. Each point represents the mean ± SEM of four experiments from individual cell preparations obtained from each donor. Effects of thawing, gender and/or estrogen treatment on growth curves were analyzed by two-way analysis of variance (P = 0.0001) followed by the Tukey’s multiple comparisons test. (B) Male GM-derived hBMECs with estrogen vs. male GM-derived hBMECs without estrogen, *P = 0.0001; female WM-derived HBMECs with estrogen vs. female WMderived hBMECs without estrogen, °P = 0.0001. (C) Male GM-derived hBMECs withdrawal of estrogen vs. male GM-derived hBMECs re-addition of estrogen, *P = 0.0002; male GM-derived hBMECs re-addition of estrogen vs. female WM-derived hBMECs readdition of estrogen, **P = 0.0007. GM: Gray matter; hBMECs: Human cerebral microvascular endothelial cells; WM: white matter.
WM- and GM-derived hBMECs express surface constitutive but not tumoral endothelial markers
Both female and male patient WM- and GM-derived hBMECs (freshly isolated and after thawing) were stained by immunofluorescence for specific surface antigen marker of different endothelial tumor entities. Immunofluorescence staining of all markers was merged with the nuclear Hoechst dye, and compared to their staining in brain endothelial hCMEC/D3 cells activated with 10 ng/mL TNF-α used as positive control (Weksler et al., 2005). All the cultured hBMECs before and after thawing (Figure 5A
) showed a detectable expression of PECAM-1 or CD31, which is regarded as the best constitutive endothelial marker. No statistically significant difference (P
= 0.9999) in CD31 expression was observed if all types of hBMECs were compared each to other, whereas highly significant differences were observed in comparison with the positive control, as shown in the immunofluorescence quantification (Figure 5B
). Both female WM- and male GM-derived hBMECs were stained for cluster of differentiation 54 (CD54) or ICAM-1 (Figure 5C
), a 90 kDa member of the immunoglobulin superfamily, which was constitutively expressed in both brain microvascular endothelial cells and umbilical vein endothelial cells (Huber et al., 2006), but it was also normally activated in aged endothelial tissue (Goncharov et al., 2020). TNF -alpha activated hCMEC/D3 cells were a valuable positive control for CD54 marker (Figure 5C
), showing a fluorescence intensity 1.5-fold higher than female WM-derived hBMECs (P
= 0.0004) and 1.8-fold higher than GM-derived-hBMECs (P
= 0.0001), whereas CD54 fluorescence intensity was 1.3-fold higher in female WM-derived hBMECs than GMderived hBMECs (P
= 0.0167). All the cultured hBMECs showed negativity for glycoprotein E-selectin (CD62E), a cell adhesion molecule (CAM), expressed on brain tumor endothelium (Miebach et al., 2013; Jassam et al., 2017), whereas TNF-α activated hCMEC/D3 cells were positive for control of CD62E staining (Figure 5D
), confirming the upregulation of CD62E in hCMEC/D3 cells and cancer cells in response to a TNF-α inflammatory stimulus, as reported by other authors (Weksler et al., 2005; Jassam et al., 2016; Dunst et al., 2017).
Figure 4|Cell viability. (A) Phase contrast images of bright fields of the hemocytometer and (B) quantification of the live/dead trypan blue exclusion assay, referred to male GM- and WM-derived hBMECs and female WM-derived hBMECs, each of them splitted after thawing. Orange arrows indicate dead cells. Scale bar = 50 µm. No statistical differences (NS) in viability resulted from oneway analysis of variance (P = 0.1035) followed by Bonferroni’s multiple comparisons test, performed on three aliquots of cells deriving from three independent cultures (n = 3) of each male GM- and WM-derived hBMECs and female WM-derived hBMECs, respectively. GM: Gray matter; hBMECs: Human cerebral microvascular endothelial cells; WM: white matter.
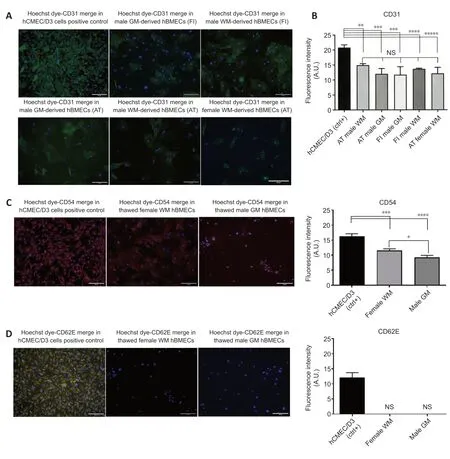
Figure 5|Visualization and quantification of immunofluorescence staining of CD31, CD54 and CD62E markers in representative cultures of male GM- and WMderived hBMECs and female WM-derived hBMECs. (A) Constitutive expression of the endothelial marker CD31 or PECAM-1 was shown for all the types of hBMECs both after fresh isolation and after thawing from cryopreservation. TNF-α activated hCMEC/D3 cells were used as positive control for staining of CD31. CD31 primary antibody was stained in green by fluorochrome-conjugated goat anti-mouse secondary antibody Alexa Fluor 488 and nuclei were stained by the blue Hoechst 33342 dye. (B) Immunofluorescence intensity quantification (in arbitrary units; A.U.) of CD31-stained hBMECs in comparison to the TNF-α activated hCMEC/D3 cells, CD31 positive control. Scale bars: 50 µm. **P = 0.0075, ***P = 0.0002, ****P = 0.0016, *****P = 0.0003. (C) CD54 or ICAM-1 exhibited a low expression in male GM-derived hBMECs and female WM-derived hBMECs compared with the positive control TNF-α activated hCMEC/D3 cells, as shown by fluorescence intensity quantification. CD54 primary antibody was stained in red by fluorochrome-conjugated goat anti-mouse secondary antibody Alexa Fluor 633 and nuclei were stained by the blue Hoechst 33342 dye. Scale bars: 50 µm. *P = 0.0167, ***P = 0.0004, ****P = 0.00001. (D) CD62E or E-Selectin was clearly seen as being negative both for male GM-derived hBMECs and female WM-derived hBMECs in comparison with the TNF-α activated hCMEC/D3 cells, as shown by fluorescence intensity quantification. CD62-E primary antibody was stained in orange-yellow by fluorochrome-conjugated goat anti-mouse secondary antibody Alexa Fluor 568 and nuclei were stained by the blue Hoechst 33342 dye. All the endothelial markers were merged with the nuclear Hoechst 33342 dye. Scale bars: 50 µm. Each point represents the mean ± SEM of four experiments from individual cell preparations obtained from each donor. Significance was calculated by one-way analysis of variance followed by Dunnet’s post hoc test, comparing each marker staining in hBMECs with its staining in hCMECs/D3 positive control. AT: After thawing; FI: Fresh isolation; GM: gray matter; hBMECs: Human cerebral microvascular endothelial cells; WM: white matter.
Discussion
The main findings of this study are the following: (1) adhesion and proliferation yield in culture of hBMECs not only depend on whether the cells are derived from subcortical WM or cortical GM, but are also gender-specific; (2) β-estradiol 17-acetate improves adhesion and proliferation of hBMECs freshly isolated from postmenopausal female WM, whereas adult or older adult male hBMECs freshly isolated from GM and WM show constitutive adhesion and proliferation even in the absence of estrogen; (3) male GM- and WM-derived hBMECs when seeded after thawing do not survive to cryopreservation, but exposure to β-estradiol 17-acetate counteracts this viability decline.
Our work describes the isolation from surgical specimens and the culture of hBMECs from female and male neurosurgery patients following a procedure similar to the one reported by Perrière et al. (2005) for young rats. HBMECs isolated from postmenopausal female patients did not produce adherent and proliferating cell cultures in the absence of 10 nM β-estradiol 17-acetate, whereas, following anin vitro
estrogen replacement treatment, a significant renewal of hBMEC viability and proliferation was induced by the hormone. The 10 nM concentration of β-estradiol 17-acetate has shown the lowest effective concentration of estrogen that can stimulate endothelial cell growthin vitro
, as also observed in our previous study (Pavan et al., 2001). Moreover, this is not a high estrogen concentration for anin vitro
study, considering a recent report indicated that 17β-estradiol is suitable to evoke the ability of granulosa cells (GCs) to proliferatein vitro
, when administered even at micromolar concentrations (Ciesiółka et al., 2017). In particular, β-estradiol 17-acetate was employed instead of 17β-estradiol as the best suitable ester prodrug identified in estrogen replacement therapy (Woolfson et al., 1999), belonging to the family of endogenous alkyl esters of estradiol showing the recognized physiological role as long-acting estrogens (Alemany, 2021). Therefore, the first novelty of our approach consists in the isolation of hBMECs from WM of female patients with a sufficient cell density to create a replicative culture with a prolonged life-span before and after cryopreservation, because of β-estradiol 17-acetate, accompanied by an irreversible loss of viability after estrogen starvation in culture. Usually, it seems to be easier to isolate capillaries from GM, because it has a softer texture than WM and quite a lot of WM is needed due to the less dense vasculature of WM compared to GM (Brown et al., 2018). Moreover, we can speculate that 10 nM β-estradiol 17-acetate was essential for recovery of WM-derived hBMECs of female patients both after fresh isolation and after thawing because of its anti-apoptotic effect promoting the cell adhesion molecule interaction with collagen type I substrate (Alvarez et al., 1997; Hunter et al., 2019). In this regard, Alvarez et al. (1997) previously demonstrated that the anti-apoptotic effect of 17β-estradiol in human aortic endothelial cells is mediated by improving cell interaction with the substrate, which strongly affects the fate of cultured endothelial cells. These cells, deriving from a female donor, when starved of estrogens for 7 days and then re-exposed to regular medium, failed to recover and continued to deteriorate; otherwise, if endothelial cells were maintained in medium supplemented with 10 nM 17β-estradiol, the same concentration applied in our experiments, they were able to reach confluency when switched back to regular medium. Therefore, also the constitutive capability of adhesion and proliferation shown by WM-derived hBMECs of three male patients could be explained by genderrelated differences in the expression of cell adhesion molecules on cultured hBMECs, as reported elsewhere (Hunter et al., 2019).Despite the spontaneous proliferative vitality of the male GM- and WMderived hBMECs after isolation, they failed to grow if seeded after thawing. However, after exposure to β-estradiol 17-acetate a massive response in terms of adhesion to the substrate and proliferation rate by the male GM-derived hBMECs seeded after thawing was observed. This behavior firstly suggests an intrinsic sensitivity of the male endothelium to 17β-estradiol, presumably based on the testosterone-derived estradiol production reported in male vascular endothelium (Villablanca et al., 2013). Moreover, it may depend on gender-specific metabolic differences in endothelial cells of human BBB, as extensively reviewed by Weber and Clyne (2021). These authors stated that although littlein vitro
research has investigated sexual dimorphisms in hBMEC metabolism, studies in human umbilical vein endothelial cells (HUVECs) obtained from male-female twin sets demonstrated that male HUVECs had higher mitochondrial respiration and lower glycolysis/mitochondrial respiration ratios than female HUVECs. Furthermore, a positive correlation between 17β-estradiol binding and upregulation of rate-limiting glycolytic enzymes was also demonstrated in female HUVECs (Weber and Clyne, 2021). In addition, 17β-estradiol was found to greatly improve the mitochondrial function in cultured hBMECs acting on estrogen receptor alpha (Razmara et al., 2008). Since cryopreservation has been shown to induce severe damage to the mitochondrial functions of endothelial cells (Lauridsen et al., 2019), a plausible hypothesis is that male GM and WM-derived hBMECs having preponderant mitochondrial respiration do not require estrogenic support after fresh isolation, but the protective effect of estrogen on mitochondrial activity becomes necessary after thawing. On the other hand, the situation is different for female WM-derived hBMECs, for which a decrease in glucose metabolism in female brains is associated with declining estrogen receptor expression following the menopausal transition (Weber and Clyne, 2021), Finally, we can assume that these gender-specific responses depend on the types of the estrogen receptors differently expressed in the hBMECs of male and female patients (Lu et al., 2016). This aspect will be further investigated to better understand how to activate signaling cascades in hBMECs to promote a stronger adhesion coating of the neural prosthesis. These results emphasize the gender-specific estrogen hormone-related effects on vascular health. The latter aspect may not be negligible in predicting the efficacy and duration of a potential β-estradiol 17-acetate-eluting device at the brain vascular level. Our results may match the recent findings of Chen et al. (2020), whereby mouse mBMECs are exquisite sensors of age-related circulatory cues and that they can be rejuvenated with acute exposure to systemic factors. In our context, we may argue that 17β-estradiol could be a crucial gender-specific factor to determine the ability of capillary endothelial cells to mediate reversible and non-autonomous mechanisms of brain aging. In fact, BBB represents an important target for the neuroprotective functions of different systemic agents, among which 17β-estradiol is the most effective in protecting brain microvascular endothelium, preventing inflammation-induced tight junction breakdown by modulating ICAM-1 (Maggioli et al., 2016). At the same time, the known anti-inflammatory action of estrogen on astrocytes and microglial cells is carried out through their neuroprotective action against the detrimental effects of glial scars (Martin-Jiménez et al., 2019).Lack of malignant properties in both female and male patient WM- and GMderived hBMECs (freshly isolated and after thawing) were highlighted both by “contact inhibition” behavior shown by hBMECs at their confluence and by immunofluorescence staining for specific surface antigens of endothelial cells from different tumor entities. The constitutive staining of CD31 found in hBMECs derived from both female WM and male GM could be attributable to normal features of aged BBB microvessels, hence even the non-statistically significant differences in CD31 expression between all types of hBMECs observed before and after cryopreservation might suggest that CD31 is gender- and tissue source-independent in older adult donors, when compared with the upregulation of this marker in TNF-α activated hCMEC/D3 cells (Weksler et al. 2005). Further support to these features is the negative staining for E-selectin or CD62E, a cell-surface glycoprotein expressed by endothelial cells of high-grade gliomas, as well as by hCMEC/D3 cells upon cytokine activation (Weksler et al., 2005), but unexpressed in endothelial cells from normal brain or low-grade gliomas (Miebach et al., 2006). Notably, the lower fluorescence intensity for CD54 staining in male GM-derived hBMECs compared with CD54 staining in female WM-derived hBMECs could be attributable to the gender-specific differences of cell adhesion molecule expression in human BBB endothelial cells, outlined by Hunter et al. (2019).
Limitations
Although we have established the lack of malignant properties in WM- and GM-derived hBMECs of female and male patients, their source from peritumor resections may limit the clinical significance of this translational study. However, the availability of resections obtained only from patients during scheduled tumor neurosurgery still allowed to standardize the optimal conditions to create viable cultures of brain endothelial cellsin vitro
, suitable for application to any other type of neurosurgical resection, which will improve in the future the predictive value of this experimental model.Conclusi
onIn conclusion and in terms of the future, the complete recovery of freshly isolated and/or cryopreserved viable autologous hBMECs might make it possible to bypass further surgical sampling from each patient for subsequent cell therapies. In addition to the relevance of hBMEC cultures for the study of their physiological role at the BBB, hBMECs can also be used as a biomimetic coating for implanted neural devices (e.g. microrecording or microstimulating systems). These cells could prevent local reactions and glial scars, therefore allowing long-lasting two-way communication between the device and the neural population of interest. For this reason, it will be useful to evaluate whether the prevention of inflammatory phenomena triggered by the foreign implant can be closely linked to the mimic of the BBB by the hBMEC coating or whether autologous origin of hBMECs could be the necessary and sufficient requirement. Finally, a sustained release of the long-acting estrogen β-estradiol 17-acetate along with suitable growth factors could be considered in biomimetic implants, when aiming to improve survival of grafted hBMECs and their re-anastomosis into the surrounding tissues.
Author contributions:
Conceptualization: LF, BP; methodology: BP, SG; investigation: LF, SG, BP; writing – original draft: BP and SG; writing – review & editing: LF, BP; funding acquisition: LF; resources: LF, PDB; supervision: LF, PDB, BP. All authors contributed to the submitted version of the manuscript and approved the final version of the manuscript.
Conflicts of interest:
No conflicts of interest, financial or otherwise, are declared by the authors.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Alisa Morss Clyne, University of Maryland, USA; Steven Levy, MD Stem Cells, USA; Paulo Lizano, Beth Israel Deaconess Medical Center, USA.
Additional file:
Open peer review reports 1, 2, and 3.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

