Potential role of PANoptosis in neuronal cell death: commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons”
Yanyan Sun, Changlian Zhu
Extensive neuronal cell death occurs during nervous system development to remove surplus, unwanted, and damaged cells. This is a highly regulated physiological process that plays a pivotal role in nervous system homeostasis and normal development. In some brain regions, more than half of the neurons are removed during normal development without interfering with the remaining cells. This gene-regulated neuronal cell deletion process is called programmed cell death (Fricker et al., 2018). However, under pathological conditions such as brain ischemia or hemorrhage and neurodegenerative and neuroinflammatory disorders, neuronal cell death may occur through gene-regulated cell death or accidental cell death in specific brain regions depending on the severity of the pathological stimuli. Accidental cell death is an uncontrolled or unavoidable biological process that involves immediate breakdown of cellular structures resulting from severe physiochemical or mechanical insult. Regulated cell death is closely integrated with signaling cascades and molecular processes that can be altered by pharmacological or genetic interventions (Cui et al., 2021).
Types of neuronal cell death:
Neuronal cell death has been classified into three major types – namely apoptosis, autophagic cell death, and necrosis – according to their morphological characteristics, biochemical changes, and functional aspects. Apoptosis is considered to be the predominant mechanism of regulated cell death, especially during normal brain development processes, but also under pathological conditions (Li et al., 2020). Autophagic cell death is a form of regulated cell death that refers to a self-sacrificing mechanism that degrades cellular contents. Necrosis is characterized by the swelling of the cell, and thus the integrity of the cell membrane is lost and the intracellular contents leak out, which is considered an accidental and uncontrolled form of cell death. In recent years, other types of regulated cell death have been described as alternative cell death pathways, including pyroptosis, necroptosis, and ferroptosis. Cumulative evidence supports that these regulated cell death mechanisms not only have different features and pathways, but also engage in extensive cross-talk with each other (Wang and Kanneganti, 2021;Figure 1
).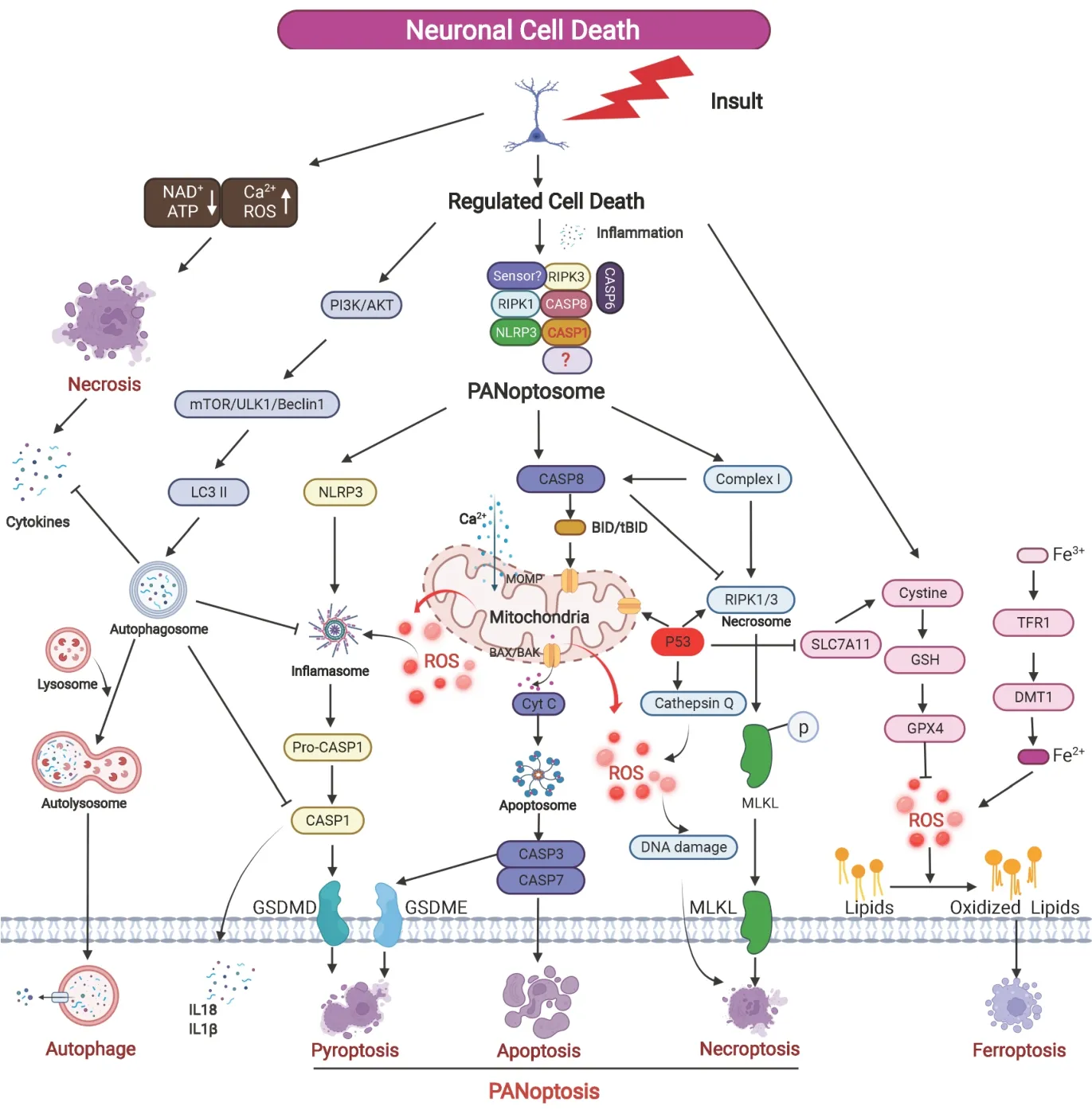
Figure 1|Graphic representation of the different types of neuronal cell death after insult. Brain insult induces accidental necrosis and regulated neural cell death. Necrosis leads to the release of cytokines that initiate inflammation and regulated cell death. PANoptosome formation after insult with specific sensor to recruit NLRP3, RIPK3, RIPK1, and CASP 1, 6 and 8 to induce GSDMD and GSDME -mediated pyroptosis, caspase-dependent apoptosis, and MLKL-mediated necroptosis. ROS involves multiple regulated cell death pathways such as ferroptosis and PANoptosis. Autophagy can inhibit pyroptosis through the degradation of CASP1, cytokines, and the NLRP3 inflammasome. CASP8 can activate BID (forming tBID) which can trigger MOMP release of pro-apoptotic proteins such as CytC and also prevent the induction of necroptosis by cleaving key proteins. AKT: Protein kinase B; ATP: adenosine triphosphate; CASP: caspase; CytC: cytochrome C; DMT: N, N-dimethyltryptamine; GPX4: glutathione peroxidase 4; GSDMD: gasdermin-D; GSDME: gasdermin-D; GSH: glutathione; LC3: light chain 3; MLKL: mixed lineage kinase domainlike protein; MOMP: mitochondrial outer membrane permeabilization; NAD: nicotinamide adenine dinucleotide; NLRP: nucleotide-binding and leucine-rich repeat protein; PI3K: phosphatidylinositol-3 kinase; RIPK: receptor-interacting protein kinase; ROS: reactive oxygen species; tBID: truncated BH3 interacting-domain death agonist; TFR: follicular regulatory T.
Pyroptosis is a type of regulated inflammatory cell death that relies on caspase-1 activation, and with morphological features of cell swelling and membrane rupture. It subsequently releases inflammatory intracellular contents such as interleukin-1β and interleukin-18. Pyroptosis depends on gasdermin D (GSDMD) protein to form membrane pores, release inflammatory responses and execute cell death (Kesavardhana et al., 2020), which relate to the pathogenic mechanisms of inflammation, neurodegenerative diseases, stroke, and brain injury. Necroptosis is a form of regulated necrosis with morphological hallmarks similar to necrosis, and it is a caspase-independent mechanism and initiated by the activation of death receptors such as tumor necrosis factor receptor. The main factors involved in necroptosis-related signal transduction include receptor-interacting protein kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain-like protein (MLKL). Transgenic models and pharmacologic inhibition have shown that RIPK1, RIPK3, and MLKL are involved in various neuropathological conditions (Yuan et al., 2019). Ferroptosis is a newly identified irondependent regulated oxidative cell death that is morphologically and biochemically distinct from other types of regulated cell death, without chromatin condensation or fragmentation, but with a shrinking mitochondrial membrane, increased membrane density, reduced or complete absence of mitochondrial cristae, and outer mitochondrial membrane rupture, which has been shown involved in multiple neurological disorders.
Interaction of different regulated cell death pathways:
Regulated cell death mechanisms such as apoptosis and autophagy play important roles in normal brain development; however, aberrant activation of regulated cell death such as apoptosis, pyroptosis, necroptosis, and ferroptosis is a common feature of neurological diseases and may be especially prominent in the developing brain. The molecular mechanisms underlying these distinct forms of regulated cell death are not independent, and recent evidence indicates that there are complex interplays and that the crosstalk between these processes (Cui et al., 2021). Pyroptosis and necroptosis are lytic, inflammatory types of regulated cell death that require the membrane damaging GSDMD and MLKL proteins, respectively. Thus caspase-1 activation in neurons may be associated with either pyroptosis or necroptosis. The pyroptosis and apoptosis pathways are closely interconnected and are mutually regulated on different levels from pathway initiation to final execution. Both genetic and biochemical data suggest that the caspase 8/RIPK1 platform is critical in the regulation of apoptosis, pyroptosis, and necroptosis (Schwarzer et al., 2020). The crosstalk between necroptosis and ferroptosis has been elucidated in multiple sites, including RIP3 and phosphatidylethanolamine-binding protein 1 interaction, RIP1, and heat shock protein 90 (Lamade et al., 2022). Increasing evidence suggest the tight interplay between the different molecular mechanisms regulating the signaling pathways of regulated cell death, and the different cell death processes might modulate each other via mutual mechanisms, which makes therapeutic targeting and clinical translation especially challenging (Bedoui et al., 2020). Therefore, it is of major importance to identify novel molecular regulators and cell death commitment points that function as triggering mechanisms and key decision points linking the different pathways of regulated cell death.PANoptosis:
PANoptosis has been identified as a form of regulated cell death that consists of apoptosis, pyroptosis, and necroptosis occurring simultaneously under the pathophysiological conditions of infectious disease (Malireddi et al., 2019). These pathways are interconnected by shared regulatory proteins named the PANoptosome, and many of the PANoptosome components have been implicated in neurological disorders (Samir et al., 2020), which opens up promising perspectives for the development of therapeutic strategies for neurological disorders. PANoptosis has been confirmed in infectious diseases that are associated with inflammation and immune activation, but there is no report yet with experimental evidence to show that PANoptosis occurs in neurons, even though the literature suggests such a possibility (Yan et al., 2022). It is thus worth investigating whether PANoptosis exists in the nervous system in order to identify the master regulator of PANoptosis to develop inhibitors to target multiple cell death pathways.In the present issue, Yan et al. (2023) report that apoptosis, pyroptosis, and necroptosis can be induced in retinal neurons, as indicated by the expression of active caspase-3, GSDMD, and MLKL in retinal neurons, and this was identified as PANoptosis-like cell death. In this study, pretreatment with selective individual inhibitor of apoptosis, pyroptosis, or necroptosis reduced regulated cell death. However, combinations of the inhibitors were not always better than the use of a single inhibitor, which suggests the existence of other signaling cascades or that other types of regulated cell death also occur either concurrently or as a backup and thus might extend the scope of PANoptosis. However, some points should be taken into consideration in this study. For example, the TUNEL assay labels all free 3′-hydroxyl termini, and it should be considered to be a method for the detection of DNA damage in general rather than being specific for apoptosis. In addition, it is noteworthy that functional crosstalk exists between cell death pathways. For example, the protein inhibitor Z-VAD-FMK that inhibits cysteine aspartate-specific proteases promotes necroptotic signaling via RIPK3-dependent MLKL phosphorylation (Samir et al., 2020). Thus, we should consider confirming not only the existence of PANoptosis-like cell death, but also the network of connected signaling pathways. The authors in this study reported expression changes of key proteins in PANoptosis-like cell death in retinal neurons after insult, which included hallmarks for apoptosis, pyroptosis, and necroptosis. Although these results indicated that apoptosis, pyroptosis, and necroptosis exist in retinal neurons, there was no evidence to suggest that all three regulated cell death pathways occur simultaneously or sequentially in the same neuron. Furthermore, the existence of the PANoptosome and the master molecular regulator that can regulate the three cell death pathways are still unclear in this paper. These gaps make the data more limited and provide only a partial interpretation, and thus the authors refer only to “PANoptosis-like” cell death.
Potential PANoptosis in developing brain:
The immature brain possesses programmed cell death machinery, and regulated cell death is more pronounced after insult, for example, in response to neonatal hypoxia ischemia (HI) brain injury. HI induces a broad range of regulated cell death processes such as apoptosis, pyroptosis, and necroptosis along with various hybrid forms of cell death (Fricker et al., 2018). HI induces microglial activation and immune cell infiltration as well as the production of cytokines such as tumor necrosis factor-α, interferon-γ, and interleukin 1β. Such sustained aseptic inflammation and immune responses are keys to many aspects of the pathogenesis and pathophysiology of neuronal cell death and brain damage. Additionally, clinical studies have shown that inflammation is associated with adverse neurological outcomes. Thus, both experimental and clinical studies have confirmed that inflammation plays important roles in neuronal cell death and brain injury in the immature brain after HI insult. PANoptosis is an inflammation-regulated cell death pathway (Lee et al., 2021), suggesting that PANoptosis is much more potentially involved in neurons of neonatal HI brain injury.In summary, these studies suggest that a high potential for PANoptosis exists in neurons under pathological conditions. Currently, there are still many unanswered questions regarding this issue, more research on PANoptosis will broaden our understanding of the fundamental processes of neuronal cell death and molecular targets and that will be leading to the development of novel therapeutics.
Related work was supported by the National Nature Science Foundation of China (81901335 to YS, U21A20347 to CZ), China Postdoctoral Science Foundation (2020M672288 to YS) and Henan Postdoctoral Research Grant (201902007 to YS).
Yanyan Sun, Changlian Zhu
Institute of Neuroscience and Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China (Sun Y, Zhu C)Department of Human Anatomy, School of Basic Medicine, Zhengzhou University, Zhengzhou, Henan Province, China (Sun Y)
Henan Key Laboratory of Child Brain Injury and Henan Pediatric Clinical Research Center, Institute of Neuroscience and Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China; Center for Brain Repair and Rehabilitation, Institute of Neuroscience and Physiology, University of Gothenburg, Göteborg, Sweden (Zhu C)
Correspondence to:
Changlian Zhu, MD, PhD, changlian.zhu@neuro.gu.sehttps://orcid.org/0000-0002-5029-6730 (Changlian Zhu)
Date of submission:
February 13, 2022Date of decision:
March 29, 2022Date of acceptance:
April 27, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346483
How to cite this article:
Sun Y, Zhu C (2023) Potential role of PANoptosis in neuronal cell death: commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons”. Neural Regen Res 18(2):339-340.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Jie Gong, Nantong University, China; Songlin Zhou, Jiangsu Key Laboratory of Neuroregeneration, Nantong University, Co-innovation Center of Neuroregeneration, China.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

