Perspective on inflammatory cytokines in open spinal dysraphism
Friederike Knerlich-Lukoschus
Myelomeningocele (MMC) is a severe form of spinal dysraphism. Due to the failure of neural tube closure during early embryonic development, the affected part of the spinal cord is left open like a book at the back of the affected child. This malformed part of the spinal cord is not covered by its protective mesodermal and ectodermal derived layers. Consequently, the exposed neural tissue (i.e., the neural placode) is prone to injury during further intra-uterine development. Former investigations in sheep MMC models and ultrasound examinations in human fetuses demonstrated progressively decreased limb function during the later fetal course (Stiefel and Meuli, 2007). As a possible morphological correlate, Stiefel and Meuli (2007) demonstrated progressive tissue destruction of the initially intact appearing unfolded neural placode in curly tail/loop tail mouse fetuses. These observations were consistent with the hypothesis of secondary damage of the neural placode (so-called “second hit hypothesis”) (Heffez et al., 1990). According to this hypothesis, the “first hit” is considered the primary structural defect, which is due to faulty developmental processes. The size and location of the spinal cord abnormality within the spinal axis are important in determining the initial functional status. The assumed ongoing toxic and mechanical damaging impacts on the exposed neural placode are viewed as the “second hit”. The second hit presumably leads to additional deficits at and below the lesion level. These processes might also be responsible for further sequelae, like the development of secondary tethered cord syndrome (TCS), that typically occur during the later clinical course of the affected child. As in spinal cord injury (SCI), the second hit presumably induces further cellular and molecular lesion cascades in the placode, which are summarized under the term “third hit” (Figure 1A
).This perspective article—which is based on recent investigations on human and rat-derived MMC specimens—focuses on potential molecular inflammatory mediators of such hypothesized “third hit” lesion cascades in the placode. In the first approach, pro-inflammatory cytokines were investigated in human MMC placode fragments which were obtained during the initial surgical reconstruction of the spinal cord and its surrounding tissue layers in the first days after birth (Kowitzke et al., 2016). In these studies, the ligand/receptor pairs interleukin (IL)-1β/IL-1R1 and tumor necrosis factor-α (TNF-α)/TNF-R1 were found to be elevated. To investigate if these mediators became detectable during the fetal development, we established the retinoic acid MMC rat model according to Danzer et al. (2011). Briefly, time-dated Sprague-Dawley rats were gavage-fed with all-trans retinoic acid (60 mg/kg) dissolved in olive oil at E10. Control animals received olive oil only. Fetuses from both groups were obtained and investigated at E16, E18, and E22 (Cohrs et al., 2021) (Figure 1B–E
exemplarily depicts immunohistochemical staining for IL-1β in fetal rat spinal cord and MMC-placodes at E16 and E22). In a further approach, we examined the expression of pro-inflammatory mediators in spinal tissue which was obtained during untethering surgery of patients who developed a secondary TCS in their later clinical course after being operated for MMC early after birth (Cohrs et al., 2019).To summarize the results, cellular gliosis with significantly elevated glial fibrillary acidic protein (GFAP)- and Vimentin-immunoreactivity became evident in human neuroepithelial MMC placode tissues that were obtained during repair surgeries early after birth (Kowitzke et al., 2016). Also, round, partially clustered CD3-, CD11b-, and CD68-positive cells were detectable in MMC cases indicating the presence of cellular inflammatory reactions (these cell types were not detectable in respective control specimens). All investigated MMC specimens exhibited significantly higher IL-1β, IL-1R1, and TNF-α immunoreactivities compared to the immunoreactivity level in normal spinal cord controls (Figure 1F
andG
). As in the immunohistochemical analyses, IL-1β-, IL1-R1-, and TNF-α-mRNA levels were found to be higher in the placodes of the MMC-group compared to expression level in control SC tissues. TNF-R1 was also elevated in the MMC specimen, but this did not reach statistical significance. Immunofluorescence staining confirmed that these cytokines were co-expressed with cellular markers of glial, inflammatory, and neuronal cells (as shown for IL-1β and IL-1R1 inFigure 1H
andI
).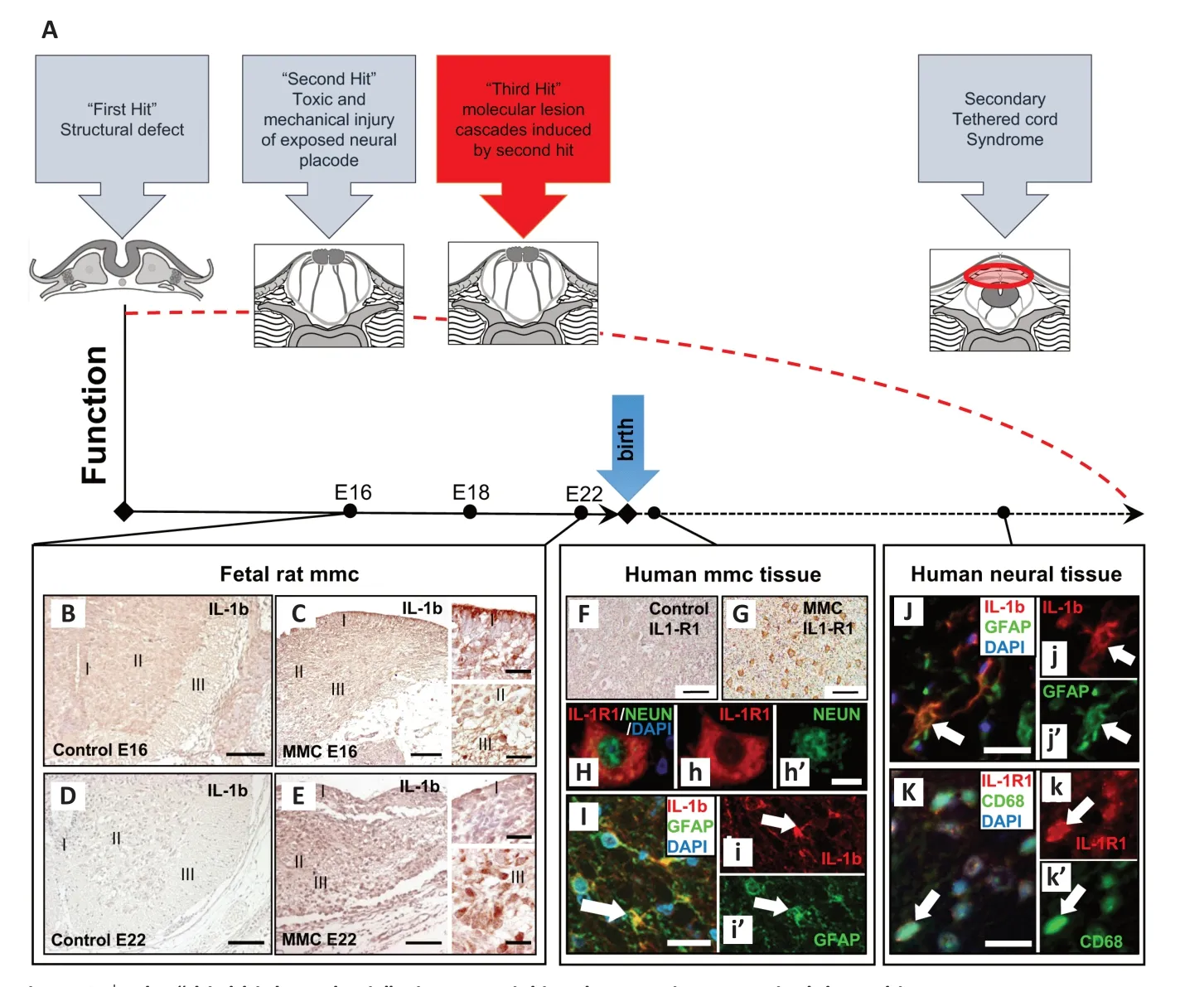
Figure 1|The “third-hit hypothesis”—its potential involvement in open spinal dysraphism. (A) The main points of the “two-hit hypothesis”, which is supplemented by a “third hit” that is postulated to be induced by the second hit. After impaired neurulation (“fist hit”), the spinal cord (SC) placode is left uncovered and thus unprotected at the back of the child. Traumatic external impacts (“second hit”) to this initially functional tissue induce molecular cascades (“third hit”) that lead to a progressive decline of neural function during the further developmental time course. One focus of our studies was to examine potential inflammatory mediators of the “third hit” in rat fetal and human postnatal myelomeningocele (MMC) tissues. Further, the problem of open dysraphism associated sequelae such as development of secondary tethered cord syndrome (TCS) is captured in the scheme. (B–E) Immunohistochemical staining for the proinflammatory cytokine IL-1β in fetal rat spinal cord tissue: interleukin (IL)-1β diaminobenzidine staining in controls and MMC placodes on fetal day E16 (B and C) and E22 (D and E) in different anatomical regions (marked as I, II, and III in intact spinal cord controls and in the disrupted morphology in MMC specimens) (I = ependymal layer of the central canal, II = gray matter, III = white matter). (F, G) IL-1 receptor (IL-1R1) staining patterns in human control spinal cord (F) and human MMC tissue (G) obtained during repair surgeries early after birth of affected children. IL-1R1 was, among others, co-labeled with the neuronal marker NeuN (Neuron-specific nuclear protein; Neurons) (H depicts merged images; h depicts channel for IL-1R1 labeling (red) and h’ the channel for NeuN labeling of the neuronal nucleus (green)). (I) It depicts merged immunofluorescence labeling of IL-1β (I; red) and GFAP (glial fibrillary acidic protein) (I; green). DAPI (4′,6-Diamidin-2-phenylindol; for nucleic acid staining; blue). Similar staining patterns were seen in tissue specimen obtained during surgeries of patients who developed secondary tethered cord syndrome in their later clinical course after being operated for MMC defects in their first days of life. (J, K) Merged immunofluorescence labeling of IL-1b (red in J) and GFAP (green in J’) and IL-1R1 (red in K) with the inflammatory cellular marker CD68 (green in K’). Arrows in immunofluorescence staining depict cellular co-localization. Scale bars in A, B, C, D, E, F: 100 µm. Scale bars in the enlarged detail pictures of B and D, and in G, H, I, J: 20 µm). Adapted from Cohrs et al. (2019) and Kowitzke et al. (2016) with permission.
In rat fetal MMC tissues, there was strong immunoreactivity for Nestin and Vimentin at E16 and E18 in cells exhibiting long immunoreactive processes that traverse through the neuroepithelium. At E22 there was a shift with less nestin- and more GFAPimmunoreactive cells appearing in the placodes. GFAP reactive astroglia thereby appeared in a morphologically active state with thickened cellular processes compared with non-reactive astroglia in control specimens. In addition to astrogliosis, round inflammatory cells became detectable at E18 and E22. IL-1β was significantly elevated on mRNA-level on E22 in MMC fetuses, while its receptor was found induced on day E16 and E22 in MMC placodes. The respective immunostaining exhibited strong expression in distinct regions of the placode with co-staining of inflammatory and neuronal markers. TNF-α and TNF-R1 exhibited similar expression profiles on E22. This inflammatory cytokine receptor/effector-pair became detectable at E16, E18, and E22, but reached a significantly elevated level only at the perinatal day E22. To summarize these new insights, pro-inflammatory molecules became detectable at a significantly elevated level in the late fetal and perinatal time-course in MMC rats (Cohrs et al., 2021).
To cover another aspect of open spinal dysraphism, the same inflammatory markers were investigated in neural tissue obtained from patients who suffered from secondary TCS some time after being operated for MMC defects soon after birth. These specimens exhibited strong astrogliosis with morphological signs of cellular reactivity and CD3, CD68, and CD11b immunoreactivities. Thus, similar to the findings in MMC specimens, there were signs of a distinct inflammatory cellular reaction. Further, TUNEL and PARP-positive cells indicated the appearance of apoptotic cell death in the examined materials. Along with these findings, TNF-α and IL-1β and their main receptors exhibited strong immunostaining in respective neural tissue samples, which was co-expressed with GFAP, CD68, and NeuN (Cohrs et al., 2019) (Figure 1J and K). Therefore, neuroinflammatory processes seem to also play a crucial role in the development of MMC-associated sequelae such as secondary TCS. However, the question as to when exactly these mediators are induced in the long-term course after initial MMC repair surgeries remains unanswered. The excessive cytokine immunoreactivity may reflect a chronic ongoing or acute inflammatory reaction, which, together with other mechanical and hypoxia-related factors, might underlie the pathogenesis of symptomatic retethering. Further functional studies are needed to evaluate the significance of these findings.
The presented studies demonstrated a potential role of pro-inflammatory cytokines such as IL-1β and TNF-α in the late fetal, perinatal, and further post-natal timepoints in MMC neural placodes of rat and human origin. Along with other factors, these cytokines are well-known molecular mediators of secondary lesion cascades, which are induced after traumatic SCI. Under pathological conditions, these mediators exceed their normal expression level and become involved in pro-inflammatory and pro-apoptotic cascades, which have the potential to further damage primarily intact tissue (summarized under the term “second lesion” in the neurotrauma literature). Because mechanical injury to the neural placode is considered one crucial factor of the “second hit”, one can postulate that similar processes are induced in the neural placode. Strong gliosis and the appearance of inflammatory cells in the neuroepithelia of fetal rat and newborn human MMC specimens indicated that such damaging processes take place in open spinal dysraphism. It may be that limited intra-uterine space in addition to changes of amniotic fluid consistency increases the probability of damaging insults to the exposed neural epithelium. This may explain the elevated cytokine expression level at later fetal and prenatal time points in our studies. With their proinflammatory and pro-apoptotic properties, induction of TNF-α and IL-1β may promote further neuronal damage and thus decline neural function.
To date, the therapeutic options for open spinal dysraphism involve surgical repair of the dysraphic defect in the first hours after birth or during prenatal fetal surgery in specialized centers. In order to improve the long-term outcome of children born with MMC, special attention has been directed to refining the surgical techniques to meticulously reconstruct each malformed layer. Especially in fetal surgery handling the very delicate and fragile fetal neural tissue remains a challenge under the already complex circumstances in terms of fetal and maternal intraoperative needs. Careful reconstruction of the placode and its meninges protects the placode from environmental damaging influences, and is considered a prerequisite to effectively prevent re-tethering. However, recent publications indicate that even after such careful surgical approaches during intrauterine closure of MMC, important problems remained such as the development of early TCS (Weaver et al., 2021).
An alternative approach to surgical reconstruction of the anatomical defect in utero is the application of patches on the fetal placode to protect the exposed neural tissue from outer harmful impacts and promote cellular coverage during further development. Ongoing research has attempted to optimize the regenerative capacity of the patches by altering cellular composition and consistency of the grafts. For example, Mann et al. (2019) demonstrated different properties of patches, which were derived from acellular dermal matrixversus
cryopreserved human umbilical cord. The latter improved organized cellular outgrowth of cells into the outer surface of the graft (i.e., promoted “meningeal cell tropism”) and reduced the acute inflammatory reaction between the placode and repair site. The acellular dermal matrix graft was associated with a more inflammatory and astroglial reaction to the inner and outer surface of the patch, which may promote scar formation with resulting adhesion (tethering) of the patch to the placode (Mann et al., 2019). In the light of our findings of cytokine expression and astrogliosis in the placode, it would be interesting to investigate the influence of both patch types on the underlying placode, which was not addressed by the cited group so far. In this context, findings of another group were of interest as they demonstrated a reduction in caspase-3 (a marker for apoptotic processes), Iba1 (a cellular inflammatory marker), and GFAP expression in fetal ovine MMC placodes after applying a reverse thermal gel patch (Bardill et al., 2022). This type of patch has the capability to undergo a spontaneous temperature-induced phase transition from liquid to gel consistency, which provides advantages in regard to potential application during in-utero MMC repair surgery.Considering the presented data of our studies and those in the current literature, application of specifically TNF-α-blocker or IL-1R1-antagonist proteins during the initial repair surgery for MMC or patch application may provide an adjuvant approach to potentially prevent further damaging of the placode and prevent long-term complications such as secondary TCS. Therefore, specifically anti-TNF strategies might become attractive candidates for therapeutic trials: Activated TNF-α initiates an induction of further cytokines and associated intracellular signaling pathways. Vice versa blockage of TNF-α reduces activation of diverse other cytokines and their potentially detrimental activities. Also, anti-TNF drugs have been already Food and Drug Administration-approved in chronic inflammatory diseases, though with sometimes critical side effects (Esposito and Cuzzocrea, 2011). Considering SCI, perispinal application of the TNFantagonist etanercept in the very early time-course after injury resulted in interruption of proapoptotic signaling cascades (Esposito and Cuzzocrea, 2011)). Interestingly, peripheral administration of IL-1R antagonist attenuated the inflammatory response after contusive SCI in mice (Yates et al., 2021). Considering the presented findings on cytokine expression in fetal and human MMC-placode tissues it would be interesting to examine the actual effects of such biopharmaceutical agents on the tertiary lesion cascades under the controlled settings of an MMC animal model. Thereby, the relevance of the outlined data on fetal cytokine expression in the murine MMC model has to be still confirmed in respective agematched material of human origin.
Despite the current lack of functional data, our studies have identified specific pro-inflammatory cytokines as crucial mediators of proposed tertiary lesion cascades in MMC placodes. These aspects should be considered in future approaches to protect neural components of the placode and its potential function, and prevent the development of further problems such as secondary TCS. This could be in form of combining surgical approaches with an adjuvant application of targeted antagonists to cytokine receptors and neuroprotective substrates. Considering the latter, placental mesenchymal stromal cells have been shown to promote anti-inflammatory and neuroprotective effects in SCI treatment and fetal MMC repair (Kulubya et al., 2021), making them interesting candidates for future multitargeted therapeutic approaches to open spinal dysraphism.
This perspective was written on behalf of our group with special thanks to Gesa Cohrs and Janka Held-Feindt (Department of Neurosurgery, University of Schleswig-Holstein, Kiel, Germany), inspiring principal investigators and warm-hearted co-workers. Particular thanks go to Bea Drucks, Ann-Kathrin Blumenröther, and Jan-Philip Sürie, hardworking students who always gave inspiring inputs during work on their theses.
Friederike Knerlich-Lukoschus
Department of Neurosurgery, Section Pediatric Neurosurgery, University Medical Center Gӧttingen, Gӧttingen, Germany
Correspondence to:
Friederike Knerlich-Lukoschus, MD, PhD, friederike.knerlich-lukoschus@med.uni-goettingen.de.
https://orcid.org/0000-0003-4907-015X (Friederike Knerlich-Lukoschus)
Date of submission:
December 23, 2021Date of decision:
February 17, 2022Date of acceptance:
February 26, 2022Date of web publication:
July 1, 2022https://doi.org/10.4103/1673-5374.343901
How to cite this article:
Knerlich-Lukoschus F (2023) Perspective on inflammatory cytokines in open spinal dysraphism. Neural Regen Res 18(2):329-330.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Ramesha Papanna, The University of Texas Health Science Center at Houston (UTHealth), USA; Fang Gao, Fourth Military Medical University, China.
Additional file:
Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

