Schwann cell extracellular vesicles: judging a book by its cover
Steven L. Gonias, Wendy M. Campana
Schwann cells (SCs) are essential in the development of the peripheral nervous system (PNS), in PNS injury, and the aging PNS. SCs comprise greater than 90% of the nucleated cells in peripheral nerves (Campana, 2007). Myelinating SCs insulate large-diameter axons, forming a 1:1 relationship and promoting saltatory conduction of axonal action potentials. Non-myelinating SCs ensheathe clusters of smaller diameter axons in Remak bundles. Both types of SCs provide essential metabolic and trophic support to axons, which is essential for maintaining axon integrity in healthy peripheral nerves.
Following an injury to the PNS, Wallerian degeneration occurs in the distal segment of the injured nerve, preceding functional nerve regeneration. SCs play a central role in this process. Perhaps by sensing macromolecules released by damaged axons, SCs undergo transdifferentiation into the repair phenotype (Jessen and Mirsky, 2019). This process is guided by well-characterized cell-signaling pathways, which culminate in the activation of c-Jun and ERK1/2. Many properties of repair phenotype SCs are essential for Wallerian degeneration and functional PNS regeneration. Almost immediately after PNS injury, SCs decrease the expression of genes encoding myelin-associated proteins and activate processes that lead to the disassembly of existing myelin. SC reprogramming in PNS injury also induces the expression of genes encoding diverse cytokines, including but not limited to tumor necrosis factor alpha (TNFα), leukemia inhibitory factor, and interleukin-1β (Shamash et al., 2002). These cytokines regulate SC physiology in autocrine pathways and control intraneural inflammation by functioning as chemoattractants and regulators of gene expression in inflammatory cells. For Wallerian degeneration and functional regeneration to proceed effectively, cytokine levels in the injured PNS must be regulated both spatially and temporally.
Exosomes and other extracellular vesicles (EVs) are well-described mediators of intercellular communication (van Niel et al., 2018). Exosomes range in size from 40–150 nm and are formed by inward budding of late endosomes to form multivesicular bodies. Other EVs, such as shedding microvesicles and apoptotic bodies, are formed at the plasma membrane and tend to be somewhat larger than exosomes. EVs carry bioactive macromolecules, including mRNAs, microRNAs, and proteins, which may be transferred from EVgenerating cells to target cells following EV uptake. Cargo transfer via EVs constitutes a powerful mechanism by which EV-generating cells regulate gene expression and cell physiology in target cells. Blood and cerebrospinal fluid contain exosomes and other EVs, produced by diverse cell types. Therefore, EVs may regulate cell physiology in target cells via autocrine, paracrine, and endocrine pathways. EVs also regulate the physiology of EVgenerating cells by providing a mechanism for the release of bioactive macromolecules.
The activity of microRNAs, which are transported as EV cargo, has been of great interest because individual microRNAs may regulate the translation of over 200 different mRNAs. The microRNAs in small EVs released by SCs have diverse effects on PNS neurons. For example, mir-21a, which is released in EVs generated by Repair Phenotype SCs, promotes the regeneration of targeted neurons (López-Leal et al., 2020). Mechanisms of EV activity involving less well-described forms of cargo also may be important in the injured PNS. For example, SC EVs may transfer ribosomes together with mRNAs to associated axons and thereby drive protein synthesis in axons, independently of the neuronal cell body (Court and Alvarez, 2016).
Our laboratories are interested in EV bioactivities associated with the EV plasma membrane. There are already exciting examples in which EV surface macromolecules regulate cell physiology and pathophysiology. EV plasma membrane-associated integrins target EVs to specific cell types and trigger cell signaling in target cells (Hoshino et al., 2015). Neutrophil elastase, which associates with the external surfaces of EVs, degrades extracellular matrix proteins and thereby remodels tissue (Genschmer et al., 2019). EVs derived from cancer cells may express tumor-associated antigens, allowing these EVs to capture cancertargeting antibodies (Capello et al., 2019). Finally, we have shown that EVs harvested from human plasma carry abundant non-pathogenic cellular prion protein, which engages a receptor system in macrophages to regulate innate immunity and inflammation (Mantuano et al., 2022). The complete spectrum of activities attributable to EV plasma membranes probably reflects both intrinsic and extrinsic membrane proteins. The latter is associated with the external EV surface.We recently demonstrated a novel mechanism by which EVs produced by SCs may regulate cell physiology, involving the EV plasma membrane. SCs in primary culture produce EVs that are highly enriched in tumor necrosis factor receptor-1 (TNFR1; Sadri et al., 2022). In immunoblotting experiments, both TNFR1 and TNFR2 were identified in extracts of cultured SCs isolated from neonatal rats. Using available antibodies, the TNFR2 signal was significantly more intense than the TNFR1 signal. When we used the identical primary antibodies under the same conditions to examine SC EV extracts, the TNFR1 signal was profoundly amplified whereas TNFR2 was undetectable. We determined that each SC EV carries over 1000 copies of full-length TNFR1 monomer. TNFR1 accounted for as much as 2% of the total protein of the SC EVs.
SC EV-associated TNFR1 was biologically active, binding TNFα and preventing TNFα from initiating cell-signaling in cultured SCsin vitro
andin vivo
, when injected into sciatic nerves (Sadri et al., 2022). We interpreted these results to indicate that SC EVs express TNFα “decoy activity” and may inhibit TNFα from engaging TNFα receptors in diverse cells in the injured PNS. Furthermore, by purging TNFR1 from the SC, EV formation shifts the balance between TNFR1 and TNFR2 in the EV-generating cell in favor of TNFR2. This is important because TNFR2 lacks a death domain and thus, may function preferentially in tissue repair and regeneration (Faustman and Davis, 2013). Our results suggest that SC EV formation may be critical in mediating transitions in the SC phenotype required to support PNS regeneration, as opposed to Wallerian degeneration. Furthermore, by preventing TNFα from engaging receptors in macrophages in the injured PNS, SC EVs may facilitate a transition in macrophage populations from cells with M1-like properties to cells with M2-like properties (Kroner et al., 2014). This transition in macrophage phenotype also may favor processes associated with tissue repair and regeneration. Pathways by which selective sequestration of TNFR1 into SC EVs may facilitate repair of the injured PNS are diagrammed inFigure 1
.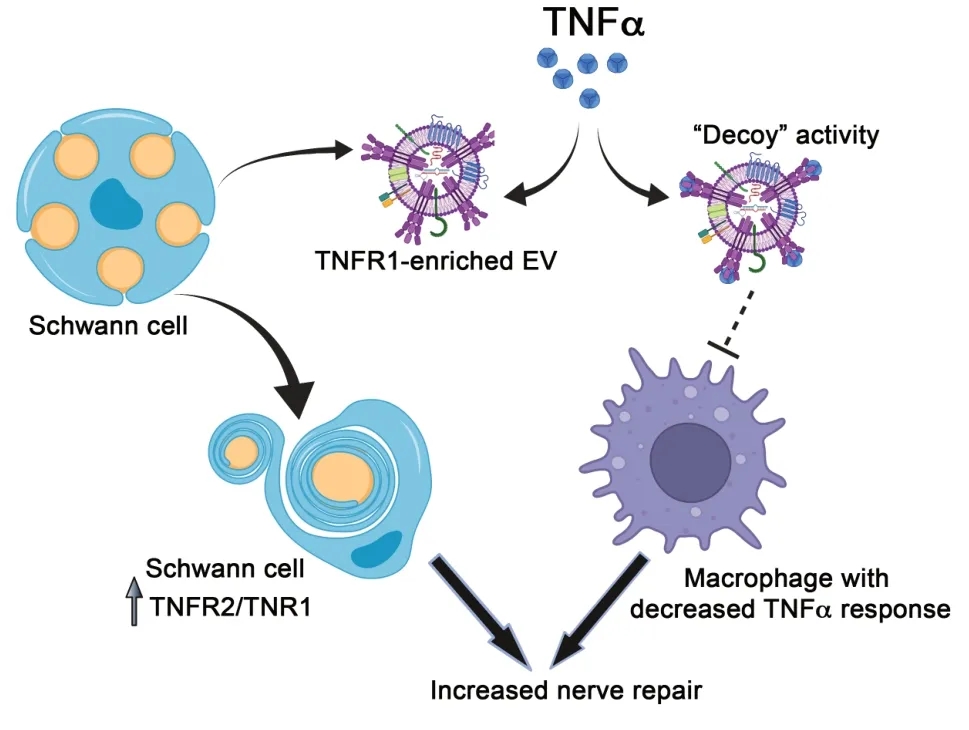
Figure 1|Selective sequestration of TNFR1 into Schwann cell extracellular vesicles may regulate the response to PNS injury. Selective sequestration of TNFR1 into SC EVs alters the balance between TNFR1 and TNFR2 in the EV-generating cell. The increase in the ratio of TNFR2/TNFR1 may alter the response of the SC to TNFα. The TNFR1-enriched SC EV functions as a TNFα decoy, providing a second layer of TNFα regulation. In the presence of SC EVs, less TNFα is delivered to various cells including macrophages in the injured peripheral nerve. As a result, macrophages also may undergo phenotypic modulation in favor of processes involved with nerve repair. EV: Extracellular vesicle; PNS: peripheral nervous system; SC: Schwann cell; TNFα: tumor necrosis factor alpha; TNFR: tumor necrosis factor receptor.
Soluble forms of TNFR1 and TNFR2, which are shed from cell surfaces by the transmembrane protease, ADAM17/TACE, have been recognized as regulators of TNFα activity for some time; however, Hawari et al. (2004) demonstrated that full-length TNFR1 in association with EVs may be the principal form of TNFR1 found in plasma. Although SC EVassociated TNFR1 may function as a powerful TNFα decoy in the injured PNS, the activity of SC EVs in TNFα regulation may be bimodal. Because the association of TNFα with EV-associated TNFR1 is reversible, when the concentration of soluble TNFα begins to decrease in the injured PNS microenvironment, TNFα may be released from SC EVs, causing the decrease in free TNFα to be less precipitous.
The discovery of cytokines as spatial and temporal regulators of the response to PNS injury revolutionized the cell-cell communication field in PNS biology. TNFα is expressed early in the course of PNS injury and is important in orchestrating the subsequent expression of many secondary cytokines (Campana, 2007). Our work on SC EVs suggests that EVs may provide a novel layer of regulatory control in PNS injury. Many questions remain regarding the role of this unique EV mechanism in PNS injury. To what extent is selective sequestration of TNFR1 into EVs replicated by SCsin vivo
? If TNFR1 is excluded from EV biogenesis pathways, would this compromise SC viability? Furthermore, do SC EVs regulate the relative proportions of different inflammatory cells at sites of injury in the PNS? The availability of well-characterized rodent models of PNS injury will provide a unique opportunity to learn more about the activities of SC EVs under pathological conditions.The capacity of SCs in culture to selectively sequester TNFR1 into EVs also raises questions regarding inflammatory states in general. Is selective sequestration of TNFR1 into EVs a unique property of SCs or do other cell types mimic this process? Do TNFR1-enriched EVs represent a novel class of anti-inflammatory agents? If so, are there advantages to naturally produced SC EVs compared with EVs engineered using HEK293 cells to function as TNFα decoys (Duong et al., 2019)? The latter question may be related to the problem of EV targeting, which is incompletely understood. Macromolecules displayed on the surfaces of EVs target the EVs and their powerful cell-regulatory cargo to specific cell types (van Niel et al., 2018). Understanding the cell surface proteome of SC EVs is an important future objective.
In summary, SC EVs carry abundant TNFR1 that is capable of reversibly binding TNFα. SC EV TNFR1 may play an important role in the initiation, maintenance, and resolution of neuroinflammation by regulating TNFα activity in PNS injury. In addition, selective sequestration of TNFR1 into EVs may shift the balance of TNFRs in SCs in favor of TNFR2 so that the response to TNFα is altered in the EV-generating cell. Because TNFα directly activates nociceptors in the PNS (Campana, 2007), SC EVs also may emerge as exciting regulators of pain states.
We acknowledged all contributions to the scientific areas addressed in this perspective, yet were limited by allowable citation numbers.
This work was supported by 1I01 RX003363 from the Veterans Administration (to WMC).
Steven L. Gonias, Wendy M. Campana
Department of Pathology, University of California, San Diego, CA, USA (Gonias SL)Department of Anesthesiology, Program in Neurosciences, University of California; The Veterans Administration San Diego Healthcare System, San Diego, CA, USA (Campana WM)
Correspondence to:
Wendy M. Campana, PhD, wcampana@health.ucsd.edu.https://orcid.org/0000-0002-9039-1396
(Wendy M. Campana)
Date of submission:
January 29, 2022Date of decision:
March 21, 2022Date of acceptance:
April 29, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346478
How to cite this article:
Gonias SL, Campana WM (2023) Schwann cell extracellular vesicles: judging a book by its cover. Neural Regen Res 18(2):325-326.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

