The Wnt/β-catenin signaling: a multifunctional target for neuroprotective and regenerative strategies in Parkinson’s disease
Annalucia Serafino, Mauro Cozzolino
Parkinson’s disease (PD) is the most frequent movement disorder and the second most prevalent age-related neurodegenerative disease (ND) worldwide. From the clinical point of view, it is characterized by severe motor complications, including uncontrollable resting tremors, rigidity, bradykinesia, and postural instability. These motor symptoms are caused by the selective and progressive degeneration of midbrain dopaminergic neurons in the subtantia nigra pars compacta and their striatal projections (Kalia and Lang, 2015), which leads to a substantial reduction in dopamine production. Motor features of PD are associated with the accumulation of pathological aggregates of α-synuclein into Lewy bodies, considered the most typical hallmarks of the disease. Non motor symptoms, including the progressive impairment of cognitive, autonomic, and mood functions, are additional PD-associated clinical complications, consequent to damage in other regions of the central and peripheral nervous system (Kalia and Lang, 2015; Schapira et al., 2017). Indeed, besides dopaminergic neuron degeneration, it is now clear that dysfunction of glial components and other kinds of neurons also participate in PD pathogenesis and progression.
Current and emerging therapeutic aspects in PD:
Currently, a disease-modifying therapy for PD is not available, and treatments are focused on moderating symptoms through pharmacotherapy with levodopa, alone or combined with dopamine receptor agonists and monoamine oxidase-B or catechol-O-methyltransferase inhibitors. This pharmacological strategy does not prevent dopaminergic neuron damage but only controls primary motor symptoms at the early stages of PD. With disease progression, the responsiveness declines and the long-term use causes undesirable side effects including dyskinesias and motor fluctuations. Thus, the development of new and more effective therapeutic strategies is required and the deepening of the molecular mechanisms underlying PD onset is a fundamental step to reach this goal. Until now, the mechanisms triggering this ND are still largely unknown. Only about 5% of PD cases are familial forms, that have been associated to autosomal dominant or recessive mutations inLRRK2
,SNCA
,VPS35
,PINK1
,Parkin
, andDJ-1
genes (Lill, 2016). The vast majority of cases (about 95%) are sporadic, and the underlying causes have been related to various environmental factors leading to neuroinflammation, including pollutants and life styles, oxidative stress, dysfunctions of mitochondria, and changes in neurotransmitter receptors (Helley et al., 2017; Guo et al., 2018). The demonstrated role of such multiple factors that synergistically might contribute to the pathophysiology of PD, supports the emerging concept that this ND, similar to other neurodegenerative disorders such as amyotrophic lateral sclerosis, is effectively a multifaceted syndrome. Under this point of view, the idea of “one-diseaseone target” followed in the past decades might explain the failure of PD-modifying therapies tested so far.Therapeutic perspectives and challenges:
On this ground, synergic therapeutic approaches and/or the targeting of multifunctional pathways possessing both neuroprotective and regenerative potentialities might be a winning strategy to overcome this multifaceted feature of PD and for developing an effective therapy. Potential targets for developing PD-modifying therapies include neuroinflammation, mitochondrial dysfunction and oxidative stress, LRRK2 kinase activity, calcium channel activity, autophagy-lysosomal pathway as well as α-synuclein accumulation and aggregation (Kalia and Lang, 2015). Among the currently explored synergic therapeutic approaches, strategies combining the treatment with neuroprotective molecules, to slow down the disease progression, with stem-cell-based therapies for stimulating nerve repair, seem the most promising. These neuroregenerative methodologies may imply the grafting of exogenous stem cells, differentiatedin vitro
orin vivo
after transplantation, or the stimulation of endogenous neural progenitors for improving the release of trophic, differentiating, and growth factors for brain repair (Bonaventura et al., 2021; Silva et al., 2022). Further,in vitro
andin vivo
evidence has recently revealed that the use of stem-cell secretome, a mixture of immune/neurotrophic factors and vesicular fractions released by neural and non-neural stem cells that acts in a paracrine fashion, could be beneficial for PD treatment (Willis et al., 2020). The chance of success of such synergic therapeutic approaches might be increased if we concomitantly target multifunctional pathways able to restore neuron functions and to stimulate the circuit reconstruction in the injured brain.The Wnt/β-catenin pathway as an attractive therapeutic target for PD:
The Wnt/β-catenin (or canonical Wnt) signaling is an evolutionarily conserved pathway that has an essential role not only in normal embryonic development but also in the preservation of adult tissue homeostasis. As schematized inFigure 1A,
in the central nervous system this pathway has a neuroprotective capacity and regulates several aspects of neuronal functions including differentiation, synapse formation and neurogenesis. Furthermore, numerous recent evidence indicates that the up-regulation of the Wnt/β-catenin pathway is able to re-activate neurogenesis and stimulate the inherent selfrepair capacities in the injured brain (Marchetti et al., 2020). Owing to its multifunctional and crucial role in the central nervous system, dysregulation of the Wnt/β-catenin signaling has been suggested as a new pathological mechanism leading to PD and other NDs (Serafino et al., 2020). In the healthy brain, and specifically midbrain, the balancing between active (Wnt-ON) and non-active (Wnt-OFF) Wnt/β-catenin signaling in dopaminergic neurons is regulated by the microglial/astrocytic system, that actively contributes to the regulatory and self-adaptive circuit against oxidative stress caused by neurotoxins, inflammatory cytokines, growth factor deprivation and aging. Microglial/astrocytic cells are a major source of Wnt ligands and express a variety of Wnt pathway Frizzled receptors, that contribute to bidirectional astrocyte-microglia and glia-neuron crosstalk. Dysregulation of this crosstalk, consequent to neuroinflammation, causes decreased neuroprotection and neuron loss, which ultimately may result in neurodegeneration. Thus, strategies aimed to control the activation state of glial cells could also be useful for restoring the Wnt-ON/Wnt-OFF balancing in the injured brain.Therapeutic strategies targeting the Wnt/β-catenin signaling:
Pharmacological and cellular therapies, based on the activation/modulation of this signaling in both neurons and glial cells, have been shown to be neuroprotective, to promote endogenous neurogenesis and to improve neuro-restoration in PD experimental models (Colini Baldeschi et al., 2018; Serafino et al., 2020; Giovannini et al., 2021). Moreover, it has been demonstrated that appropriate levels of Wnt signaling are essential for improving both quantity and quality of stem cell- or reprogrammed cellderived midbrain dopaminergic neurons to be employed in transplantation-based reparative approaches for PD. Multiple strategies are currently explored in this area(Figure 1B).
Wnt/β-catenin signaling activators such as Wnt1 or Wnt1 agonists can improvein vitro
the differentiation of pluripotent stem cells into dopaminergic neurons, or can be employed for reprogramming somatic cells into dopaminergic progenitors/neurons, before cell transplantation. Moreover, Wnt signaling modulators can be used to regulate the functions of endogenous, reprogrammed, or transplanted dopaminergic progenitors/neurons. As an alternative reparative approach, the use of stem cell secretome for the treatment of various pathological conditions, also including NDs, is currently receiving great attention. Very early evidence indicates that secretome released by neural stem cells contains Wnt pathway-related molecules such as β-catenin and Wnt ligands and that secretome from glial cells upon activation of canonical Wnt signaling contains numerous potential neurite growth-promoting factors (Musada et al., 2020; Torres et al., 2021). Thus, the activation/modulation of this signaling could also be useful for improving the neurodegenerative potential of secretomebased therapeutic strategies for PD and other NDs.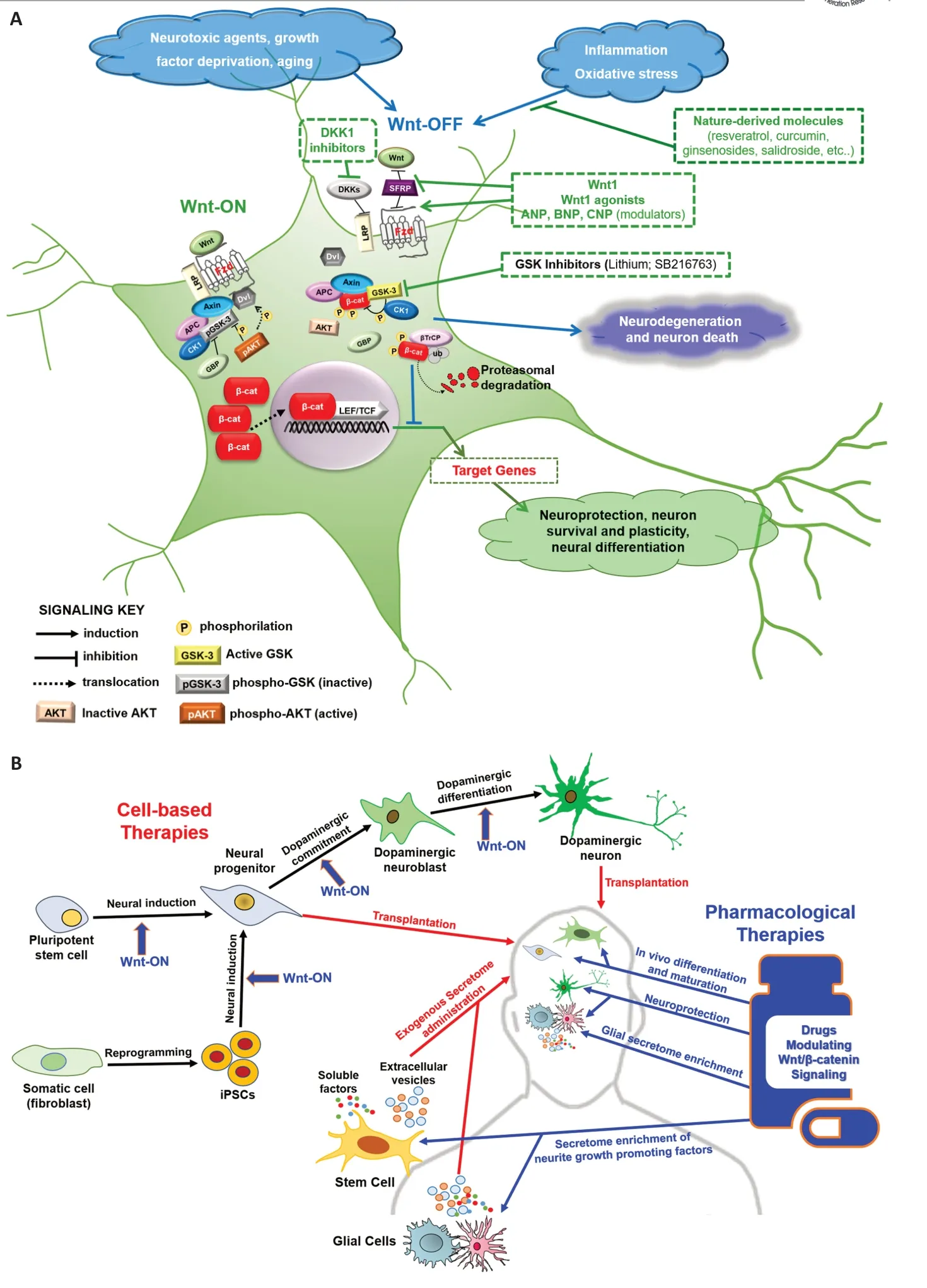
Figure 1| Schematic representation of the Wnt/β-catenin pathway in the CNS (A) and multiple strategies of targeting the Wnt/β-catenin signaling in cellular and pharmacological therapies for PD (B).(A) In intact midbrain, Wnt/β-cat signaling functions as a microenvironmental sensor that balances cell survival and death. Wnt ligands (Wnt1, Wnt2, Wnt3, Wnt3a, Wnt8, and Wnt8a), upon the binding to the Fzd receptor and the LRP5/6 co-receptors, activate the pathway (Wnt-ON) by blocking the GSK-3β-induced phosphorylation and degradation of β-catenin at the proteasome. Stabilized β-catenin accumulates in the cytoplasm and translocates into the nucleus where, by acting as a co-activator for TCF/LEF-mediated transcription, it triggers the expression of target genes involved in neuroprotection, neuron survival and plasticity, and neural differentiation. Conversely, negative regulators of Wnt signaling, such as SFRPs or DKK, oxidative stress, inflammation, neurotoxic agents, growth factor deprivation, or aging antagonize the Wnt/β-catenin signaling in neurons. In this state of Wnt-OFF, the β-catenin excess is rapidly phosphorylated by GSK-3β at the APC/axin/GSK-3β destruction complex, and then subjected to ubiquitin-proteasomal degradation. As a consequence, the transcription of Wnt target genes involved in neuron survival is inhibited. The main pharmacological approaches targeting the signaling for achieving beneficial effect in PD are highlighted in dotted rectangles. (B) In cell-based therapies, Wnt/β-catenin signaling activators can improve in vitro the differentiation of pluripotent stem cells or iPSCs towards dopaminergic neurons before cell transplantation, or regulate the maturation and functions of transplanted dopaminergic progenitors/neurons. Activation of the signaling in stem cells and glial cells by drugs modulating the signaling might increase the content of neurite growth-promoting factors in the released secretome both in vitro, useful for exogenous secretome administration, and in vivo. In the pharmacological approaches, molecules able of selectively modulating different events of the Wnt/β-catenin signaling can be neuroprotective, by acting on both neurons and glial cells, and promoting dopaminergic differentiation of endogenous neural stem cells, thus improving neuro-restoration in PD. APC: Adenomatous polyposis coli; β-TrCP: E3 ubiquitin ligase; CBP: CREBbinding protein; CK: casein kinase; CNS: central nervous system; DKK: Dickkopf; Dvl: Dishevelled; Fzd: frizzled; GBP: GSK3-binding protein; GSK: glycogen synthase kinase; iPSCs: induced pluripotent stem cells; LEF: lymphoid enhancer factor; LRP: LDL receptor-related protein; PD: Parkinson’s disease; SFRPs: secreted frizzled-related proteins; TCF: T-cell factor; ub: ubiquitination.
As it concerns the pharmacological targeting of the Wnt/β-catenin signaling as a possible therapeutic approach, there are increasing and compelling data obtained from preclinical studies on cellular and animal models of PD and other NDs, employing both synthetic and natural molecules. Furthermore, some Wnttargeting compounds are under evaluation in clinical trials for many neurological disorders, including PD, even if their efficacy is not directly or specifically related to an action on the signaling (Serafino et al., 2020). Among the bioactive modulators of the Wnt/β-catenin signaling that are under examination as potential therapeutics for PD(Figure 1A),
there are drugs targeting molecules involved in upstream events, such as Wnt agonists and modulators (e.g. Wnt 1, Wnt2, Wnt3, Wnt3a, Wnt8, and Wnt8a), or DKK1 and GSK inhibitors, and numerous nature-derived compounds, including resveratrol, curcumin, ginsenosides and salidroside, that seem to promote neuronal differentiation and to possess neuroprotective ability (Serafino et al., 2020). In this regard, our group has recently demonstrated that three vascular-derived hormones, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), widely expressed in mammalian central nervous system - where they contribute to many brain functions including neuroprotection, neural development and differentiation - possess neuroprotective ability inin vitro
models of PD, by functioning as Wnt modulators (Colini Baldeschi et al., 2018; Giovannini et al., 2021). Moreover, the three natriuretic hormones are able to improve the dopaminergic differentiation and maturation of hiPSCs-derived neural progenitors, concomitantly activating the canonical Wnt signaling (Giovannini et al., 2021). Even if the mechanism through which these natriuretic peptides trigger the Wnt/β-catenin pathway has not been completely clarified, preliminary indirect evidence suggests that they might act as Wnt signaling modulators through a frizzled-related modality, rather than through an action on the specific NPR receptors (Colini Baldeschi et al., 2018; Giovannini et al., 2021). These data support the relevance of exogenous atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide as attractive therapeutics for both neuroprotection and neuro repair for PD, as well as for other NDs such as Alzheimer’s disease and amyotrophic lateral sclerosis, for which a role of aberrant Wnt signaling has been demonstrated (Serafino et al., 2020). Interestingly, these natriuretic hormones are endogenously produced and released by astroglial and microglial cells in the midbrain, where they seem to participate in dopaminergic neurons/glia communication and to have key roles in regulating neuroinflammation and neuroprotection. Our results, therefore, suggest that the autocrine and paracrine regulatory role ascribed to the glia-released natriuretic peptides in the midbrain could be also exerted through the modulation of the canonical Wnt signaling. Thus, strategies aimed to control the release of these hormones by the microglial/astrocytic system could be beneficial for restoring the Wnt-ON/Wnt-OFF balancing in the PD brain.Conclusions and remarks:
In conclusion, as depicted in this work, the multifunctional nature of the Wnt/β-catenin pathway makes the targeting of this signaling one a highly attractive strategy to overcome the multifaceted feature of PD and for developing a disease-modifying therapy(Figure 1B)
. As described above, multiple approaches can be followed to exploit the multifunctional properties of this signaling, including both pharmacological and cellbased therapies. The established role of the Wnt/β-catenin signaling in adult neurogenesis strengthens the chance that molecules targeting this signaling could be effective not only in contrasting the PD progression, but also in stimulating the endogenous neural stem cells niches and counteracting dopaminergic neuron loss. Additionally, secretome-based therapeutic strategies for PD could be made more effective by up-regulating,in vitro
orin vivo
, the canonical Wnt signaling in stem cells or glial cells.However, some limitations must be kept in mind when targeting a signal transduction pathway critical for adult tissue homeostasis as well as for both normal and cancer stem cell maintenance, such as the Wnt/β-catenin signaling. Indeed, the use of drugs targeting such a crucial pathway could function as a double-edged sword. For example, molecules that irreversibly activate the Wnt signaling might be protective and reparative in NDs, but could promote cancer onset and metastases.Vice versa
, agents that antagonize Wnt signaling might have a beneficial effect on cancer outcomes but, could lead to neurotoxic complications. The use of bioactive molecules able to modulate rather than irreversibly activate the pathway might limit these risks, since, as modulators, they might effectively revert aberrant Wnt signaling in neurodegenerative conditions, restoring the Wnt-ON/Wnt-OFF balancing in the brain without interfering with the key role that this pathway has in adult tissues or promoting cancer. Furthermore, increasing our knowledge on this pathway and on the crosstalk with other signaling pathways will indeed offer new clues to develop safe Wnt-targeting therapeutic strategies, that might hopefully improve the current therapeutic approaches and open up a new perspective for developing diseasemodifying treatments for PD and NDs.This work was supported by Institute of Translational Pharmacology, National Research Council (IFT-CNR), Projects DSB.AD007.088 to AS.
Annalucia Serafino, Mauro Cozzolino
Institute of Translational Pharmacology - National Research Council of Italy, Rome, Italy
Correspondence to:
Annalucia Serafino, PhD, annalucia.serafino@ift.cnr.it.https://orcid.org/0000-0002-1142-4752 (Annalucia Serafino)
Date of submission:
January 11, 2022Date of decision:
February 21, 2022Date of acceptance:
March 4, 2022Date of web publication:
July 1, 2022https://doi.org/10.4103/1673-5374.34390
8How to cite this article:
Serafino A, Cozzolino M (2023) The Wnt/β-catenin signaling: a multifunctional target for neuroprotective and regenerative strategies in Parkinson’s disease. Neural Regen Res 18(2):306-308.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Ana Flávia Fernandes Ferreira, Universidade de Sao Paulo, Brazil.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

