An asymmetrically substituted dithieno[3,2-b:2′,3′-d]pyrrole organic small-molecule hole-transporting material for high-performance perovskite solar cells
Jingwen Jia, Yue Zhang, Liangsheng Duan, Quanping Wu, Yu Chen,*, Song Xue
1 Tianjin Key Laboratory for Advanced Mechatronic System Design and Intelligent Control, School of Mechanical Engineering, Tianjin University of Technology, Tianjin 300384, China
2 Tianjin Key Laboratory of Organic Solar Cells and Photochemical Conversion, Department of Applied Chemistry, Tianjin University of Technology, Tianjin 300384, China
Keywords:Hole-transporting materials DTP Perovskite Photovoltaic performance
ABSTRACT Hole-transporting materials play a vital role in terms of the performance of perovskite solar cells(PSCs).The dithieno[3,2-b:2′,3′-d]pyrrole(DTP),an S,N-heterocyclic building block,has been proved to be desirable for molecular design of hole-transporting materials in PSCs.We developed an asymmetrically substituted DTP small-molecule (JW12) and a reference compound (JW11).The asymmetrical structure of JW12 leads to different absorption properties and electron distribution.The device in a planar n-i-p architecture using JW12 shows a much higher PCE (18.07%) than that based on JW11 (15.46%), which is also better than the device based on spiro-OMeTAD (17.47%).We hope our research can provide a new perspective in molecular design of organic HTMs for perovskite solar cells.
1.Introduction
Solar energy has been utilized in many ways since ancient times due to its sustainability,easy access and vast amount.For the past five decades, various solar cells have been successfully developed to convert solar energy into electricity.Perovskite solar cells(PSCs)first emerged in 2009 with a power conversion efficiency of 3.8%.Researchers all over the world have put much endeavor and now the certified record efficiencies are approaching 30% [1-4].PSCs have the advantages of good solution processability,weak hysteresis effect and low cost of preparation, thus they exhibit great prospects for large-scale commercialization and are gaining more and more concern.In PSCs, the hole-transporting material (HTM) has an important impact on the interfacial interaction of perovskite crystals, the extraction and transport of holes, and the stability of the devices[5-8].Inorganic,organic small-molecule and polymeric HTMs are developed for highly efficient and stable PSCs.However,inorganic hole-transporting materials require high-temperature operation[9,10], and polymeric HTMs usually are not easy to purify [11-13].Among all the efforts to improve PSC’s performance,organic small-molecule HTMs have drawn much attention due to facile synthesis, easy purification, flexible modification, low cost,and noticeable efficiency [14-19].
Various organic small-molecule HTMs have been synthesized and exhibit extraordinary performances, which are mainly analogues of spiro-OMeTAD with symmetric conjugated structures.Efficient HTMs of asymmetric structures have been less reported in perovskite solar cells[20-23].Chenet al.developed asymmetric three-dimensional (3D) HTMs based on propeller-shaped triphenylethylene compounds and achieved power conversion efficiency (PCE) of PSCs rivaling that obtained from spiro-OMeTAD[24].Qinet al.fabricated a PSC incorporating asymmetric sulfonyldibenzene-based HTMs with a high PCE close to that incorporating spiro-OMeTAD[25].The distorted conformation of asymmetric central cores results in good intermolecular π-stacking and charge transport,as well as smooth and uniform surfaces covering perovskite.
DTP has been employed in organic field-effect transistors [26],dye-sensitized solar cells [27], small-molecule organic solar cells[28], polymers [29], photovoltaics and sensitive photodetectors[30]for its conjugated plane, excellent electron-donating ability and flexible structural modifications.DTP-based HTMs are reported in both conventional n-i-p PSCs and planar p-i-n PSCs[31,32].DTP,or N-aryl substituted DTP,provides adjustable energy levels, good solubility, film morphology and intermolecular packing properties[26,33].Liet al.reported new HTMs through combinations of a diphenylamine-substituted carbazole arm and different DTP compounds [34].They found out that their HTMs incorporatingN-(4-methoxyphenyl) DTP are superior toN-(4-hexyl) DTP enabling a PCE over 17%.Tanget al.applied DTPcored small molecules to fabricate dopant-free PSCs enabling efficiency over 20%[32,35].Therefore,DTP is a good building block for organic HTMs.It is worthwhile to explore the structure-efficiency relationship in DTP-based HTMs so as to produce PSCs with high efficiencies and good stability.
Herein, a molecular structure of asymmetric peripheral 4,4′-di methoxytriphenylamine and bis(4-(dimethylamino)phenyl)vinyl moieties appended to dithieno[3,2-b:2′,3′-d]pyrrole (DTP) central core is investigated.We applied organic small-molecule HTMs based on this structure in planar n-i-p PSCs, and they exhibited a PCE more than 18%.We found PSCs fabricated with our asymmetrical HTMs are superior to those based on a reference compound we prepared through a similar route.The DTP core is believed to be prerequisite for achieving high efficiency and the asymmetrical substitutions reduce intermolecular aggregation.
In our work,the asymmetrical HTM,JW12,is 4,4′-(6-(2,2-bis(4-methoxyphenyl)vinyl)-4-(4-methoxyphenyl)-4H-dithieno[3,2-b:2′,3′-d]pyrrole-2,3-diyl)bis(N,N-bis(4-methoxyphenyl)aniline).JW11, 4,4′-(2-(4′-((4′-(2,2-bis(4-(dimethylamino)phenyl)vinyl)-[1,1′-biphenyl]-4-yl)(4-methoxyphenyl)amino)-[1,1′-biphenyl]-4-yl)ethene-1,1-diyl)bis(N,N-dimethylaniline), is JW12′s reference structure, which was prepared through a similar synthesis procedure.JW12 possesses a conjugated DTP core with 4-methoxyphenyl as the lateral substituents and is end-capped with two 4,4′-dimethoxytriphenylamine groups on one side and a bis(4-methoxyphenyl)vinyl group on the other side.JW11 has a 4-methoxytriphenylamine core end-capped with bis(4-(dimethyla mino)phenyl)vinyl groups.The structures of our studied HTM(JW12) and the reference compound (JW11) are shown in Fig.1,and they are applied in PSCs to explore the structure-efficiency relationship of planar n-i-p PSCs.We hope our research can provide experimental basis and a different perspective to obtain highly efficient PSCs.
2.Materials and Methods
2.1.Materials
Tetrahydrofuran (THF) andN,N-dimethylformamide (DMF)were dried before use.The reagents came from Energy Chemical,J&K Chemical, TCI and Sigma-Aldrich, and they were used with no further purification.
2.2.Fabrication of PSCs
Perovskite solar cells of conventional planar structure, n-i-p type (ITO/SnO2/perovskite/HTM/Au), were fabricated according to the following steps: after having been etched by Zn powder and diluted HCl solution,indium tin oxide(ITO)coated substrates were washed in turn with soap, deionized water, acetone and isopropanol.Subsequently, the dry ITO substrates were treated by oxygen plasma for 15 min.Then they were covered with SnO2(4000 r·min-1,30 s)and sintered at 150°C for 30 min[36].The precursor solution of the perovskite working as light-harvesting layer,preprepared according to the literature [21,34], was spin-coated(1000 r·min-1,10 s and 6000 r·min-1,30 s),and 100 μl chlorobenzene was added exactly at 25 s while spin-coating.After the substrates were being sintered at 100 °C for 1 h in a nitrogen-filled glove box and cooled down for several minutes, the solutions of spiro-OMeTAD(70 mmol·L-1in CB),JW11 and JW12(40 mmol·L-1in CB) were doped with 0.5 eq Li-TFSI, 0.03 eq FK209 and 3.3 eq TBP, then spun on perovskite layers (4000 r·min-1, 30 s).In the end,Au counter electrodes were deposited on the top through vacuum evaporation.
2.3.Device characterization
Current-voltage (J-V) characteristics of PSCs were measured under standard AM 1.5G solar simulator (Oriel 91160-1000(300 W)) with Zennium electrochemical workstation (ZAHNER,Germany), the light intensity was calibrated by an Oriel reference solar cell.All devices were measured both forwardly (-0.1 V to 1.2 V) and reversely (1.2 V to -0.1 V) under the scan speed of 100 mV·s-1.Other measurement information could be found in Supporting Information (SI).
2.4.Synthesis of JW11 and JW12
The synthesis routes of JW11 and JW12 are shown in Fig.2.The syntheses of intermediates 1-18 have been reported[37-40].General procedures for the syntheses,1H and13C NMR spectra of JW11,compound 19 and JW12 are given in SI.
3.Results and Discussion
3.1.Optical-electrochemical characteristics and theoretical calculation
Normalized UV-Vis absorption and fluorescence emission spectra of JW11 and JW12 are illustrated in Fig.3(a).The spectral data of JW11 and JW12 are tabulated in Table 1.The maximum absorption peaks (λabs) of JW11 in DCM solution lies at 395 nm, by contrast, λabsof JW12 is blue-shifted to 385 nm, which means that the asymmetric structure of JW12 results in less conjugation compared with symmetric JW11.The same trend happened when measured in film,but only 3 nm blue shifted,as seen in Table 1.On the other hand, the absorption peaks of JW11 are almost the same when measured in solution and in solid-state film.λabsof JW12 in solid-state film is obviously red-shifted in contrast with that of JW12 in solution, which means stronger molecular interaction happened in solid-state film [41].
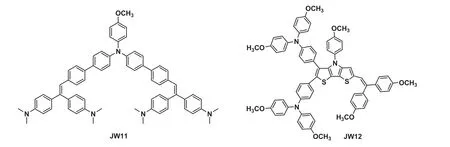
Fig.1. The molecular structures of JW11 and JW12.
The optical band gap values (E0-0) of JW11 and JW12, are 2.86 eV and 2.88 eV, respectively, determined by the formula,E0-0=1240/λint.λintis the wavelength where normalized absorption and emission spectra in CH2Cl2cross.JW11 shows smallerE0-0,manifesting that JW11 has larger π-conjugation than JW12, which is consistent with the fact that λabsof JW11 displays a red shift compared with that of JW12 both in CH2Cl2and in solid-state film.
The cyclic voltammetry (CV) curves of JW11 and JW12 are shown in Fig.3(b) with ferrocene as an internal standard.Fig.4 shows each layer’s energy level in a planar n-i-p PSC and it clearly displays that their energy levels are fit for those of the perovskite.The related electrochemical data can be found in Table 1.The energy levels of highest occupied molecular orbitals (EHOMO), are-5.51 eV for JW11 and -5.49 eV for JW12, through the formula,EHOMO= -4.7 -Eredox.Eredoxwas standardized with ferrocene(0.63 Vvs.NHE).They are pretty close and both more positive than the valence band energy of the perovskite (-5.65 eV), which suggests that it is convenient for JW11 and JW12 to extract holes from perovskite materials.The energy levels of lowest unoccupied molecular orbitals (ELUMO) are -2.65 eV for JW11 and -2.61 eV for JW12, using the formula,ELUMO=EHOMO+E0-0.Both of them are much higher than the conduction band energy of perovskite(-4.05 eV),implying that JW11 and JW12 can greatly prevent electrons from transferring back to HTMs.

Fig.3. (a) The UV-Vis absorption and fluorescence emission spectra in CH2Cl2 solution.(b) Cyclic voltammogram of JW11 and JW12.

Table 1Optical and electrochemical characteristics

Fig.4. Energy levels in n-i-p PSCs.
It is essential for molecular design of organic HTMs to balance charge transport and film-forming property by incorporating conjugated DTP core with distorted substitutes.To gain insight on the influence of geometric optimization structures and electron distribution on JW11 and JW12, density function theory (DFT) calculations were performed for the studied HTMs at B3LYP/6-31G(d)level of theory.The representative dihedral angles are displayed in Fig.S1 of SI.The electron density distributions in JW11 and JW12 are depicted in Fig.5.The dihedral angles between the central core and the end-capped groups are -34.1° (JW11) and 46.9°,38.8°and 14.3°(JW12),while the dihedral angles between the central core and 4-methoxyphenyl moiety are 38.1° (JW11) and 4.2°(JW12).Apparently, JW12 has more highly planar DTP core with more distorted ending groups compared with JW11.For JW11,the electron density distribution of its HOMO is localized on the entire molecule due to smaller dihedral angles between its central core and end-capped groups,while that of its LUMO is transferred without localization on 4-methoxyphenyl moiety.For JW12, the electron density distribution of its HOMO is mainly localized on the DTP core and one more planar 4,4′-dimethoxytriphenylamine group and bis(4-methoxyphenyl)vinyl group, while that of its LUMO is transferred on DTP and bis(4-methoxyphenyl)vinyl side.The electron-rich DTP core brings different electron transitions by introducing asymmetric ending groups.
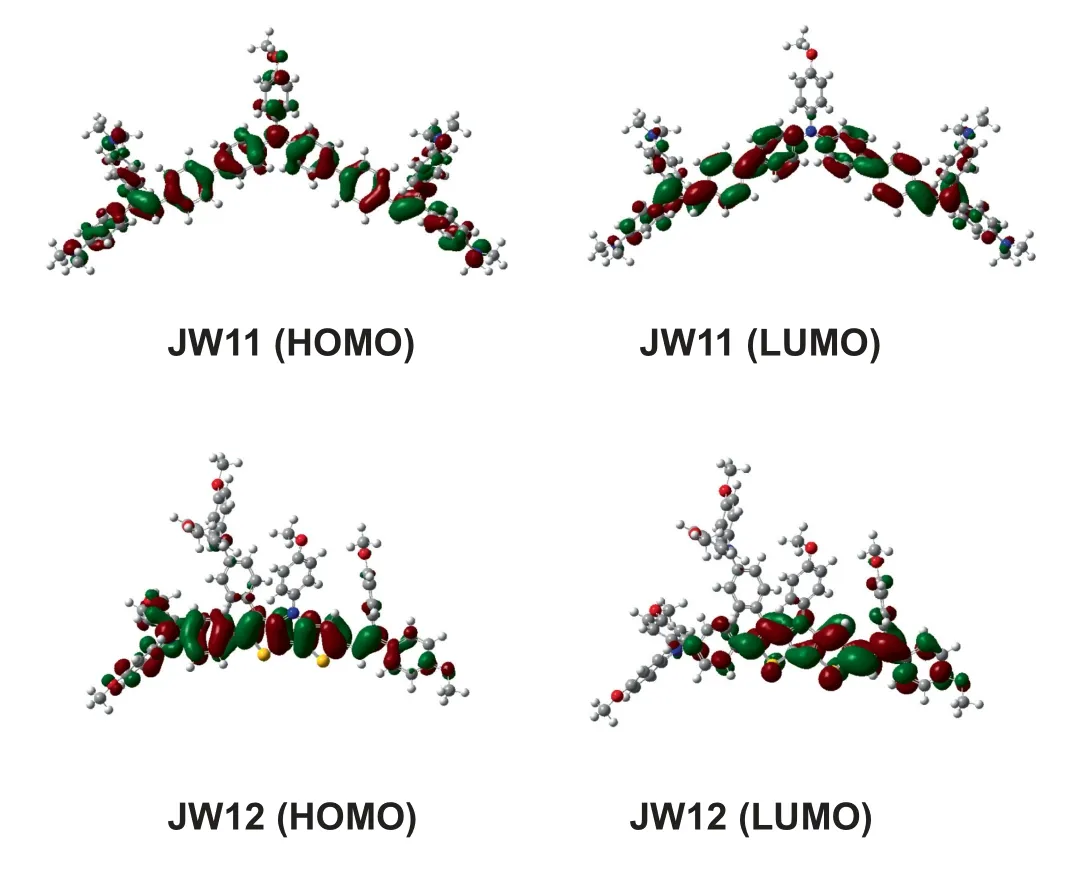
Fig.5. Molecular orbital surfaces of JW11 and JW12.
3.2.Device performance
The device efficiencies and corresponding photovoltaic performance parameters of PSCs based on JW11, JW12 and spiro-OMeTAD are summarized in Table 2.The forward-scan photocur-rent density-voltage (J-V) curves are depicted in Fig.6(a).As seen from the table,PSCs of JW12 outperform those of JW11 and spiro-OMeTAD with a decent PCE of 18.07%,short-circuit current density(Jsc)of 22.93 mA·cm-2,open-circuit voltage(Voc)of 1.11 V,and fill factor (FF) of 0.71.Both Fig.6(a) and Table 2 clearly demonstrate that open-circuit voltages (Voc) and fill factors (FF) of PSCs with JW12 and spiro-OMeTAD are almost the same, short-circuit current density (Jsc) of JW12 is slightly higher than that of spiro-OMeTAD, leading to a difference of 0.6% in PCE.Compared with JW11, JW12 has an obvious advantage, with a gap of 2.61% in PCE.These all indicate that JW12 is a very competitive candidate for HTMs in PSCs.

Table 2J-V parameters of PSCs with different HTMs: JW11, JW12 and spiro-OMeTAD
Incident photon-to-current conversion efficiency(IPCE)spectra of conventional planar n-i-p PSCs with JW11, JW12 and spiro-OMeTAD as HTMs are illustrated in Fig.6(b).A wide spectra response is observed in the range of 350-750 nm,yielding a broad IPCE plateau above 82%.IPCEs of JW12 and spiro-OMeTAD are higher than that of JW11,which brings out better short-circuit current densities.The photovoltaic performance of PSCs with JW11 and JW12 differs due to their molecular structures.The steadystate photoluminescence (PL) measurements were performed to obtain the spectra of perovskite films with or without capping the studied HTMs, as demonstrated in Fig.6(c).The peak PL value happened at 757 nm, when excited at 500 nm.A remarkable quenching of the steady-state photoluminescence (SSPL) could be seen from the figure for all the three HTMs.The SSPL quenching efficiencies of JW11, JW12 and spiro-OMeTAD are 83.8%, 86.1%and 84.3% respectively, which suggests that the best holeextraction and transfer take place at the interface of JW12 and the perovskite among PSCs with these three HTMs, achieving the biggest PCE of 18.07%.Fig.6(d) indicates the cross-sectional scanning electron microscope (SEM) image of the device.As displayed in Fig.6(d), the structure is dense and even.The thickness of the perovskite layer is around 400 nm,and the HTM layer shows some pin holes.
The photovoltaic performance of PSCs with JW11, JW12 and spiro-OMeTAD was tested by forward and reverse scan to see how the hysteresis of devices functioned.The result is tabulated in Table 3.Both the fill factor and PCE of PSCs are higher when tested in forward scan than the opposite.

Table 3J-V parameters of PSCs with JW11, JW12 and spiro-OMeTAD scanned forwardly and reversely
3.3.The stability of PSCs
To achieve stable and efficient perovskite solar cells, good heat resistance of hole transport materials is preferred.We performed thermogravimetric analysis (TGA) and the result is shown in Fig.7(a).Thermal decomposition temperatures (Td) of JW11 and JW12 are 424 °C and 235 °C, respectively.JW11 and JW12 were further purified by recrystallization in sample pretreatment.The higherTdvalue of JW11 is possibly attributed to its good crystallization in solid state.

Fig.6. (a)J-V curves.(b)Incident photon-to-current conversion efficiency(IPCE)curves and integrated current density from IPCE curves.(c)Steady state photoluminescence(PL) spectra of JW11, JW12 and spiro-OMeTAD.(d) Cross-section scanning electron microscope (SEM) image.

Fig.7. (a) Thermogravimetric analysis and (b) DSC curves of JW11 and JW12.
Thermal stability of hole transport material has great influence on the life of perovskite solar cells.When operating at higher temperatures for a long time, the heat both inside and outside of the devices will change the shape of the HTMs, which is generally transformed from an amorphous state into a crystalline state,affecting the quality of the film, accordingly, the charge transfer inside the devices will also be influenced.An important parameter to measure film morphology is glass transition temperature (Tg).When the device temperature is higher thanTg, the velocity of molecular movement increases sharply, promoting the transformation to crystalline state.Fig.7(b)illustrates the curves of differential scanning calorimetry(DSC)thermograms of JW11 and JW12.Both of their glass transition temperatures are much higher than the common operating temperatures of PSCs, which is around 80°C.Tgof JW11 is 130 °C, with 5 °C higher than that of JW12 (125°C).
4.Conclusions
Based on our previous work,an asymmetrically substituted DTP small molecule (JW12) and a reference compound (JW11) were synthesized to enhance the efficiency of PSCs.The asymmetrical structure of JW12 leads to different absorption properties and electron distribution.The maximum absorption peak of JW12 in solidstate film is obviously red-shifted in contrast with that in solution,implying stronger molecular interaction in solid-state film.Both HOMO and LUMO levels of JW11 and JW12 are suitable for valence and conduction band of perovskite.Through DFT calculation, we have proved that JW12 has more highly planar DTP core with more distorted ending groups compared with JW11.The electron-rich DTP core brings different electron transitions by introducing asymmetric ending groups.PSCs of JW12 outperform those of JW11 and spiro-OMeTAD with a decent PCE of 18.07%,Jscof 22.93 mA·cm-2,Vocof 1.11 V, and FF of 0.71.Based on these results, we believe JW12 is a very competitive candidate for PSCs.And a lot more could be done to optimize device architectures and fabrication process to get highly-efficient PSCs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Scientific Research Project of Tianjin Municipal Education Committee (2017KJ261).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.052.
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Conjugated microporous polymer membranes for chemical separations
- Atomic layer deposition of TiO2 on carbon-nanotubes membrane for capacitive deionization removal of chromium from water
- Application of pulsed chemical vapor deposition on the SiO2-coated TiO2 production within a rotary reactor at room temperature
- Process monitoring of the Au-S bond conversion in acetylene hydrochlorination
- Tuning the properties of Ni-based catalyst via La incorporation for efficient hydrogenation of petroleum resin
- Synthesis and mechanism analysis of a new oil soluble viscosity reducer for flow improvement of Chenping heavy oil
