Tuning the properties of Ni-based catalyst via La incorporation for efficient hydrogenation of petroleum resin
Qunhong Liu, Jiangtao Yang, Hongwei Zhang, Hongming Sun, Shuzheng Wu, Bingqing Ge,Rong Wang, Pei Yuan,*
1 Research Center of Chemical Fertilizer Catalyst, Fuzhou University, Fuzhou 350002, China
2 College of Chemical Engineering, Fuzhou University, Fuzhou 350116, China
Keywords:Ni-based catalyst Layered double hydroxides Promotion effect Petroleum resin Hydrogenation
ABSTRACT The hydrogenation of petroleum resin (PR) is an effective process to prepare high value-added hydrogenated PR(HPR).However,the preparation of non-noble metal-based catalysts with high catalytic activity for PR hydrogenation still remains a challenge.Herein, a La promoted Ni-based catalyst is reported through the thermal reduction of quaternary NiLaMgAl-layered double hydroxides (NiLaMgAl-LDHs).The incorporation of La is beneficial to the reduction and stability of Ni particles with reduced particle size,and the increased alkalinity effectively mitigates the breakage of molecular chains of PR.As a result,the La promoted Ni-based catalyst exhibits high catalytic activity and excellent stability for PR hydrogenation.A hydrogenation degree of 95.4% and 96.1% can be achieved for HC5PR and HC9PR with less reduced softening point, respectively.Notably, the hydrogenation degree still maintains at 92.7% even after 100 hours’reaction,much better than that without La incorporation or prepared using conventional impregnation method.
1.Introduction
C5and C9petroleum resins (C5PR and C9PR), polymerized from the C5and C9fractions produced during the production of ethylenevianaphtha cracking,are widely used in various applications such as paints, coatings, inks and rubber.However, due to the presence of olefinic double bonds and aromatic rings, these resins exhibit several shortcomings including poor thermal stability, malodour,and susceptible to oxidation, which limit their applications in high-end fields [1,2].The hydrogenation of C=C bonds and aromatic rings in PR is an efficacious process to obtain hydrogenated PR (HPR) which possesses lower bromine numbers as well as higher heat and weathering resistance.High-quality HPR has attracted great attention in food hygiene and high-grade ink field,and the ever-increasing market demand of HPR can be apparently observed [3,4].
HPR is usually obtained through hydrogenation of PR under high temperature and pressure conditions in the presence of noble metal catalysts such as Pd supported catalysts [5,6].Although Pdbased catalysts show a high hydrogenation activity, their application is restricted by the high cost, scarcity and poor tolerance to sulfur impurity in PR.Recently, Ni-based catalysts have attracted extensive interest for various catalytic reactions due to their good thermal stability, low cost, and appropriate catalytic activity [7-10].The application of Ni-based catalysts for PR hydrogenation has also been reported [11-13].Wang and co-workers prepared an eggshell Ni catalyst supported on a fluid catalytic cracking catalyst residue through an incipient wetness impregnation method,showing good hydrogenation activity for C5PR [14].The bromine value of the resin was reduced from 27 to 0.98 g Br2/100 g after hydrogenation, while the softening point showed only a slight decrease from 98 to 91 °C.Wei and co-workers reported Nibased catalyst supported on an activated fluid catalytic cracking catalyst residue (FC3R), in which PEG1000 was used to modify the Ni particles to improve the dispersion[15].The bromine value of C9PR was decreased from 46.1 to 0.72 g Br2/100 g, indicating C9PR can be hydrogenated deeply by the PEG1000 modified Ni/FC3R catalyst at relatively high temperature (270 °C).However,the particle size of the active component is still too large(>13 nm of NiO particles), which will deteriorate the hydrogenation activity to some extent and decrease the utilization of the metal atoms.Wang and coworkers constructed a nano MOFderived Ni catalyst, in which the Ni exhibited a small particle size of 5.6 nm and were embedded inside the carbon matrix [16].The catalyst with a high Ni loading of 53.1% (mass) achieved a hydrogenation activity of 96.0% under mild reaction conditions (150 °C,6 MPa,2 h) for C9PR.This work suggests reducing the particle size of active metals is an effective way to improve the activity of Nibased catalyst for PR hydrogenation.Nevertheless, high metal loading and encapsulation of Ni particles in carbon matrix reduced the utilization of the active metal atoms and limited large-scale application.The development of more facile and economical catalytic systems for PR hydrogenation is an active area of research.
Recently,the layered double hydroxides(LDHs)have been used to prepare Ni-based catalysts [17-19].The LDHs derived catalysts manifest many advantages such as high surface-to-volume ratio,uniform metal ions dispersion, efficient exposure of active sites,and easy manipulation of chemical compositions.Liang and coworkers reported a highly dispersed Cu/Ni-Al2O3catalysts using LDHs as precursors[20].With a small size of 6.3 nm and loading of 34.0% for active metals, the catalyst manifested a hydrogenation activity higher than 98% for dicyclopentadiene resin at 250 °C and 8 MPa of H2pressure.Ren and co-workers reported that a promoter can not only reduce the particle size of Ni metals, but also improve the anti-poisoning performance of the Ni/Al2O3catalyst[21].The catalytic activity for PR hydrogenation was improved and the life of the catalyst increased by about 3 times with Cu as the promoter.It should be noted that these Ni based catalysts in most reports uses Al2O3as the support.It is well known that Al2O3is acidic, and the strong acidity may accelerate the crack of C—C bond at elevated temperature.Therefore,more efforts should be devoted to tune the structural properties of the catalysts to further improve the catalytic performance for PR hydrogenation.
In this work, we report a Ni based catalyst with excellent dispersion derived from MgAl-LDHs precursors for PR hydrogenation.The structural parameters and properties of the catalyst is further tunedviaLa incorporation.The incorporation of La can not only improve the dispersion of Ni particles, but also promote the electron transfer to Ni active sites as well as improve the alkalinity of the catalyst.The catalytic performance of Ni/LaMgAl-mixed metal oxides catalyst (pre-Ni/LaMgAl-MMO) derived from LDHs and with La as the promoter, pre-Ni/MgAl-MMO catalyst without La incorporation, and the Ni/LaMgAl-MMO prepared with a conventional impregnation method (imp-NiLa/MgAl-MMO) was compared for PR hydrogenation.The bromine number of hydrogenated C5PR (HC5PR) and hydrogenated C9PR (HC9PR) is reduced from 16.40 and 24.62 g Br2/100 g to 0.75 and 0.96 g Br2/100 g, respectively, corresponding to hydrogenation degree of 95.4% and 96.1%, better than those with other catalysts.In addition, the decline of softening point for HC5PR and HC9PR was mitigated.These results suggest regulating the structural properties of Nibased catalysts is the key to the efficient hydrogenation of the petroleum resins.
2.Experimental
2.1.Materials
Mg(NO3)2·6H2O, Ni(NO3)2·6H2O, La(NO3)3·6H2O, Al(NO3)3·9H2O and urea were purchased from Aladdin (Shanghai China).The raw PR was purchased from the Shandong Qiwei Chemical Co.,Ltd.,and the bromine numbers of the C5PR and C9PR are about 16.42 and 24.60 g Br2/100 g, the softening point are 108 and 122 °C, and the Gardner color are 4.0 and 9.4, respectively.The commercial Ni catalyst was purchased from Liaoning Haitai Technology Development Co., Ltd with 35% (mass) Ni and 65% (mass) Al2O3.Cyclohexane (AR), ethanol (AR), benzene (AR), acetic acid (AR), lithium bromide monohydrate(CP)were obtained from Sinopharm Chemical Reagent Co., Ltd.All the reagents were used as received without further purification, High purity hydrogen, nitrogen helium and carbon dioxide were supplied by Fuzhou Huaxinda Gases Industry Co., Ltd.
2.2.Catalyst preparations
2.2.1.Ni and Ni-La catalysts prepared with LDHs as the precursor
Quaternary NiMgAlLa-LDHs precursor was fabricated through a precipitation method with the hydrolysis of urea.Specifically, Mg(NO3)2·6H2O (0.04 mol), Ni(NO3)2·6H2O (0.02 mol),La(NO3)3·6H2O(0.0015 mol),Al(NO3)3·9H2O(0.02 mol)and urea(0.135 mol)were firstly dissolved in deionized water (150 ml) to make a mixed salt solution.Subsequently, the solution was kept under vigorous stirring at 95°C in water bath for 18 h.After aging for 6 h,the precipitate was filtered,washed with deionized water,and dried at 90°C overnight to obtain the quaternary NiLaMgAl-LDHs precursor.The precursor was then calcined at 600 °C in air for 6 h to obtain the mixed metal oxides(denoted as pre-NiLaMgAl-MMO).Afterwards,the pre-NiLaMgAl-MMO was reduced at 800 °C in 10% (vol) H2/Ar flow(100 ml·min-1)for 4 h,and the target catalyst was denoted as pre-Ni/LaMgAl-MMO.The catalyst without La was also prepared using the similar procedure and denoted as pre-Ni/MgAl-MMO.In addition, we also prepared binary MgAl-LDHs precursor as the support.
2.2.2.Ni-La catalysts prepared by co-impregnation method
For comparison, the Ni-La catalyst was prepared by coimpregnation method using MgAl-LDHs as the support.Specifically, Ni(NO3)2·6H2O (0.021 mol) and La(NO3)3·6H2O (0.001 mol)were dissolved in 20 ml deionized water to get mixed salt solution.Afterwards,the solution was added dropwise to the 5 g MgAl-LDHs support, followed by drying at 90 °C overnight.The thermal treatment procedure was the same as that in the section 2.2.1.The obtained catalyst denoted as imp-NiLa/MgAl-MMO.
2.3.Characterizations
Power X-ray diffraction (XRD) patterns were recorded using D/max Ultima X-ray diffractometer operated with Cu Kα(λ = 0.179 nm) radiation in the 2θ range from 5° to 90°.Transmission electron microscopy (TEM) images with energy-dispersive spectroscopy (EDS) were obtained by using a JEOL 2100 transmission electron microscope operated at 200 kV.The high angle annular dark field scanning transition electron microscopy (HAADFSTEM) images with phase mapping scans by energy-dispersive spectroscopy(EDS)were obtained using a Tecnai F20 transmission electron microscope.X-ray photoelectron spectra (XPS) were performed on Thermo Scientific Escalab 250Xi X-ray photoelectron spectroscope equipped with an Al Kα radiation, and the data was calibrated by C 1s (284.8 eV) and fitted using Avantage software.The actual loading amounts of Ni and La in the synthesized catalysts were determined by Aglient 720 Inductively coupled plasma optical emission spectrometer (ICP-OES).The BET surface area and pore structures data were measured by N2adsorption-desorption experiments in Micromeritics ASAP 2460 at-196°C after pretreatment in vacuum at 200 °C for 6 h.
Temperature programmed reduction (H2-TPR) profiles were obtained in an AutoChem 2920 device (Micromeritics, USA)equipped with a thermal conductivity detector(TCD).The calcined hydrotalcites were first outgassed at 300°C for 0.5 h in pure Ar and then reduced using 10%(vol)H2-Ar at a heating rate of 10°C·min-1from 30 °C to 900 °C.CO2temperature programmed desorption(CO2-TPD) was performed using the same apparatus (AutoChem 2920).The catalysts were first degassed for 0.5 h at 300°C in pure He and then cooled to 30 °C.Subsequently, a mixture of 10% (vol)CO2-He was fed for 1 h in order to saturate their surface with CO2,followed by the desorption of physically adsorbed CO2under a flow of pure He for 15 min.Then,the catalysts were heated in the range from 30 °C to 900 °C under pure He atmosphere at rate of 10 °C·min-1and the evolution of CO2was followed with the aid of a TCD detector.
2.4.Hydrogenation of PR
The hydrogenation of PR solution (containing 10% (mass) of C5PR or C9PR in cyclohexane) was carried out in a fixed-bed micro-reactor with the continuous flow filling 2.5 ml catalysts(0.42-0.85 mm).The catalysts were activated in H2(50 ml·min-1)at 300 °C for 1 h to make sure the high reduction degree.The bromine number of HPR was measured by a BR-1 bromine number detector (made in China Guorui) to determine the hydrogenation degree of the PR.Softening point of the hydrogenated products was determined by an automatic asphalt softening point tester(SYD-2806F, made in China Changji).The Gardner color scale was measured according to a Lovibond Gardner scale 3000 comparator using Gardner color number method (50% (mass) solution in toluene).FT-IR, UV-vis and NMR spectra were used to observe the change of unsaturated bonds of PR before and after hydrogenation.1H NMR spectra were taken on a Bruker AVANCE III 400 MHz with CDCl3as solvent.IR spectra were performed on Nicolet iS50 spectrometer with KBr pellet and recorded in the range of 400-4000 cm-1.
The hydrogenation degree(HD)of was calculated using the following formula:
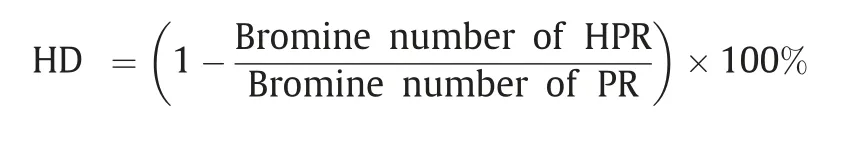
2.5.Computational details
All the DFT calculations in the generalized gradient approximation (GGA) were performed using the Vienna Ab Initio Simulation Package(VASP)[22]with the Perdew-Burke-Ernzerhof(PBE)function[23].A kinetic energy cutoff of 400 eV was used for expanding the electronic wave functions and the ionic cores were described with the projector augmented-wave (PAW) method [24].The Ni(1 1 1)surface was modeled by a periodic three-layers 5×5 supercell slab, with the bottom two layers fixed in DFT calculations.A vacuum layer of 2.0 nm was employed in the (1 1 1) surface slabs along thez-direction, the Brillouin zone was sampled at the Γpoint only for the calculations.A small oxidized La cluster with 4 La atoms and 6 O atoms are located on the Ni(1 1 1)surface,which is denoted as Ni (1 1 1)-La.
3.Results and Discussion
3.1.Material characterization
XRD patterns of the NiLaMgAl-LDHs, NiMgAl-LDHs and MgAl-LDHs were shown in Fig.1(a).It can be seen that the MgAl-LDHs precursors exhibit characteristic reflections peaks for wellcrystallized hydrotalcite at 2θ = 11°, 24°, 35°, 39°, 44°, 60° and 62°, corresponding to the 003, 006, 009, 015, 018, 110 and 113 planes, respectively.The NiMgAl-LDHs and NiLaMgAl-LDHs precursors show similar diffractions with MgAl-LDHs, suggesting the successful formation of the hydrotalcite layered structure after the addition of Ni and La[25].It should be noted that the intensity of the diffraction peaks for NiLaMgAl-LDHs after the addition of La became much weaker than the other two precursors, suggesting the crystallinity is decreased.This is probably because the ionic radius of the La3+is larger than Mg2+, Ni2+and Al3+ions, and the Al-O,Ni-O and Mg-O octahedrons are distorted when La3+enters the lattice of the layered structure,thus decreasing the crystallinity of NiLaMgAl-LDHs [26,27].Fig.1(b) displays the XRD patterns of the three samples after calcination in air.Three major diffraction peaks at 2θ = 37.5°, 43.6° and 63.3° are revealed in the diffractograms that are proper to the NiO (JCPDS 71-1179), MgO (JCPDS 87-0652)and Ni-MgO(JCPDS 24-0712)solid solution.No peak corresponding to Al2O3is detected,which is probably due to the high dispersion of the Al species and/or the incorporation of Al into the Ni-Mg-O phase to form the Ni-Mg-Al-O solid solution [28,29].The XRD patterns of the reduced catalysts are presented in Fig.1(c).In addition to the diffraction peaks of the mixed metal oxides, three new signals at 2θ = 44.5°, 51.7° and 76.1° that are originated from metallic nickel also appeared in all samples.No diffraction peak belonged to La species can be found in pre-Ni/LaMgAl-MMO,which is due to the low concentration or small particle size of La spices.The peaks at 20.5°, 22.0° and 36.1° are assigned to Ni-La mixed metal oxide phase in imp-NiLa/MgAl-MMO, indicating that the Ni and La species aggregate to form relatively large particles on the surface of the support.Additionally, the particle size of Ni on pre-Ni/LaMgAl-MMO calculated at the peak of 44.5° using the Scherrer equation is 9.5 nm, which is much smaller than that of pre-Ni/MgAl-MMO (11.7 nm) and imp-NiLa/MgAl-MMO catalysts(25.2 nm) with similar Ni content.These results suggest the particle size of Ni can be effectively controlled by using Ni-containing LDHs as the precursor and La as the promotor.Fig.S1 displays the SEM images of three precursors and theirs derived catalysts.All the three LDHs precursors exhibit a predominantly hexagonal lamellar morphology, similar with the typical Mg-Al hydrotalcitelike compounds.After calcination and reduction, the obtained pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO inherit the lamellar morphology of the precursors and the surface of the reduced catalysts become rough and porous.In contrast, the lamellar structure is destroyed for imp-NiLa/MgAl-MMO.N2sorption results suggest the BET surface of pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO are 151 and 147 m2·g-1, respectively, higher than that of imp-NiLa/MgAl-MMO(120 m2·g-1)(Table S1 and Fig.S2).The total pore volume of three samples also exhibit the same tendency as surface area.It should be noted that large mesopores can be identified from both pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO, while no distinguished peak is observed from the pore size distribution for imp-NiLa/MgAl-MMO.These results imply that the active sites of pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO are more accessible to the reactants than those of imp-NiLa/MgAl-MMO,which is probably due the blockage of pores by Ni-La oxide particles at such high loading prepared by co-impregnation method.
HRTEM was carried out to investigate the detailed nanostructures of the three catalysts.Uniform Ni nanoparticles are homogeneously distributed on the support with excellent dispersion in Ni/LaMgAl-MMO (Fig.2(a)).The HRTEM image in Fig.2(b) shows the lattice fringes with a spacing of 0.203 nm, which corresponds to the(1 1 1) interplane spacing of Ni,can be distinguished.The particle size distribution is calculated by randomly selected 100 particles,showing that the average particle size of Ni on pre-Ni/LaMgAl-MMO is 8.2 nm with narrow distribution (Fig.2(c)).The Ni particles on pre-Ni/LaMgAl-MMO catalyst without La incorporation also shows good dispersion as shown in Fig.S3.The average particle size is 10.5 nm with a little wider distribution than that of pre-Ni/LaMgAl-MMO.For comparison,severe aggregation of the metal particles on imp-NiLa/MgAl-MMO prepared with impregnation method is observed from Fig.S3.The particle size of Ni is arranged from several nanometers to 40 nm with an average size of 22.1 nm,which is much larger than those in pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO.The metal dispersion of the three catalysts is characterized by CO pulse adsorption analysis.As shown in Table 1,pre-Ni/LaMgAl-MMO exhibits the best metal dispersion of 11.5%,higher than other two catalysts.The Ni active sites of pre-Ni/LaMgAl-MMO and pre-Ni/MgAl-MMO are 0.437 and 0.393 mmol·g-1based on the CO chemisorption measurement,suggesting the number of Ni active sites is increased after La incorporation.These results suggest that judicious selection of LDHs as the catalyst precursors and appropriate promoter incorporation are of significance in reducing the particle size and improving the dispersion of active metals on the supported catalysts.The formation of small particle size and high metal dispersion is probably because the internal limiting effect of the LDHs precursor during the structural topology transformation can effectively inhibit the migration and aggregation of the active metals [25].HAADF-STEM analysis was performed to further study the element distribution of pre-Ni/LaMgAl-MMO catalyst (Fig.2(d)-(i)).It can be clearly observed that Ni was granular and distributed on the sheet in isolation.Elemental mapping suggests that La, Mg, Al, and O elements are uniformly distributed throughout the catalyst sheet.This result indicates that Ni and La are successfully incorporated in the layered structure, and Ni with a lower reduction temperature is reduced and extracted from the layered structure to form small particles on the lamellar support during reduction process.
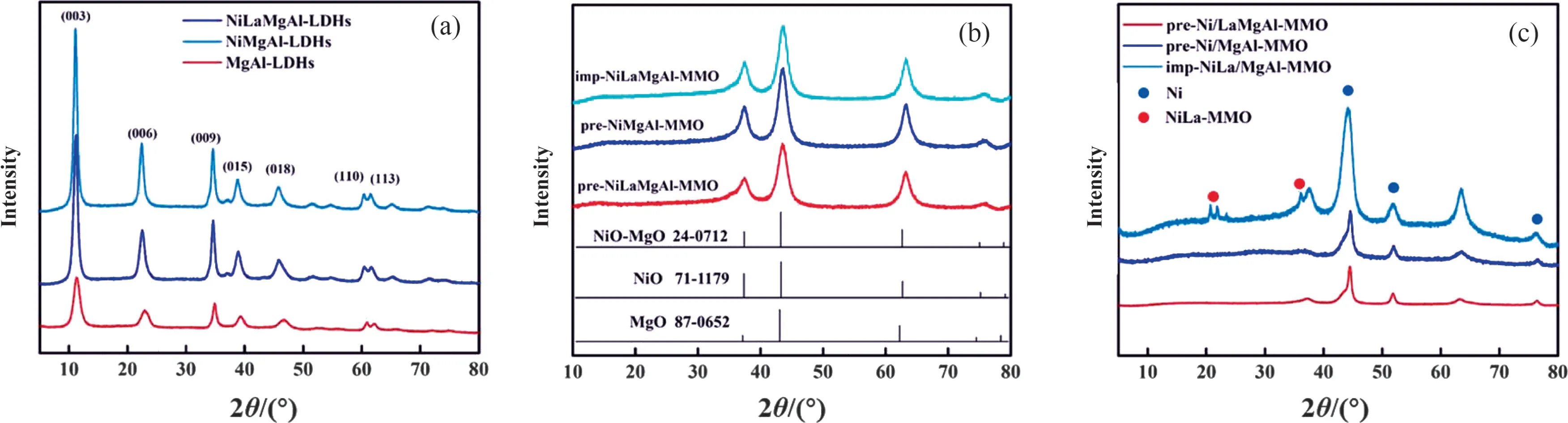
Fig.1. XRD patterns of as prepared LDHs precursors (a), calcined MMO in air (b) and reduced catalysts (c).
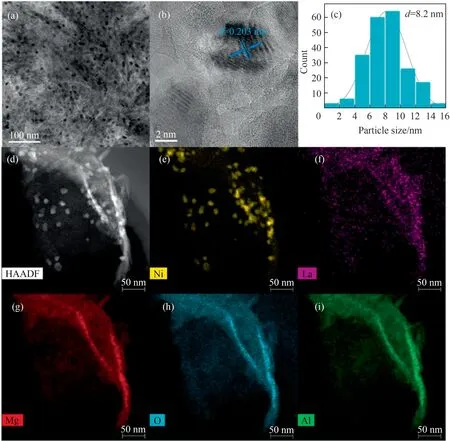
Fig.2. HRTEM images (a) and (b), Ni particle size distribution (c), and HADDF-STEM elemental mappings (d)-(i) of the pre-Ni/LaMgAl-MMO sample.
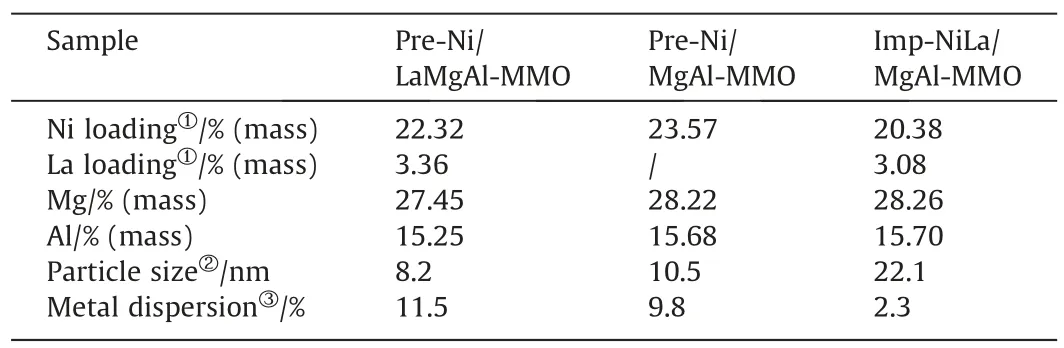
Table 1Physical and chemical properties of the catalysts
H2-TPR profiles of pre-NiLaMgAl-MMO, pre-NiMgAl-MMO and imp-NiLaMgAl-MMO after air calcination are compared in Fig.3(a).For pre-NiMgAl-MMO, hydrogen consumption begins at 400°C and ends completely at 900°C,and it exhibits a wide hydrogen consumption peak at 704°C,which is ascribed to the reduction of Ni2+species to Ni0[30].The reason of higher reduction temperature is because the metal of the LDHs precursor is oxidized during calcination,and Ni2+species are embedded in the Mg-Al composite metal oxide to form a solid solution with high thermal stability and strong interaction [31].For the pre-NiLaMgAl-MMO sample, a shoulder peak between 400°C and 450°C can be observed clearly,which is ascribed to the presence of bulk weakly-bonded NiO[32,33].This sample shows the largest hydrogen consumption at 675 °C, and the peak shifts towards lower temperature by 29 °C compared to pre-NiMgAl-MMO sample.The results indicat that the introduction of La could effectively promote the reduction of Ni2+species in the pre-NiLaMgAl-MMO sample.For comparison,the presence of La does not promote the reduction of Ni-oxide species in imp-NiLaMgAl-MMO sample where the largest hydrogen consumption peak appeared at 712 °C, which is probably because the NiO particles with large size is more difficult to be reduced.The surface basicity of pre-Ni/LaMgAl-MMO, pre-Ni/MgAl-MMO and imp-NiLaMgAl-MMO samples were detected through CO2-TPD analysis.Three typical CO2desorption peaks appear between 100°C and 700°C in CO2-TPD pattern(Fig.3(b)).These peaks represent the presence of basic sites with different intensities, weak Brønsted basic sites such as surface OH-groups(low temperature),medium-strength Lewis acid-base sites (intermediate temperature),and low-coordination surface O2-acting as strong basic sites(high temperature)[34,35].The pre-Ni/MgAl-MMO catalyst shows obvious CO2desorption peaks at 100°C and 350-650°C,indicating that it has weak and medium strong basic sites.The area of the desorption peak represents the amount of alkali.Regardless of the preparation methods, the area of CO2desorption peaks has increased significantly in the catalysts containing La, indicating that the doping of the La component increases the number of medium and strong alkaline sites, which indicates the acidic sites will be neutralized on the surface of the catalyst.Therefore, the breakage of C-C single bonds can be effectively prevented and the decrease of softening point of HPR can be inhibited when the La incorporated catalyst is used for PR hydrogenation.
XPS analysis was performed to investigate the electronic interaction between Ni and La.All the binding energies were calibrated by referencing to the C 1s binding energy (284.8 eV).The detailed binding energies of Ni and La are displayed in Table S2 for these three different catalysts.As shown in Fig.S4, the binding energies at 851.8 eV and 872.3 eV can be assigned to 2p3/2and 2p1/2of Ni0specie, respectively [36].In addition, the peak attributed to Ni2+species are also appeared at 855.0 eV.The binding energy of Ni 2p3/2of imp-NiLa/MgAl-MMO is 852.1 eV, higher than that of pre-Ni/LaMgAl-MMO.The pre-Ni/MgAl-MMO catalyst without La incorporation shows the highest binding energy of Ni 2p3/2at 852.3 eV.DFT simulation was applied to further prove the electronic interaction between Ni and La.Fig.S5A give the optimized structures of the Ni (1 1 1)-La surface.It can be clearly seen from the difference charge density plot(Fig.S5B)that there is significant charge transfer between the Ni(1 1 1)substrate and La4O6cluster.The combined Bader charge calculation result indicates that the surface 21 Ni atoms have negative charges of -0.75 |e| on Ni(1 1 1)-La surface, which confirming the charge transfer from La4O6to Ni.These results suggest there is electronic interaction between La and Ni, and La prefers to donate electrons to the Ni spices.It is worth noting that the ratio of the reduced state is quite different and the percentage of the reduced state ratio (Ni0/Ni2++-Ni0) is 39.7% (pre-Ni/LaMgAl-MMO), 30.0% (pre-Ni/MgAl-MMO),37.9% (imp-NiLa/MgAl-MMO), respectively.This indicates that La could also effectively promote the reduction of nickel oxide species from mixed oxide solid solution.Additionally, the binding energy of La 3d5/2of pre-NiLa/MgAl-MMO is 834.3 eV, indicating the La specie is in the form of La2O3.
3.2.Catalytic performance
The prepared catalysts and a commercial Ni catalyst were applied for the catalytic hydrogenation of C5PR and C9PR to produce HPR.For comparison, a blank experiment was performed without catalyst under the same reaction conditions.As shown in Table 2 and Fig.S6, the bromine numbers of HC5PR and HC9PR obtained without catalyst are 15.83 and 23.88 g Br2/100 g,respectively, indicating that the unsaturated bonds of petroleum resin have been rarely hydrogenated.Fig.4 displays the hydrogenation degree of HC5PR and HC9PR over different catalysts.The pre-Ni/MgAl-MMO catalyst has relatively high hydrogenation activity.The bromine numbers of C5PR and C9PR decrease from 16.40 and 24.62 g Br2/100 g to 0.91 and 1.21 g Br2/100 g, respectively.With the introduction of La, the activity of the Ni catalyst is further improved.The bromine numbers of HC5PR and HC9PR are reduced to 0.75 g Br2/100 g and 0.96 g Br2/100 g, respectively, corresponding to hydrogenation degree of 95.4% and 96.1%.For comparison,the bromine numbers of HC5PR and HC9PR catalyzed by imp-NiLa/MgAl-MMO only decrease to 2.03 g Br2/100 g and 2.58 g Br2/100 g, respectively with hydrogenation degree of only 87.6%and 89.5%.These results indicate that the in situ reduction from LDHs precursor is more conducive to preparing Ni based catalysts with high hydrogenation activity compared with the conventional impregnation method.The softening points of all the hydrogenated products are also displayed in Table 2.It is observed that the softening points of HC5PR and HC9PR decrease from 108 and 122°C to 92 and 105 °C in the blank experiment without any catalyst, suggesting fracture of the petroleum resin molecular chain is unavoidable at high temperature.The softening points of HC5PR and HC9PR hydrogenated by pre-Ni/LaMgAl-MMO are 86 and 95 °C, respectively, higher than other hydrogenated PR products from pre-Ni/MgAl-MMO and imp-NiLa/MgAl-MMO catalysts.This is probably because the pre-Ni/LaMgAl-MMO catalyst possesses most abundant medium-strong basic sites after La doping, which can inhibit the thermal crack of petroleum resins at high temperature during hydrogenation.It should be noted that the commercial Ni catalyst exhibits the worst catalytic activity for PR hydrogenation among all the catalysts.
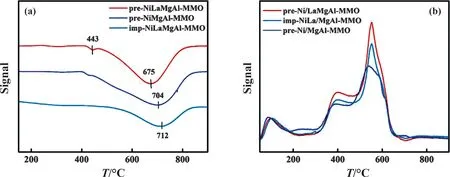
Fig.3. H2-TPR (a) and CO2-TPD (b) profiles of pre-NiLaMgAl-MMO, pre-NiMgAl-MMO and imp-NiLaMgAl-MMO samples.
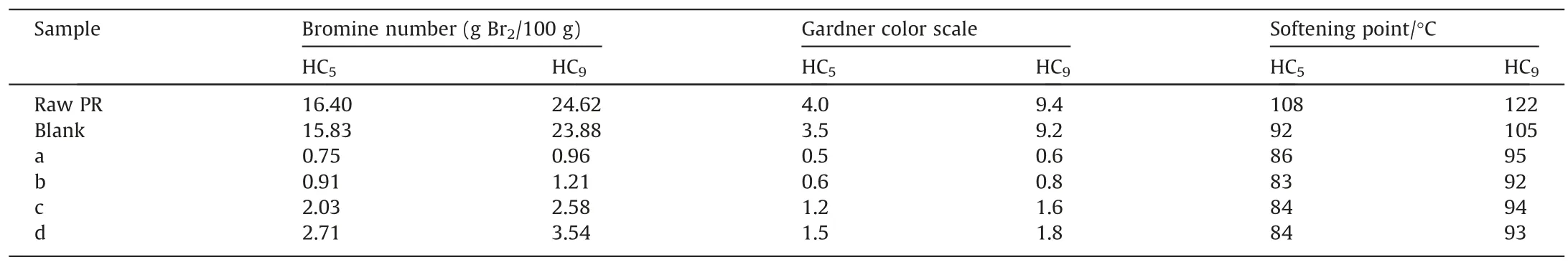
Table 2Properties of PR and HPR over the pre-Ni/LaMgAl-MMO(a),pre-Ni/MgAl-MMO(b),imp-NiLa/MgAl-MMO(c)and commercial Ni catalyst(d).Reaction conditions:220°C,5 MPa(H2 pressure), 600:1 (volume ratio of H2: PR), and 1.5 h-1 (LHSV).The hydrogenated products were sampled and analyzed after running 6 hours

Fig.4. Hydrogenation degree of HC5PR (A) and HC9PR (B) over pre-Ni/LaMgAl-MMO (a), pre-Ni/MgAl-MMO (b), imp-NiLa/MgAl-MMO (c) and commercial Ni catalysts (d).
Furthermore, in order to observe the changes more intuitively before and after hydrogenation of unsaturated bonds in PR, FT-IR characterization was performed on the hydrogenated PR prepared by three catalysts.As shown in Fig.5A, the peaks at 1458 and 1382 cm-1are assigned to the bending vibrations of the C-H of methyl or methylene and the peak at 970 cm-1in C5PR and HC5PR can be assigned to out-of-plane bending vibration of the C-H of unsaturated C=C [14,37].After hydrogenation, the intensities of the peaks at 970 cm-1decrease in HC5PR catalyzed by different catalysts.This peak of HC5PR for pre-Ni/LaMgAl-MMO is almost disappeared, suggesting the catalyst exhibits the highest hydrogenation activity,which is followed by pre-Ni/MgAl-MMO catalyst and imp-NiLa/MgAl-MMO.In Fig.5B, the bands at 1450, 1486 and 1604 cm-1are attributed to the stretching vibration of the aromatic ring skeleton.The peaks at 3040 cm-1,699 and 747 cm-1are attributed to the stretching vibration and of out-of-plane bending vibration C—H bond of aromatic ring [38].Signals from olefinic groups are also observed.After catalytic hydrogenation, these peaks decrease at different extent, similar to the C5PR results.The catalytic activities of the three catalysts for C9PR hydrogenation follow the order of pre-Ni/LaMgAl-MMO >pre-Ni/MgAl-MM O >imp-NiLa/MgAl-MMO.These results further prove that the Ni-based catalysts prepared using LDHs as the precursor and the doping of La can improve the hydrogenation activity and promote the conversion of unsaturated bonds in petroleum resins to saturated bonds.
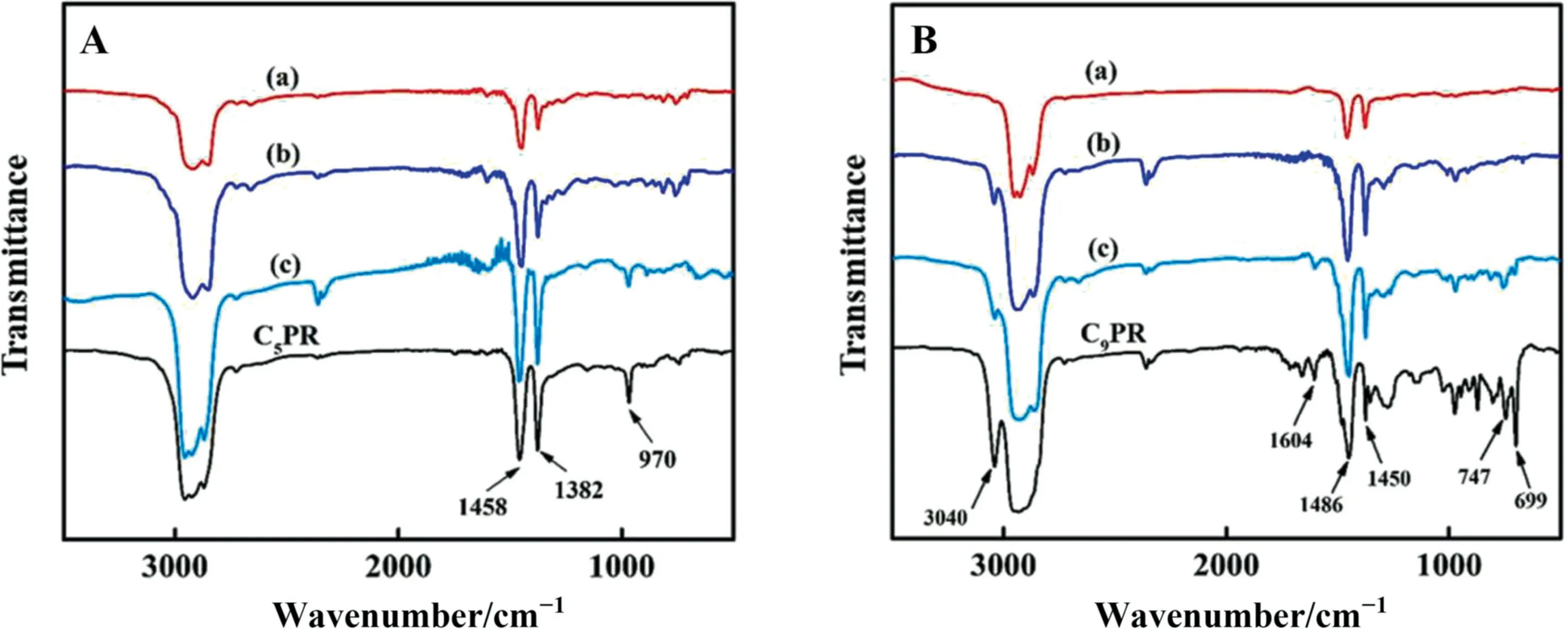
Fig.5. FT-IR spectra of HC5PR (A) and HC9PR (B) produced by pre-Ni/LaMgAl-MMO (a), pre-Ni/MgAl-MMO (b) catalysts and imp-NiLa/MgAl-MMO (c).
1H NMR was further carried out to analyze the type and number of H protons in different chemical environments in C9PR and HC9-PR.As displayed in Fig.S7,the resonance peaks at chemical shift of 0.5-3.5 are attributed to the aliphatic protons; the peaks in the range of 5.3-6.5 are listed as the olefinic protons; and the peaks around 6.5-7.3 are belonged to the aromatic protons in C9PR[38].After hydrogenation, the peaks belonging to olefinic protons and aromatic protons decrease significantly in HC9PR produced by pre-Ni/LaMgAl-MMO catalyst,indicating that the benzene rings and the olefin bonds are almost hydrogenated.For the HC9PR of pre-Ni/MgAl-MMO and imp-NiLa/MgAl-MMO, the peaks for olefin H protons are almost disappeared,but the aromatic groups are not removed completely.UV-vis was used to analyze the hydrogenation of the aromatic rings in C9PR.As shown in Fig.S8,the absorption peak at around 260 nm can be attributed to the characteristic absorption of benzene ring or its derivatives.The peak intensity is decreased significantly after hydrogenation, suggesting most aromatic groups of C9PR have been hydrogenated.The HC9PR from pre-Ni/LaMgAl-MMO shows the lowest peak intensity, indicating that pre-Ni/LaMgAl-MMO catalyst exhibits the best catalytic activity towards aromatic groups among the three catalysts.
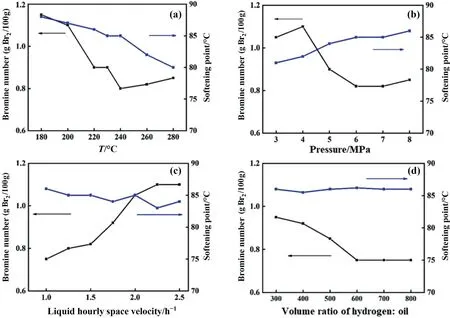
Fig.6. Effect of reaction temperature (a), hydrogen pressure (b), LHSV (c) and H2/C5PR ratio (d) on C5PR hydrogenation for pre-Ni/LaMgAl-MMO catalyst.
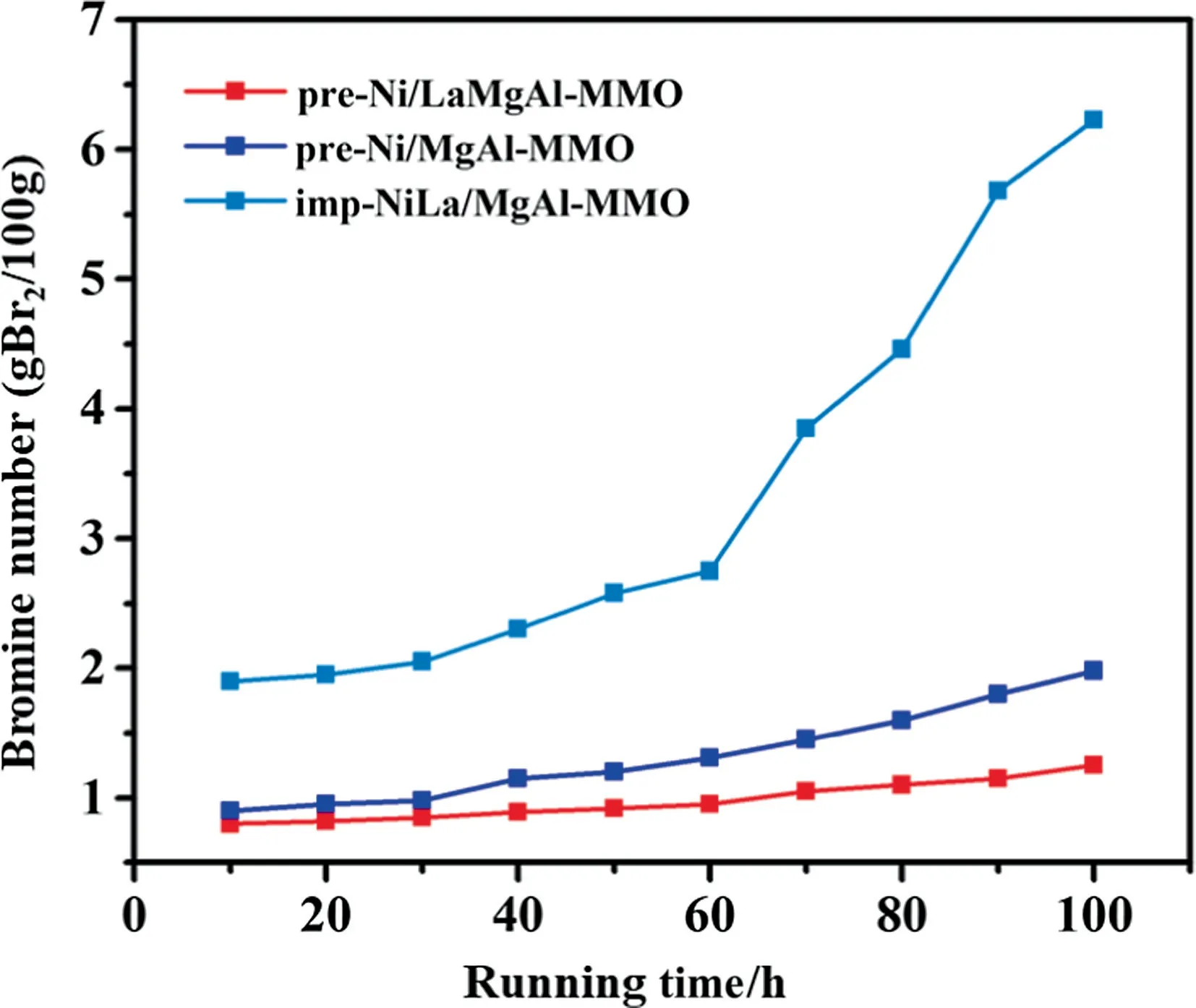
Fig.7. Stability of the different catalyst for C5PR hydrogenation.Reaction conditions: T = 240 °C, P = 6.0 MPa, LHSV = 1.5 h-1, H2/C5PR = 600:1.
The influence of reaction temperature, hydrogen pressure, liquid hourly space velocity (LHSV) and H2/C5PR ratio on the hydrogenation of C5PR for the pre-Ni/LaMgAl-MMO catalyst was investigated to obtain the optimal conditions for PR hydrogenation.Fig.6(a) shows the catalytic performance of catalyst for hydrogenation of C5PR at 180-280 °C.As the reaction temperature increases,the bromine number of HC5PR decreases gradually,indicating elevated temperature is beneficial for the hydrogenation of PR.However, the C5PR tends to degrade at high temperature,which reduces the softening point of the hydrogenated product due to the breakage of the macromolecular chain.When the reaction temperature reaches 240 °C, the HC5PR presents the lowest bromine numbers of 0.78 g Br2/100 g.The bromine number and softening point are influenced by the hydrogen pressure (Fig.6(b)).With the pressure increases, the bromine number decreases and the softening point increases gradually.A minimum bromine number of 0.80 g Br2/100 g is achieved at 6 MPa.We also explored the effect of LHSV on the hydrogenation efficiency of C5PR in the range from 1.0 to 2.5 h-1.As shown in Fig.6(c),the softening point does not change obviously with the change of LHSV,and fluctuates at around 85°C.The bromine number of HC5PR increases gradually from 0.75 g Br2/100 g at 1.0 h-1to 1.1 g Br2/100 g at 2.5 h-1.This is because the residence time of the C5PR solution in the catalyst bed becomes shorter as the LHSV increasing,resulting in an insufficient reaction.However, too low LHSV would result in a significant reduction of the product, therefore a reasonable LHSV is 1.5 h-1.The H2/C5PR ratio indicates the relative amount of hydrogen used for the hydrogenation feed.As shown in Fig.6(d), the bromine number of HC5PR decreases with the increasing of H2/C5PR ratio and reaches a minimum value of 0.75 g Br2/100 g at the ratio of 600:1.In summary,the optimal reaction conditions for the hydrogenation of C5PR over the pre-Ni/LaMgAl-MMO catalyst are 240°C,6 MPa (H2pressure), 1.5 h-1(LHSV) and 600:1 (H2/C5PR volume ratio).
The catalytic stability of the three catalysts were investigated at the optimal conditions obtained above (Fig.7).It can be observed that the bromine number of HC5PR from pre-Ni/LaMgAl-MMO increases slightly with the reaction time increasing.Even after 100 h, the value is still below 1.20 g Br2/100 g, corresponding to a hydrogenation degree of 92.7%.For comparison, the bromine number of HC5PR from pre-Ni/MgAl-MMO and imp-NiLa/MgAl-MMO increases rapidly, revealing the catalytic stability of these two catalysts is worse than that of pre-Ni/LaMgAl-MMO.
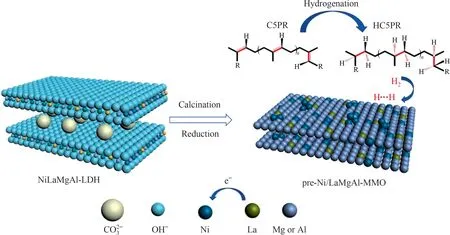
Fig.8. Schematic illustration of the fabrication procedure of pre-Ni/LaMgAl-MMO catalyst and catalytic conversion process of C5PR to HC5PR.
Based on above results and discussions, we propose our design concept and a possible reason for the superior catalytic activity of pre-Ni/LaMgAl-MMO catalyst for PR hydrogenation as follows(Fig.8): LDHs are chosen as precursor to prepare the catalysts,where Ni spices are homogeneously distributed throughout the catalysts.During thermal treatment, the Ni spices are reduced to metallic Ni, extracted from the precursor and firmly anchored on the surface of MgAl-MMO supports.The presence of La in the catalysts can not only reduce the particle size of Ni nanoparticles,but also promote the electron transfer to Ni active sites, thereby improving the H2disassociation ability.Due to the unique features of pre-Ni/LaMgAl-MMO including the large external surface and highly accessible active sites, the PR molecules are easy to diffuse and approach towards the active sites and reacts with the disassociated hydrogen.Moreover, the Ni particles prepared by in situ reduction of Ni spices of LDHs are firmly attached on the catalyst,which can prevent the migration and aggregation of the active components during hydrogenation at high temperature.Therefore,the stability of the catalyst is highly enhanced compared to the catalyst prepared by conventional impregnation method.
4.Conclusions
In summary, we report a La promoted Ni-based catalyst using quaternary NiLaMgAl-LDHs as the precursor.With the presence of La,the Ni nanoparticles in this catalyst exhibit unique properties including small particle size (~8.2 nm), excellent dispersity, more electron rich, and good stability on the supports.When used for the PR hydrogenation, the pre-Ni/LaMgAl-MMO catalyst shows much better catalytic performance than that without La incorporation or that prepared using a conventional impregnation method.A hydrogenation degree of 95.4%and 96.1%can be achieved for HC5-PR and HC9PR, respectively over pre-Ni/LaMgAl-MMO.Importantly, even after 100 h reaction, the hydrogenation degree still maintains at 92.7%, suggesting the excellent catalytic stability.Our work sheds light on the delicate control of Ni-based catalysts with tunable properties and excellent hydrogenation activity for PR, which can also be used in various catalytic reactions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(22078064)and Natural Science Foundation of Fujian Province for Distinguished Young Scholar(2018J06002).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.053.
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Conjugated microporous polymer membranes for chemical separations
- Atomic layer deposition of TiO2 on carbon-nanotubes membrane for capacitive deionization removal of chromium from water
- Application of pulsed chemical vapor deposition on the SiO2-coated TiO2 production within a rotary reactor at room temperature
- Process monitoring of the Au-S bond conversion in acetylene hydrochlorination
- An asymmetrically substituted dithieno[3,2-b:2′,3′-d]pyrrole organic small-molecule hole-transporting material for high-performance perovskite solar cells
- Synthesis and mechanism analysis of a new oil soluble viscosity reducer for flow improvement of Chenping heavy oil
