The successful use of extracorporeal membrane oxygenation combined with continuous renal replacement therapy for a cardiac arrest patient with refractory hypokalemia and diabetic ketoacidosis
Yang Li, Rui Xu, Chun-Shui Cao, Liang Huang
Department of Emergency Medicine, the First Affiliated Hospital of Nanchang University, Nanchang 330006, China Corresponding Author: Liang Huang, Email: huangliang6312@sina.com
Dear editor,
Diabetic ketoacidosis (DKA) is a common clinically severe disease, with an incidence of 24.9/1,000 among diabetes patients, and approximately 180,000 people in the United States suffer from DKA every year.The reported mortality rate associated with DKA ranges from 0.65% to 3.30%, and is commonly caused by electrolyte imbalances, especially hypokalemia and hyperkalemia.Reports indicated that hypokalemia was observed in 5.6% of patients with DKA before therapy,and the overall prevalence of hypokalemia during treatment for DKA was 38%.Several studies have shown that hypokalemia can lead to fatal dysrhythmia and even cardiac arrest, which are positively associated with increased mortality.
Extracorporeal membrane oxygenation (ECMO) can be considered for patients whose cause of cardiac arrest is potentially reversible.When DKA patients develop fatal dysrhythmia or even cardiac arrest induced by severe hypokalemia, ECMO could maintain circulation and provide time to correct electrolyte imbalances, while continuous renal replacement therapy (CRRT) could be used to restore homeostasis.
We report a case of a young male with DKA complicated with refractory hypokalemia, malignant arrhythmia and cardiac arrest. After failing to reverse his hypokalemia by providing potassium chloride intravenously in a short time and conventional organ support therapy, veno-arterial (VA)-ECMO combined with CRRT was performed.
CASE
A 31-year-old male with a height of 168 cm and a weight of 110 kg developed chest tightness and dyspnea after consuming a mass of sweet foods (honey, watermelon, sugary drink, etc., bought from the markets by his family) three days before admission to the hospital. He became unconscious one day before admission. He experienced excessive urination but did not have nausea or vomiting. The patient had no history of heart disease, diabetes, or drug use, including diuretics. His vital signs at admission were as follows: body temperature 38.9 °C; heart rate 140 beats/min; respiratory rate 26 beats/min; blood pressure 96/65 mmHg (1 mmHg=0.133 kPa) with high-dose dopamine hydrochloride (12 μg/[kg·min]). The patient presented with impaired consciousness, with pupils of equal size on both sides, 2.5 mm in diameter. He was endotracheally intubated, mechanically ventilated (volume control ventilation model, fraction of inspired oxygen 45%, tidal volume [V] 7 mL/kg, positive endexpiratory pressure [PEEP] 5 cmHO [1 cmHO=0.098 kPa], respiratory rate 16 beats/min) and had coarse respiratory sounds in both lungs but no moist rales and heart murmurs at auscultation. The initial laboratory values were as follows: blood glucose 24.9 mmol/L (normal range: 3.9-6.1 mmol/L); blood potassium 2.6 mmol/L (normal range: 3.5-5.3 mmol/L); blood sodium 141 mmol/L (normal range: 136-146 mmol/L); blood chlorine 112 mmol/L (normal range: 98-106 mmol/L); pH 7.06 (normal range: 7.35-7.45); PaCO35.1 mmHg (normal range: 35.0-45.0 mmHg); HCO9.4 mmol/L (normal range: 22.0-26.0 mmol/L); standard base excess (SBE) -18.9 mmol/L (normal range: -3-+3 mmol/L); lactate (Lac) 0.6 mmol/L (normal range: 0.5-1.6 mmol/L); urine ketones 4.0 mmol/L (normal: 0 mmol/L); and urine glucose 24.85 mmol/L (normal: 0 mmol/L). An electrocardiogram (ECG) performed at admission showed complex tachycardia with T wave changes in some leads (QRS, 104 ms) (Figure 1A). A multislice spiral computed tomography (CT) of the head, chest and abdomen showed a minor infection in the lower part of both lungs. The diagnoses at admission were DKA, shock, lung infection, metabolic acidosis, and hypokalemia. Symptomatic and supportive treatments were given immediately. An infusion of isotonic saline at a rate of 500-1,000 mL/h in the first 2-4 h was completed in the emergency room. We continued fluid resuscitation by infusing 0.9% saline at a rate of 250 mL/h and other equilibrium salt solutions. At the same time, the patient was given high-dose insulin-euglycemic therapy (continuous intravenous infusion of 0.1 U/[kg·h] insulin). Depending on the blood pressure, the maximum dose of norepinephrine reached 0.5 μg/(kg·min) to maintain hemodynamics. In addition, the ventilator provided continuous respiratory support.
After admission, a continuous central venous infusion of potassium chloride was given. Seventeen hours after his admission, 19 g of potassium chloride was infused intravenously, while a reexamination of the laboratory evaluation indicated a blood potassium level of 1.8 mmol/L; thus, we increased the rate of the potassium infusion to 2.5 g/h. At that time, the arterial blood gas analysis values were as follows: pH 7.073; PaCO41.8 mmHg; HCO11.3 mmol/L; and SBE -16.5 mmol/L. Nineteen hours after admission, ECG revealed wide ventricular tachycardia (VT) (QRS, 144 ms) (Figure 1B). Immediately after VT, ECG monitoring showed recurrent ventricular fibrillation (VF) in a short time. Twenty hours after admission, the patient suddenly developed cardiac arrest, and the arterial blood gas analysis showed that the blood potassium level was 0.5 mmol/L, even though 26 g of potassium chloride was supplemented intravenously. At this time, the amount of the fluid supplement reached approximately 5,000 mL. The arterial blood gas analysis showed pH 7.079; PaCO23.1 mmHg; HCO7.9 mmol/L; and SBE -21.6 mmol/L. Extracorporeal cardiopulmonary resuscitation (ECPR) was immediately performed in VA-ECMO mode with 21 F intubation of the right femoral vein and 17 F intubation of the left femoral artery. The flow rate was initially set to 4.8 L/min and an oxygen flow rate of 4.0 L/min. The patient undergoing ECMO support received anticoagulant therapy with intravenous unfractionated heparin (UFH) to maintain activated partial thromboplastin time (APTT) at 1.5 times baseline. The signs of bleeding of the patient were observed. After ECMO, the patient developed recurrent VF again (Figure 1C), and electrical defibrillation (biphasic wave, 200 J) was performed. After four electrical defibrillations, normal sinus rhythm returned. Reexamination of the ECG showed sinus tachycardia with a first-degree atrioventricular block (QRS, 98 ms) (Figure 1D). Two hours after ECMO, his blood pressure was 112/70 mmHg (with noradrenaline 0.8 μg/[kg·min]). As hypokalemia was considered refractory, the patient received CRRT treatment in continuous veno-venous hemodialysis (CVVH) mode. CRRT was performed using the Prismaflex hemofilter (GAMBRO, Sweden) on the ECMO, with an initial potassium concentration of 12.24 mmol/L in the replacement fluid. The concentration was gradually adjusted according to the blood potassium. CVVH was administered with blood flow rates of 180 mL/min and a CRRT dose of 26-30 mL/(kg·h). The ultrafiltration was 3,000 mL/h. Fifteen hours after ECMO, his blood potassium reached 3.6 mmol/L, with a total of 64 g potassium chloride supplemented intravenously. A summary of the progression of the patient’s serum potassium levels, potassium chloride supplementation and potassium concentration in the replacement fluid is shown in Table 1.
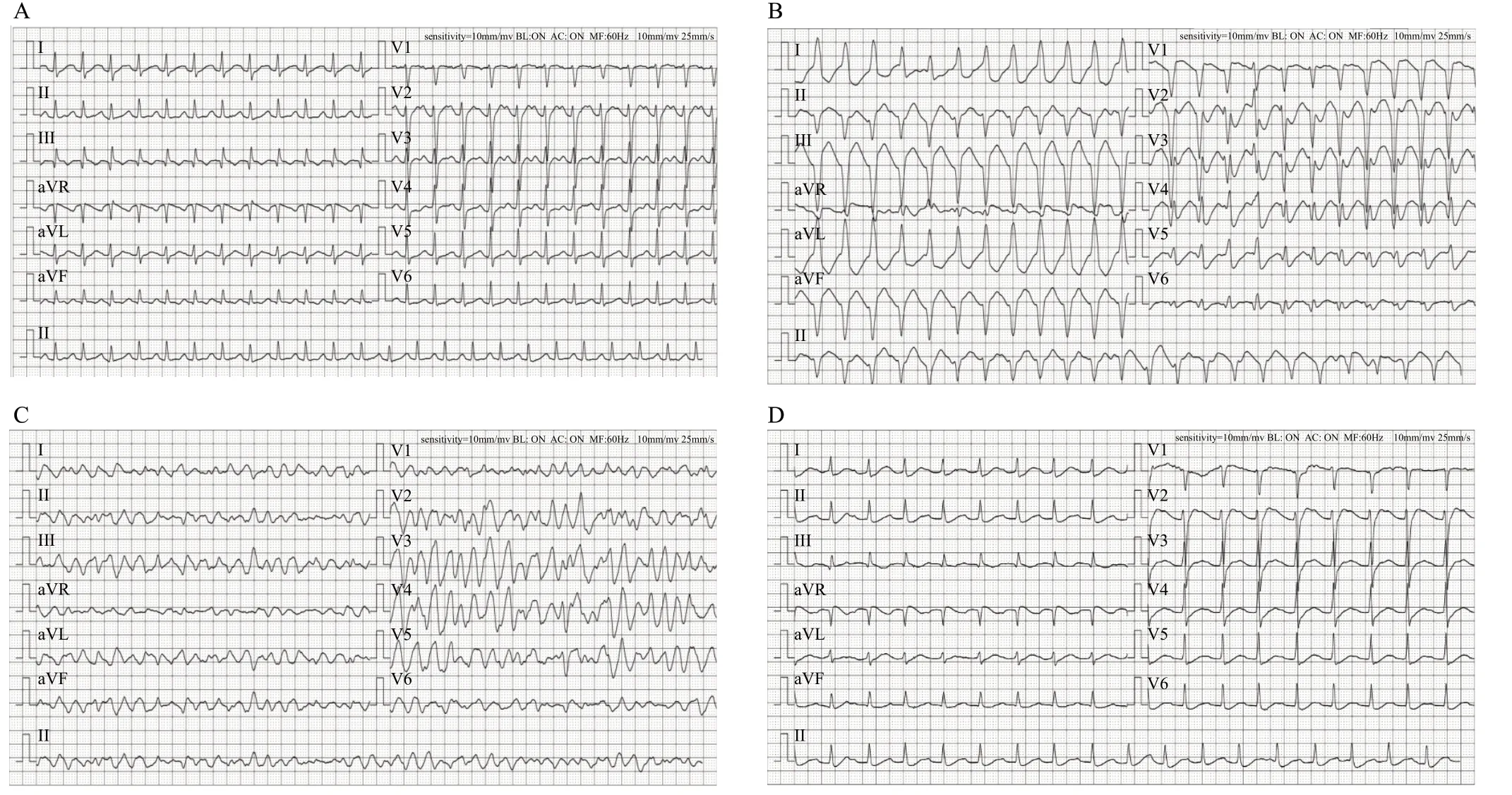
Figure 1. Serial electrocardiograms (ECG) of the patient. A: ECG on admission showed complex tachycardia (QRS, 104 ms). B: Nineteen hours after admission, ECG showed ventricular tachycardia (QRS, 144 ms). C: After ECMO, the ECG showed ventricular fibrillation. D: After ECMO and electrical defibrillation, ECG showed sinus tachycardia with first-degree atrioventricular block (QRS, 98 ms).
On the third day after ECMO initiation, the patient’s hemodynamics, heart rhythm, acid-base electrolyte balance were stable, and left ventricular ejection fraction was 53%. We withdrew ECMO and CRRT successfully. On the 8day after admission, his vital signs were stable, and he left the intensive care unit without impaired neurological function. At one-year follow-up, his blood glucose levels remained stable.
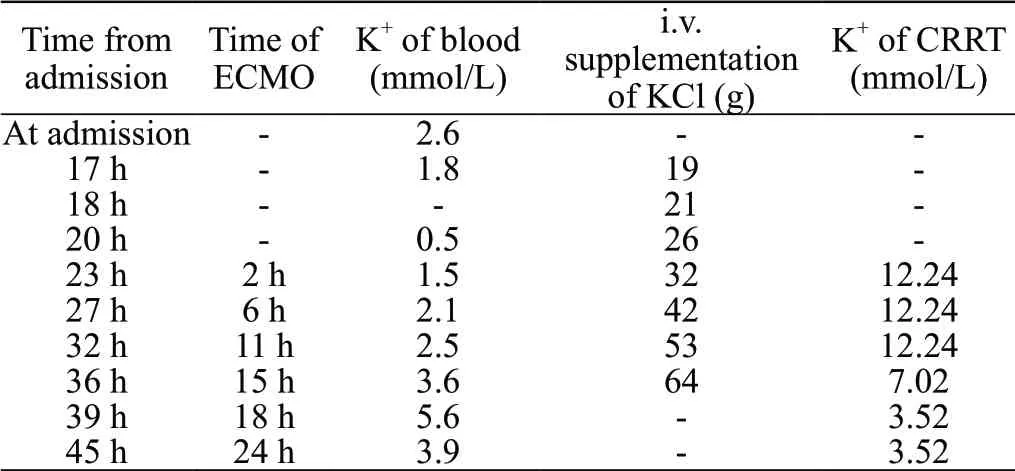
Table 1. Temporal progression of the patient’s blood K+ values, total supplementation of K+, and the concentration of K+ in the CRRT replacement fluid
DISCUSSION
The main mechanisms of hypokalemia in DKA patients are as follows. (1) Hyperglycemia leads to osmotic diuresis, resulting in potassium loss through the renal and gastrointestinal tracts. (2) Insulin increases cellular potassium uptake and results in worsened hypokalemia. (3) The aldosterone-like effect of insulin on the renal tubule further exacerbates renal potassium losses. (4) Low blood insulin levels in patients with uncontrolled diabetes lead to an increase in the renal elimination of glucose, which conversely increases the delivery of sodium to the distal nephron, thereby promoting potassium excretion.Our patient showed urorrhagia after admission because of the osmotic diuretic effect of hyperglycemia, and his urine volume increased to approximately 200 mL/h, which led to excessive potassium loss through the urine. Meanwhile, continuous insulin treatment may lead to the intracellular transfer of potassium and further aggravate hypokalemia. However, severe hypokalemia can further lead to fatal dysrhythmia. Its direct electrophysiological effects include suppression of potassium ion channel conductance, resulting in slowed conduction and delayed ventricular repolarization, and an increase in the excitability and automaticity of cardiomyocytes caused by the contribution of Na-K-ATPase inhibition to intracellular Naand Caloading.
Hypokalemia is a well-known common cause of arrhythmia and a potentially reversible cause of cardiac arrest, but there is no clear indication of CRRT for hypokalemia. Syed et alreported a case of DKA complicated with refractory hypokalemia corrected by CRRT. After admission, the patient was given continuous intravenous potassium supplementation, but the blood potassium level still decreased, with a new T-wave inversion and an obvious U-wave. At this time, CRRT was applied successfully and quickly to maintain electrolyte balance, and the patient’s heart rhythm recovered. Therefore, we consider hypokalemia-induced arrhythmia as an indication for CRRT. However, when hypokalemia-induced cardiac arrhythmias are persistent and malignant, CRRT cannot correct hypokalemia in a short time. Thus, advanced life support is required for emergencies.
When cardiac arrest occurs in patients with a reversible cause, such as hypokalemia, ECPR can improve prognosis and maintain favorable neurological function more effectively than conventional cardiopulmonary resuscitation (CCPR).Wang et alreported a case of persistent cardiac arrest caused by profound hypokalemia that was successfully rescued with ECMO, and outpatient follow-up revealed normal cardiac and lung function and good neurological function. A meta-analysis comparing the impact of ECPR or CCPR on the outcomes of patients with cardiac arrest showed that the survival rate and the proportion of patients reaching the Glasgow Pittsburgh performance category (CPC) scores of 1 or 2 in the ECPR group were higher than those in the CCPR group on the day of discharge, 3-6 months after discharge and one year after discharge.Moreover, a multicenter study of ECPR for cardiac arrest showed that all survivors in the ECPR group were discharged with favorable neurological outcomes (CPC 1 or 2), suggesting that ECPR has advantages in maintaining good neurological function while rescuing patients with cardiac arrest.
When ECMO stabilizes the perfusion of organs and tissues, severe electrolyte disturbance is one of the indications for ECMO combined with CRRT. The unique advantages of CRRT therapy combined with ECMO to correct electrolyte disorders are as follows: (1) ameliorates electrolyte disturbances; (2) effectively improves fluid balance; and (3) enhances the removal of inflammatory mediators and decreases inflammatory cytokine levels.
CONCLUSIONS
Some cases of DKA can lead to refractory hypokalemia, the development of fatal dysrhythmia, and even cardiac arrest. When conventional supportive therapy fails, the early implementation of ECMO combined with CRRT therapy might be a safe and effective measure that can improve outcomes and maintain favorable neurological function.
Funding: Science and Technology Program of Jiangxi Provincial Health Commission (20203211); Primary Research & Development Plan of Jiangxi Province (20203BBGL73166); Applied Research Cultivation Plan of Jiangxi Province Department of Science and Technology (20212BAG70016).
Ethical approval: Not needed.
Conflicts of interest: The authors declared no conflict of interest.
Contributors: YL and RX performed the initial literature review, data analysis, and wrote the manuscript; LH and CSC assisted in analyzing the case and critically revised the manuscript to the final form. All authors approved the final version for submission.
 World Journal of Emergency Medicine2022年4期
World Journal of Emergency Medicine2022年4期
- World Journal of Emergency Medicine的其它文章
- Comparing the precision of the pSOFA and SIRS scores in predicting sepsis-related deaths among hospitalized children: a multi-center retrospective cohort study
- Neutrophils inhibit CD8+ T cells immune response by arginase-1 signaling in patients with sepsis
- One-step laparoscopic pancreatic necrosectomy verse surgical step-up approach for infected pancreatic necrosis: a case-control study
- Predicting factors for the need of extracorporeal membrane oxygenation for suicide attempts by cardiac medication: a single-center cohort study
- Global research trends in cardiac arrest research: a visual analysis of the literature based on CiteSpace
- An online survey of non-compressible torso hemorrhage: training is needed
