Serial blood concentration of polyethoxylated tallow amine and clinical presentations in acute herbicide poisoning
Jungsoo Park, Sun Cheun Kim, Youngjoon Jeon, Yong Chul Cho, Changshin Kang, Yeonho You, Hong Joon Ahn, Seung Ryu, Jihan Lee, Wonjoon Jeong
1Department of Emergency Medicine, Chungnam National University Hospital, Daejeon 35015, South Korea
2Department of Emergency Medicine, College of Medicine, Chungnam National University, Daejeon 35015, South Korea
3Forensic Toxicology Division, National Forensic Service, Daejeon 34054, South Korea
4Department of Emergency Medicine, Chungbuk National University Hospital, Cheongju 28644, South Korea
Corresponding Author: Wonjoon Jeong, Email: gardenjun@hanmail.net
Most commercially available herbicides contain surfactants as co-formulants to increase adhesion and absorption by plant leaves. Ethoxylated amines, one of the most used surfactants, are non-ionic and derived from animal fats. They represent a class of surfactants with similar structural features, including polyethoxylated tallow amine (POEA). POEA is widely used in glyphosate, glufosinate-containing herbicides. In 2015, the European Food Safety Society (EFSA) concluded that POEA was more toxic than glyphosate when tested in glyphosate-based formulations.They also attributed the poisoning following ingestion by humans to the presence of POEA.
However, there are few in vivo metabolic studies on post-acute herbicide poisoning in humans. Therefore, we investigated the change in the blood concentration of POEA over time and the clinical presentations in patients with acute herbicide poisoning.
METHODS
This prospective observational study was performed at the Chungnam National University Hospital (CNUH) and Chungbuk National University Hospital. The study was performed between March 1, 2016, and February 28, 2017. Patients who ingested glyphosate- or glufosinatecontaining herbicides were enrolled in the study. The exclusion criteria were: patients under 18 years of age, patients’ blood samples without surfactants, or patients who refused to provide informed consent. This study was approved by the Institutional Review Board of CNUH (2016-03-003-002).
All patients received standard supportive therapy for acute herbicide poisoning, including gastrointestinal decontamination, activated carbon application, and supportive treatment. All aspects of patient management involved standard intensive care following our institutional intensive care unit protocols. In refractory shock, ongoing metabolic acidosis, and renal dysfunction, extracorporeal membrane oxygenation (ECMO) or continuous renal replacement therapy (CRRT) was initiated.
After obtaining informed consent from the patients or their family members, blood samples were collected at regular intervals (6 h) from admission. The samples were immediately frozen and stored at -40 °C until analysis and sent to the National Forensic Service of Korea for identifying surfactants and determining their concentrations. We also recorded patients’ age, sex, type of ingested herbicide, ingredients of herbicides, time of ingestion, amount of ingestion, initial mean arterial pressure (MAP), ventilator care, and vasopressor use based on the statements and medical records. Continuous variables are expressed as median and range (minimum to maximum), and categorical variables are expressed as descriptions.
High-performance liquid chromatography was used to detect surfactants in the patients’ blood samples. Surfactant standards and samples were prepared according to previously described methods and measured by liquid chromatography-tandem mass spectrometry.Surfactant analysis was performed using Shiseido NANOSPACE SI-2 (Shiseido Company Ltd., Japan) incorporating a Kinetex F5 100A column (100 mm × 2.1 mm, 2.6 μm) (Phenomenex, USA). Mass spectrometric detection was performed using a SCIEX 3200 QTRAP system (AB Sciex LLC, USA) equipped with an electrospray ionization source in the positive ion mode. Analytical software AB Sciex (version 1.5.1) was used for instrument settings, data acquisition, and processing. Surfactant standards (CAS no. 61791-21-6) were obtained from the chemical suppliers of a herbicide manufacturer in South Korea (Yoosung Chem. R&T Co., South Korea).
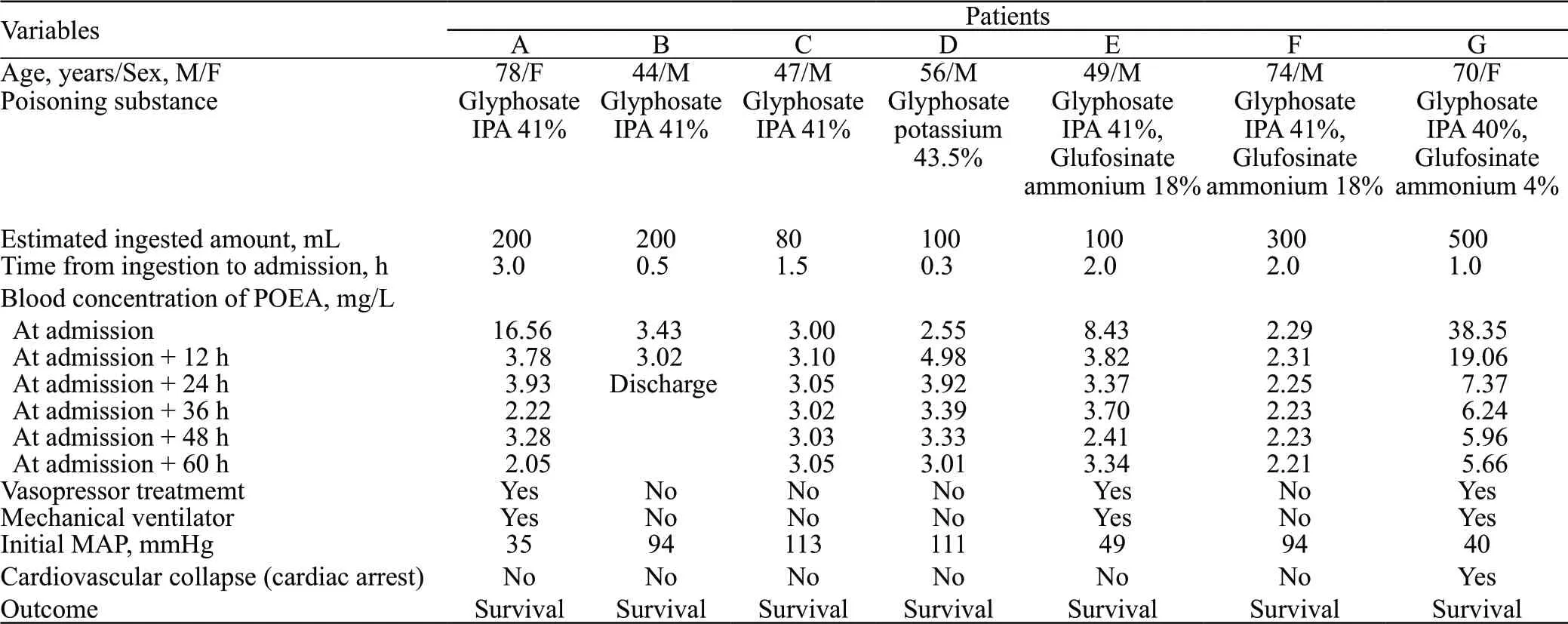
Table 1. Patients’ demographics and clinical features
RESULTS
A total of twenty-two patients visited either of the emergency departments during the study period. After excluding non-consenting patients and those who had no detectable glyphosate, glufosinate or POEA in the blood, only seven individuals remained and were included in this research.
The median age of the seven patients (five males, two females) was 56 years (range: 44-78 years). Of the seven patients, three ingested glyphosate isopropylamine (IPA) 41%, three ingested glyphosate IPA and glufosinate ammonium as a mixed agent, and one ingested glyphosate potassium 43.5%. None of the patients ingested the same product.
The estimated ingested amount was 200 (80-500) mL, and the time from ingestion to hospital admission was 1.5 (0.3-3.0) h (Table 1). None of the patients had diabetes, chronic kidney disease, or liver disease as an underlying condition. Three patients (A, E, and G) with initial concentrations exceeding 8 mg/L underwent endotracheal intubation with mechanical ventilation and vasopressor treatment; within 12 h after admission, the concentrations decreased to less than half of the initial. However, all patients with initial concentrations below 3.5 mg/L were hemodynamically stable and did not require vasopressors or ventilator treatment. Unexpectedly, the blood concentration of POEA in patient D increased after the initial concentration was recorded until 12 h after admission, and then gradually decreased with the patient being consistently hemodynamically stable throughout. Figure 1 shows the change in blood concentration over time for each patient.
Only one patient (patient G), who had the highest POEA concentration at admission, developed cardiac arrest immediately after admission. After voluntarily ingesting 500 mL of herbicide containing glyphosate IPA at 40% and glufosinate ammonium at 4%, this 74-yearold woman, was admitted to the emergency department (ED) with complaints of vomiting. Upon arrival at the hospital, she was in shock (blood pressure 60/30 mmHg, 1 mmHg=0.133 kPa), with severe metabolic acidosis and hemodynamic disturbance. She developed cardiac arrest 20 min after admission and return of spontaneous circulation (ROSC) after 3 min of cardiopulmonary resuscitation. However, low blood pressure and a systemic hyoxemia were sustained, indicating refractory shock. Veno-arterial ECMO and CRRT on continuous veno-venous hemodiafiltration (CVVHDF) mode were started to improve her shock state and metabolic acidosis at 1 h after ROSC; these treatments maintained for three days. She was treated in the intensive care unit (ICU)for approximately one month and discharged without complications.
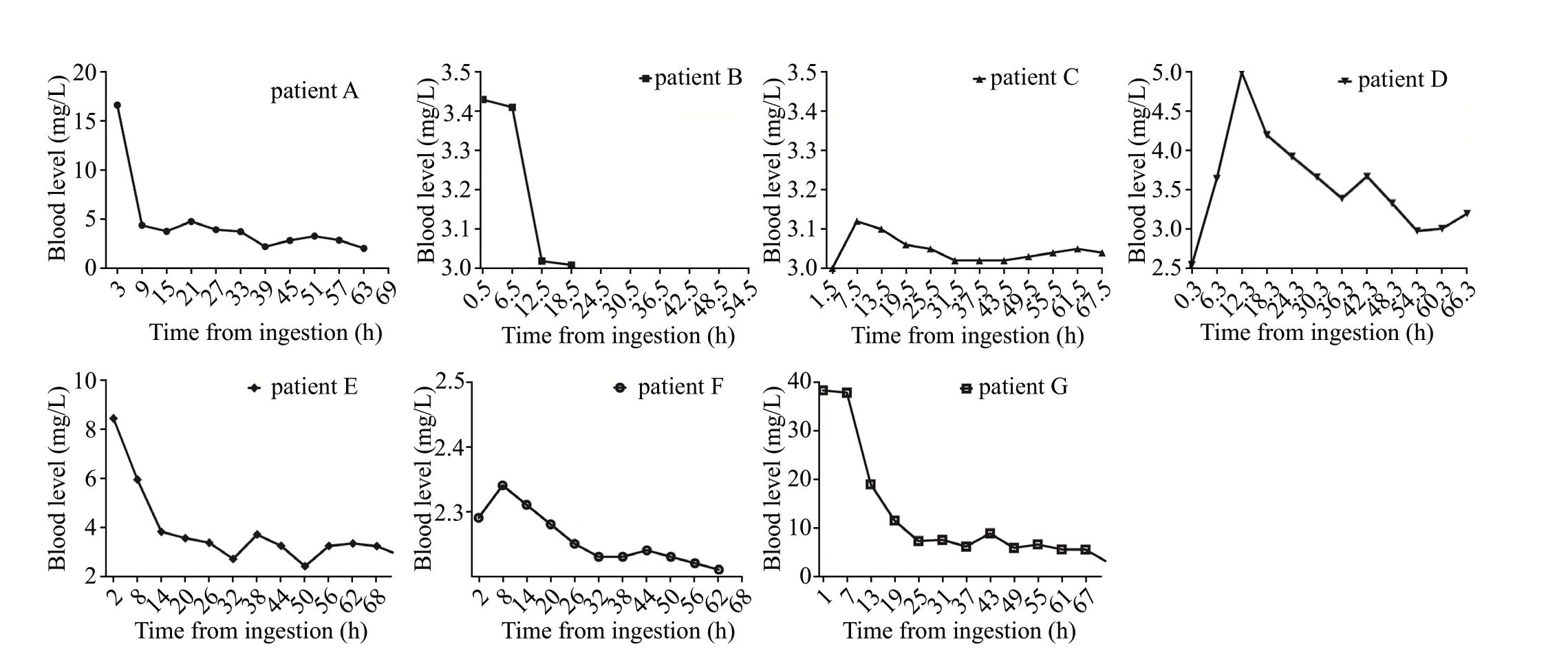
Figure 1. Changes in the blood concentration of polyethoxylated tallow amine (POEA) over time in each patient. Patient B was discharged midway.
DISCUSSION
We investigated the serial blood concentration of POEA in patients with acute poisoning with glyphosateor glufosinate-based herbicides. We found that patients with an initial blood POEA concentration exceeding 8 mg/L presented with severe symptoms, including decreased MAP and respiratory failure. In constrast, concentrations below 3.5 mg/L did not significantly aff ect the clinical presentations. In addition, it was found that the blood concentration of POEA decreased rapidly in humans.This result is consistent with the findings of a previous studybased on pharmacokinetic data of other structurally similar substances. It is expected that once absorbed, POEA is rapidly eliminated from the body within 72 h.Although the metabolic pathway is unknown, it can be inferred that the removal of POEA is possible via a normal path, considering rapid elimination was observed in the healthy people exposed to this substance. However, as observed in patient G, hemodynamic instability, including cardiac arrest, may occur when the initial concentration of POEA is high. We believe ECMO and CRRT improved this patient’s prognosis. Several cases concerning the treatment of patients with glyphosate surfactant intoxication using ECMO and CRRT have been reported.Garlich et alreported the clinical improvements and a hemodialysis clearance of 97.5 mL/min of a patient with glyphosate surfactant herbicide poisoning and the subsequent hemodialysis treatment. They considered that the molecular weight and volume distribution of POEA would be large. It would be possible to remove it through high flux dialysis rather than general hemodialysis. In our study, we considered that CRRT did not have a significant effect on the clearance of POEA in patient G, because we employed CRRT with CVVHDF mode, rather than high flux dialysis. However, regardless of the degree of removal of toxic substances, we estimated that the improvement of patients’ metabolic acidosis by CRRT had a good eff ect on the prognosis.
Among the seven patients, patient G who received CRRT was the only one who had ingested the highest dose and presented with severe metabolic acidosis and cardiac arrest. All other patients were discharged without any sequelae, without receiving blood purification treatment. They ingested relatively small amounts and did not have severe metabolic acidosis. Given these facts, we believe that if patients have a normal renal function and clinical features or their metabolic acidosis is not severe, routine conservative treatment might be sufficient. However, when severe metabolic acidosis with low blood pressure does not improve after ingestion of large amounts, life-saving therapies, such as CRRT and ECMO, are necessary for improving patient’s condition.
In patient D, the blood concentration tended to increase from the initial concentration up to 12 h. According to studies that have tested similar substances, it is estimated that the peak concentration is reached within 1-2 h after oral administration.It is plausible that substances absorbed through the gastrointestinal tract before admission are redistributed into the blood. However, the basis for these hypotheses is insufficient as this phenomenon only occurred in one patient in our study. It is expected that reliable data can be obtained through a larger-scale study on the distribution of POEA in the human body.
A limitation of this study was the small number of patients, which complicated the application of statistical analyses. In addition, toxicological investigations should be carried out to analyze the effects of single substance on certain organisms. The combined administration might lead to unclear the characteristics of an individual drug. In this study, only one surfactant was investigated, regardless of the manufacturer. EFSA indicated that POEA might affect the oral absorption of glyphosate by approximately 20% when tested alone.Thus, the action and interaction of the active ingredients or other surfactants, including surfactant-additive (glyphosate or glufosinate) and surfactant-surfactant, cannot be excluded. Therefore, failure to measure blood concentrations of glyphosate and glufosinate limited this study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethic approval: This study was approved by the Institutional Review Board of CNUH (2016-03-003-002).
Conflict of interest: The authors declare no conflict of interest.
Contributors: JSP and WJJ conceived of the presented idea. SCK and YJJ verified the analytical methods and performed the analysis. YCC, CSK and SR were involved in planning and supervised the work. YHY, HJA and JHL collected the data. WJJ wrote the manuscript with input from all authors.
 World Journal of Emergency Medicine2022年4期
World Journal of Emergency Medicine2022年4期
- World Journal of Emergency Medicine的其它文章
- Comparing the precision of the pSOFA and SIRS scores in predicting sepsis-related deaths among hospitalized children: a multi-center retrospective cohort study
- Neutrophils inhibit CD8+ T cells immune response by arginase-1 signaling in patients with sepsis
- One-step laparoscopic pancreatic necrosectomy verse surgical step-up approach for infected pancreatic necrosis: a case-control study
- Predicting factors for the need of extracorporeal membrane oxygenation for suicide attempts by cardiac medication: a single-center cohort study
- Global research trends in cardiac arrest research: a visual analysis of the literature based on CiteSpace
- An online survey of non-compressible torso hemorrhage: training is needed
