The neuroprotective effect of walnut-derived peptides against glutamate-induced damage in PC12 cells: mechanism and bioavailability
Shuguang Wang, Lin Zheng, Tiantian Zhao, Qi Zhang, Guowan Su,c,*, Mouming Zhao,*
a School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China
b Guangdong Food Green Processing and Nutrition Regulation Technologies Research Center, Guangzhou 510650, China
c Guangdong Huapeptides Biotechnology Co., Ltd., Zhaoqing 526000, China
Keywords:
Neuroprotective effects
Walnut peptides
PC12 cells
Oxidative injury
Digestive stability
A B S T R A C T
In our previous study, defatted walnut meal hydrolysate (DWMH) could attenuate D-galactose-induced acute memory de ficits in vivo, and six potent active peptides including WSREEQ, WSREEQE, WSREEQEREE,ADIYTE, ADIYTEEAG and ADIYTEEAGR were identified. The aim of this study was to investigate the possible mechanism underlying their neuroprotective effects on glutamate-induced apoptosis in PC12 cells and their digestive stability. Results showed that all these peptides could attenuate the reduction of cell viability caused by glutamate in PC12 cells, especially WSREEQEREE and ADIYTEEAGR. The addition of Arg residue in WSREEQEREE and ADIYTEEAGR might be the potential reason for their stronger protective effects. Additionally, these two peptides possibly protected PC12 cells against glutamate-induced apoptosis via activating intracellular antioxidant defence (superoxide dismutase (SOD) and glutathione peroxidase(GSH-Px)) through Kelch-like ECH-associated protein 1 (Keap1) inhibition, inhibiting ROS production,Ca2+ influx and mitochondrial membrane potential (MMP) collapse as well as regulating the expression of apoptosis-related proteins (Bax and Bcl-2). This might be due to the presence of Trp, Tyr and Arg in these two peptides. However, encapsulation of WSREEQEREE and ADIYTEEAGR should be considered based on their digestive sensibility during in vitro gastrointestinal digestion.
1. Introduction
Impairment of learning and memory is widely considered as the main clinical syndrome in many age-related neurodegenerative diseases, such as Alzheimer’s disease [1]. Apoptosis of neurons mediated by excitotoxicity has been observed in the development of memory loss [2]. For their similar morphology and physiological function to neurons, PC12 cells have been widely applied to neurological studies [3,4]. As an important neurotransmitter,glutamate plays a pivotal role in the conduction of signal in neurons and gliacytes. However, excessive amounts of extracellular glutamate can destroy the neuronal cells by increasing the accumulation of reactive oxygen species (ROS) and inhibiting the synthesis of glutathione. Meanwhile, calcium homeostasis and mitochondrial function were also reported to be involved in the process [5,6].
Bioactive peptides have been demonstrated to be capable of improving memory capacity in many studies. Chai et al. [7]found that Phe-Tyr-Tyr (FYY) and Asp-Trp (DW) fromBenthosema pterotumprotein hydrolysate possessed cytoprotective effects on H2O2-induced oxidative apoptosis in SH-SY5Y cells and D-gal-induced memory de ficits in mice. Wang et al. [8]demonstrated that peptides(GGW, VYY and LLPF) from walnut showed neuroprotective effects on glutamate-induced damage in PC12 cells.Cerebrolysin was also discovered to have neuroprotective effects on CoCl2-induced cytotoxicity in PC12 cells by getting involved in GSK-3β pathway [9]. Our previous research showed that defatted walnut meal hydrolysate (DWMH) could significantly ameliorate the behavioral performance in mice ofD-galactose-induced memory deficits, and the improvement of learning and memory performance might be closely related to its antioxidative activity [10]. Based on that, 6 peptides including WSREEQ (f281–286), WSREEQE (f281–287),WSREEQEREE (f281–290), ADIYTE (f336–341), ADIYTEEAG(f336–344) and ADIYTEEAGR (f336–345), locating in 11S globulin seed storage protein sequence of walnut (Juglans regia) by searching in UniProtKB, were identified from DWMH. Subsequently, they were verified to improve cell viability in H2O2-induced PC12 cells through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)assay without detecting their neuroprotective mechanisms [10].As discussed above, glutamate is an important neurotransmitter in central nervous system and excessive amounts of glutamate can lead to the possibility of neuronal damage and cell death. Therefore,a glutamate-induced oxidative apoptosis model was applied in this study. Meanwhile, the prerequisite for bioactive peptides to exert physiological effectsin vivois that they can survive in the digestion of the gastrointestinal (GI) tract, and then reach their target sites after absorption. Accordingly, simulated GI digestion is important parameters to be taken into consideration in the determination of the bioavailabilities and bioactivities of food-derived peptides. However, previous study mainly focused on the bioactivity of these peptides, and the specific peptides and their digestion stability still remain unknown.
Therefore, the aim of this study was to research the neuroprotective effects of these peptides derived from DWMH including WSREEQ, WSREEQE, WSREEQEREE, ADIYTE,ADIYTEEAG and ADIYTEEAGR on glutamate-induced cytotoxicity in PC12 cells. Furthermore, the possible mechanisms underlying the protective effects of the two most potent peptides including WSREEQEREE and ADIYTEEAGR were elucidated by determining the levels of intracellular antioxidant enzymes(i.e., superoxide dismutase (SOD) and glutathione peroxidase(GSH-Px)) and malondialdehyde (MDA), ROS production, Ca2+concentration, mitochondrial membrane potential (MMP, ΔΨm) and the expression of apoptosis-related proteins (i.e., Bax and Bcl-2) as well as molecular docking with Kelch-like ECH-associated protein 1(Keap1). Additionally, the digestive stabilities of WSREEQEREE and ADIYTEEAGR were detected as well.
2. Materials and methods
2.1 Chemicals
Fetal bovine serum (FBS) and RPMI 1640 medium were purchased from Gibco (Grand Island, USA), and MTT, dimethyl sulfoxide (DMSO) andL-glutamate were provided by Sigma-Aldrich(Missouri, USA). The rat pheochromocytoma PC12 cells line was offered by the Cell Bank of the Chinese Academy of Sciences(Shanghai, China). Peptides including WSREEQ, WSREEQE,WSREEQEREE, ADIYTE, ADIYTEEAG and ADIYTEEAGR were chemically synthesized by GL Biochem. Ltd. (Shanghai,China). Cerebrolysin was purchased from Cardinal Health Pharmacy(Guangzhou, China). Lactate dehydrogenase (LDH) release assay kit, ROS detection kit, MDA detection kit, GSH-Px detection kit,SOD detection kit, MMP detection kit and Fura-2 AM were bought from Beyotime Institute of Biotechnology (Shanghai, China). Other chemicals used were of analytical grade.
2.2 Cell culture and treatment
PC12 cells were cultured in RPMI 1640 medium supplemented with 10% (V/V) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 ℃ in a humidified atmosphere of 5% CO2. The coincubation model, incorporating samples and glutamate together,was chosen to assess the protective effects of peptides on glutamateinduced toxicity in PC12 cells [11]. PC12 cells were cultured in 96-well plates for 24 h at a density of 1 × 105cells/well before cells were treated with 0.10 mmol/L peptide and 32.5 mmol/L glutamate for another 24 h. In our pre-experiments, treating with 32.5 mmol/L glutamate last for 24 h could reduce the cell viability of PC12 by 40%–50% of the control group. Thus, 32.5 mmol/L was chosen as the test concentration in this study. The control and blank groups were administered with the equivalent volume of RPMI 1640 and phosphate buffered saline (PBS), respectively. All the operations were repeated more than three times under each treatment condition.
2.3 Cell viability assay
After being treated with samples for 24 h, the medium was removed, and 100 μL of 0.5 mg/mL MTT was added to each well by additional incubation at 37 °C for 4 h. Then the formazan was dissolved in 150 μL of DMSO. Absorbance of the formazan solution was measured at 570 nm with a Varioskan Flash Spectral Scanning Multimode Plate Reader (Thermo Fisher Scientific, Waltham, MA,USA). Cell viability was expressed as a relative percentage of the control group.
2.4 LDH release assay
As an indicator of the extent of cellular injury, the LDH level in the medium was evaluated in our study. The supernatant was collected at the end of treatments to determine the LDH leakage by the commercial assay kit according to the manufacturer’s instructions.The absorbance was measured at 450 nm using the Varioskan Flash Spectral Scanning Multimode Plate Reader.
2.5 Measurement of intracellular ROS
2’,7’-Dichloro fluorescin diacetate (DCFH-DA) fluorescent probe was used to evaluate the intracellular ROS in our study. At the end of treatment, cells were firstly incubated with 10 μmol/L DCFH-DA in an incubator for 30 min, and then washed twice with PBS. DCFH could be transformed into dichlorofluorescein (DCF) with strong green fluorescent at the presence of ROS. The intensity offluorescent atEx488 nm andEm525 nm were determined using the Varioskan Flash Spectral Scanning Multimode Plate Reader.
2.6 Determination of SOD, GSH-Px and MDA levels
The medium was removed and the cells were washed thrice with PBS after the cell treatments. Cells were centrifuged, collected and resuspended in PBS (200 μL) before dissociated by cell lysis buffer (200 μL RIPA lysis with 1 mmol/L phenylmethylsulfonyl fluoride), and then centrifuged for 6 min (13 000 ×g). The supernatant was used to assess the GSH-Px and SOD and MDA levels using commercial assay kit guided by the specified manufacturer’s instructions.
2.7 Molecular docking
Molecular docking was applied to estimate the interactions between peptide and Keap1, and vina was used to predict the affinity of peptide binding to Keap1 in this study. X-ray crystal structures of Keap1 (PDB ID: 6HWS and 6SP1) and peptides were obtained from protein data bank (www.rcsb.org) and ChemBio3D,respectively. The ligand, receptor and docking process were performed by AutoDock Tools.
2.8 Measurement of intracellular calcium concentration of([Ca2+]i)
At the end of treatment, PC12 cells were collected and incubated with Fluo-2/AM at a final concentration of 5 μmol/L in an incubator for 45 min, and then cells were washed and suspended in cold PBS.Additionally, adding Triton 100 (0.1%) into the cells to saturate Fura-2, and then measured at 340 and 380 nm to get the maximum fluorescence ratioRmax(F340/F380). Similarly, adding ethylenebis(oxyethylenenitrilo) tetraacetic acid ( final concentration at 5 mmol/L,pH 8.5), a high concentration of Ca2+chelating agent, into the cells to fully chelate Ca2+, and then measured at 340 and 380 nm to get the minimum fluorescence ratioRmin(F340/F380). TheFminandFmaxstand for the Fura-2 fluorescence intensity (F380) when Ca2+was zero and saturated, respectively.Kd(224 nmol/L) represents the dissociation constant of the reaction between Fura-2 and Ca2+. [Ca2+]iwas calculated as follows:

2.9 Measurement of MMP (ΔΨm)
JC-1 (5,5’,6,6’-tetrachloro-1,10,3,30-tetraethylbenzimidazolcarbocyanine iodide) could be clustered in mitochondrial matrix to form the polymers (J-aggregates) accompanying with red fluorescence if the ΔΨmis high. On the contrary, JC-1 could be distributed in mitochondrial matrix as the JC-1 monomer (monomer) accompanying with green fluorescence if the ΔΨmis low. Therefore it can be a sensitive measurement to detect the changes of mitochondrial membrane potential [12]. Brie fly, at the end of treatment, cells were incubated with JC-1 solution for 30 min in an incubator, and then centrifuged (4 min, 600 ×g) to collect the cell samples followed by washing, re- floating and analyzing with a Thermo Scientific Lumina fluorescence spectrophotometer (Thermo Fisher Scientific, Waltham,MA, USA). The ΔΨmchange was expressed as the ratio of the fluorescence intensity of monomer and aggregate.
2.10 Western blotting
Bax and Bcl-2 protein expression in cytosolic fractions was assessed. The cytosolic fractions were obtained by cell lysis. The protein content in cell sample was determined by BCA assay kit.Equal protein content (25 μg) was electrophoresed. At the end of electrophoresis, an electric transfer system was applied to transfer the protein from gel to a polyvinylidene fluoride membrane. Nonspecific binding was blocked with 5% skim milk in TBST buffer(5 mmol/L Tris-HCl, 136 mmol/L NaCl and 0.1% Tween-20, pH 7.6)for 1.5 h. The blots were incubated at 4 ℃ with antibodies against Bax (1 : 500), Bcl-2 (1 : 500) and actin (1 : 1 000) for 12 h, and then washed with 1 × TBST and incubated with horseradish peroxidaselabeled anti-rabbit or anti-mouse IgG for another 1 h at room temperature. After that they were washed 3 times again and developed using a Chemiluminescent Western Blot Imaging System (Azure Biosystems, Inc., Dublin).
2.11 In vitro simulated GI digestion
In vitrosimulated GI digestion was conducted according to the procedure of INFOGEST static method [13]with some modification.GI digestion consisted of two continuous trials including gastric phase and intestinal phase. Firstly, simulated gastric fluid (SGF)stock solution pre-heated at 37 °C was added into peptide solution(5 mg/mL) at a ratio of 1 : 1. The pH of mixture was adjusted to 3.0 with 1 mol/L HCl before CaCl2·2H2O and pepsin (2 000 U/mL in the final mixture) was added. The mixture was then incubated at 37 °C for 2 h. Secondly, simulated intestinal fluid (SIF) stock solution prewarmed at 37 °C was added to the chyme in the previous step at a 1 : 1 ratio. The system was adjusted to pH 7.0 with 1 mol/L NaOH,and then CaCl2·2H2O solution and pancreatin (100 U/mL in the final mixture) were added, the pH value was checked again and the solution was diluted to 1 × SIF with water. Next, the mixed solution was incubated at 37 °C for 2 h and finally heated in boiling water for 10 min to inactive the enzymes. All the samples were lyophilized and stored at −20 °C till use.
2.12 Peptide analysis by ultra-performance liquid chromatography-electrospray ionization/quadrupole-time-offlight tandem mass spectrometry (UPLC-ESI-QTOF-MS/MS)
UPLC-QTOF-MS/MS was conducted by a Waters Acquity UPLC(Massachusetts, USA) system connected with a Bruker IMPACT II ESI-QTOF mass spectrometer (Bremen, Germany) as described previously [14].A Waters HSS T3 column was used in this study. The mobile phase A and B were ultrapure water containing 0.1% methanoic acid and acetonitrile, respectively. The gradient program was set as: 0–10 min,95%–60% A; 10–12 min, 60%–10% A; 12–14 min, 10% A; 14–15 min,10%–95% A; 15–18 min, 95% A. Mass spectra were set at 70−1 300m/zin the positive-ion mode. The quadrupole ion energy was set at 4.0 eV,while the collision inducing dissociation energy was set at 8−50 eV.The parameters for the ESI interface were as follows: 180 °C drying gas temperature, 8.0 L/min drying gas flow, and 1.5 bar ESI nebulizer pressure. After simulated gastrointestinal digestion, digestive products(20 μmol/L, 2 μL) was loaded at a flow rate of 0.20 mL/min. The digestive peptides were identified as following: firstly, we find out all the possible digestive products derived from original peptide (WSREEQEREE and ADIYTEEAGR), and then ion chromatogram of ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS)profile of all the possible digestive products in control, gastric phase and intestinal phase were extracted. Finally, the fragments in digestive product were identified and listed. The relative content for target peptide was presented as the area of extracted ion chromatograms MS/MS.
2.13 Statistical analysis
SPSS 17.0 (SPSS Inc., Chicago, IL) with ANOVA analysis and Duncan posthoc test was used to perform the data in our study.Significant differences were expressed atP< 0.05.
3. Results
3.1 Effects of WSREEQ, WSREEQE, WSREEQEREE,ADIYTE, ADIYTEEAG and ADIYTEEAGR on cytotoxicity
Cell viability was used for investigating the protective effects of WSREEQ, WSREEQE, WSREEQEREE, ADIYTE, ADIYTEEAG and ADIYTEEAGR on glutamate-induced apoptosis in PC12 cells. Cerebrolysin, a clinically used peptidergic drug in dementia,was chosen as the positive control in this study [9]. All peptides were applied at 0.10 mmol/L, which was equivalent to 0.08, 0.10,0.14, 0.07, 0.09 and 0.11 mg/mL for WSREEQ, WSREEQE,WSREEQEREE, ADIYTE, ADIYTEEAG and ADIYTEEAGR,respectively, while cerebrolysin, a mixture of amino acids and peptides, was conducted at 0.15 mg/mL. MTT assay results showed that all samples had no cytotoxicity in PC12 cells at 0.10 mmol/L(Fig. 1A). Additionally, the viability of cells exposed to 32.5 mmol/L glutamate for 24 h decreased to (53.77 ± 2.75)% of the control group. Fig. 1B presents that a significant degree of recovery was observed after incubating with these peptides. The cell viability of WSREEQEREE and ADIYTEEAGR (0.10 mmol/L) groups increased to (76.39 ± 2.83)% and (75.79 ± 2.75)% of the control value, respectively (P< 0.05). Therefore, the most active peptides WSREEQEREE and ADIYTEEAGR were chosen to further study the underlying molecular mechanisms of their protective effects mediated by oxidative stress and related apoptosis pathways in PC12 cells.

Fig. 1 Effects of DWMH treatment on cell viability. (A) Cell viability after treatment with cerebrolysin, WSREEQ, WSREEQE, WSREEQEREE, ADIYTE,ADIYTEEAG and ADIYTEEAGR at 0.10 mmol/L, respectively. (B) Cell viability after treatment with cerebrolysin, WSREEQ, WSREEQE, WSREEQEREE,ADIYTE, ADIYTEEAG and ADIYTEEAGR (0.10 mmol/L) and 32.5 mmol/L glutamate for 24 h. (C) LDH leakage in PC12 cells. The data are represented as means ± S.D. # represents P < 0.05 versus control group. * represents P < 0.05 versus glutamate group.
3.2 Effects of WSREEQEREE and ADIYTEEAGR on LDH release
LDH release into the culture medium is regarded as an indicator of cellular impairment [15]. Glutamate insult led to a significant increase of LDH release, which was (601.09 ± 9.59)% of the control group in medium (Fig. 1C). However, treatment with cerebrolysin,WSREEQEREE and ADIYTEEAGR at 0.10 mmol/L could suppress the leakage of LDH (P< 0.05). Moreover, WSREEQEREE and ADIYTEEAGR showed stronger inhibitory activities than cerebrolysin in LDH leakage.
3.3 Effects of WSREEQEREE and ADIYTEEAGR on intracellular ROS, MDA contents and SOD, GSH-Px activities
As shown in Fig. 2A, glutamate insult resulted in a 2.20-fold higher level of intracellular ROS than that of the control group.However, treating with WSREEQEREE and ADIYTEEAGR(0.1 mmol/L) significantly reduced the ROS production to (165.21 ±6.82)% and (184.46 ± 6.08)% (P< 0.05), respectively, followed by cerebrolysin (222.50 ± 4.91)%. As shown in Figs. 2B, C, D, a decrease in SOD and GSH-Px activities and an increase in MDA content were observed in glutamate-treated PC12 cells. However, incubating with cerebrolysin, WSREEQEREE and ADIYTEEAGR (0.1 mmol/L)brought an obvious increase of SOD activity in PC12 cells from(56.33 ± 4.00) U/mg protein to (67.60 ± 3.00), (80.59 ± 2.47) and(74.10 ± 3.06) U/mg protein, respectively (Fig. 2B). Simultaneously,except for cerebrolysin group, GSH-Px levels in all administrative groups were markedly up-regulated compared with the glutamate group (Fig. 2C). Furthermore, treating with cerebrolysin,WSREEQEREE and ADIYTEEAGR could obviously reverse the negative increase in the level of MDA due to glutamate insult((33.33 ± 2.08) mmol/mg protein) (Fig. 2D).
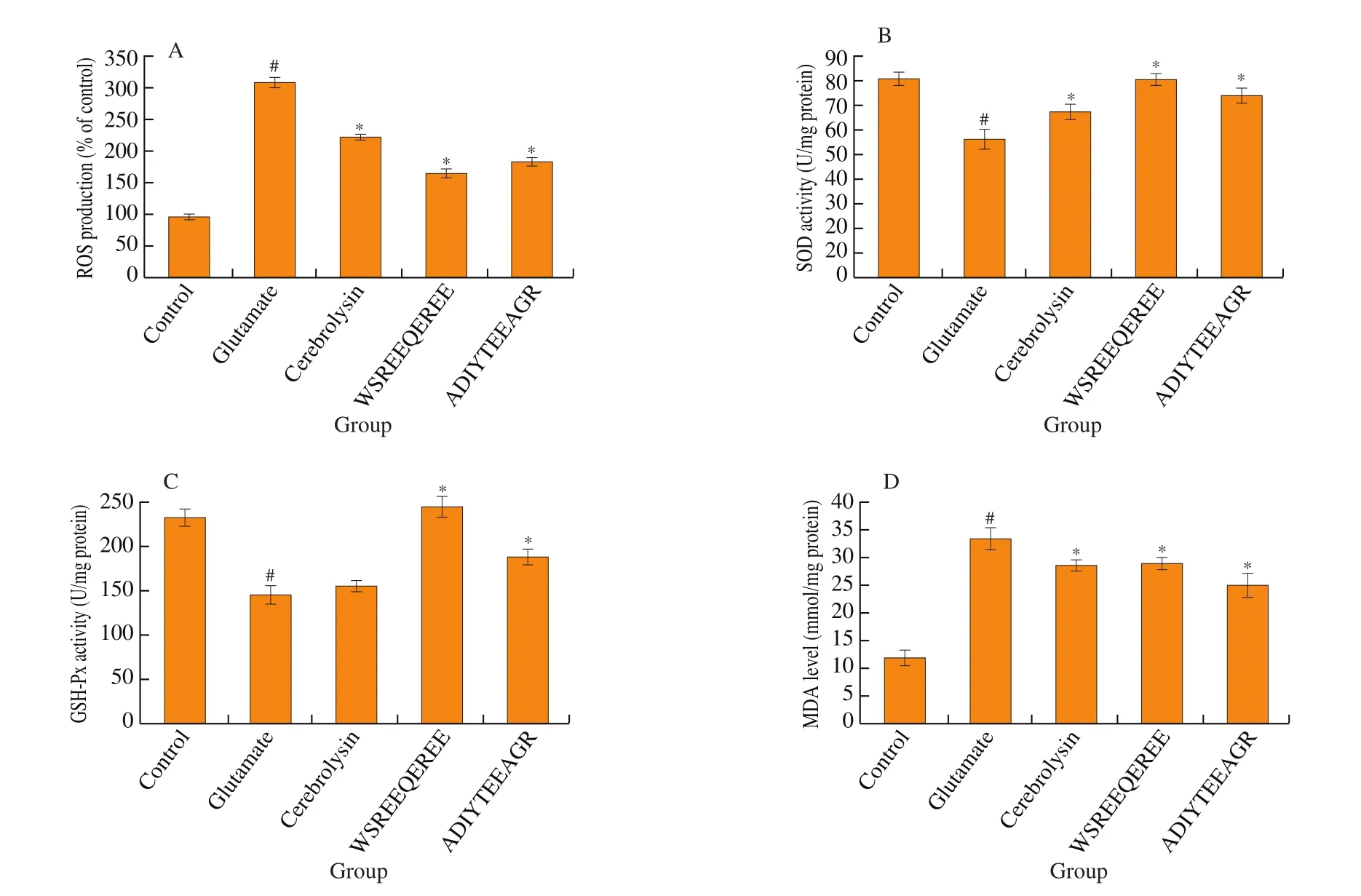
Fig. 2 Effects of WSREEQEREE and ADIYTEEAGR (0.10 mmol/L) on the glutamate-induced. (A) Intracellular ROS accumulation. (B) SOD activity downregulation. (C) GSH-Px activity down-regulation. (D) MDA levels overproduction. The data are represented as means ± S.D. # represents P < 0.05 versus control group. * represents P < 0.05 versus glutamate group.
3.4 Molecular docking analysis
The binding modes of WSREEQEREE and ADIYTEEAGR with Keap1 were obtained via molecular docking. As shown in Fig. 3 and Table 1, residues Ser431, Asn414, Ser555, Asn382 and Tyr334 of Keap1 formed five conventional hydrogen bonds with WSREEQEREE, and Tyr334 was bound to WSREEQEREE by pi-pi stacked. Simultaneously, there existed two kinds of hydrogen bonds,a conventional hydrogen bond and a carbon-hydrogen bond, between ADIYTEEAGR and Keap1. Residues Arg380, Ser363, Arg415,Arg483, Asn387, Ser602 and Ile416 of Keap1 formed conventional hydrogen bonds with ADIYTEEAGR, while Asn382, Arg380,Gly462, Arg483, Tyr525, Gly509, Ala556, Tyr334 and Tyr572 formed carbon hydrogen bonds. Additionally, ADIYTEEAGR formed pi-cation with residue Arg415 of Keap1. Based on these results, it could be found that the essential residues and hydrogen bonds were involved, indicating that peptides WSREEQEREE and ADIYTEEAGR were potent Keap1 inhibitors.
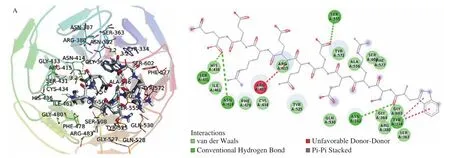
Fig. 3 The 3D and 2D molecular interactions of (A) WSREEQEREE and (B) ADIYTEEAGR with the active sites of Keap1.
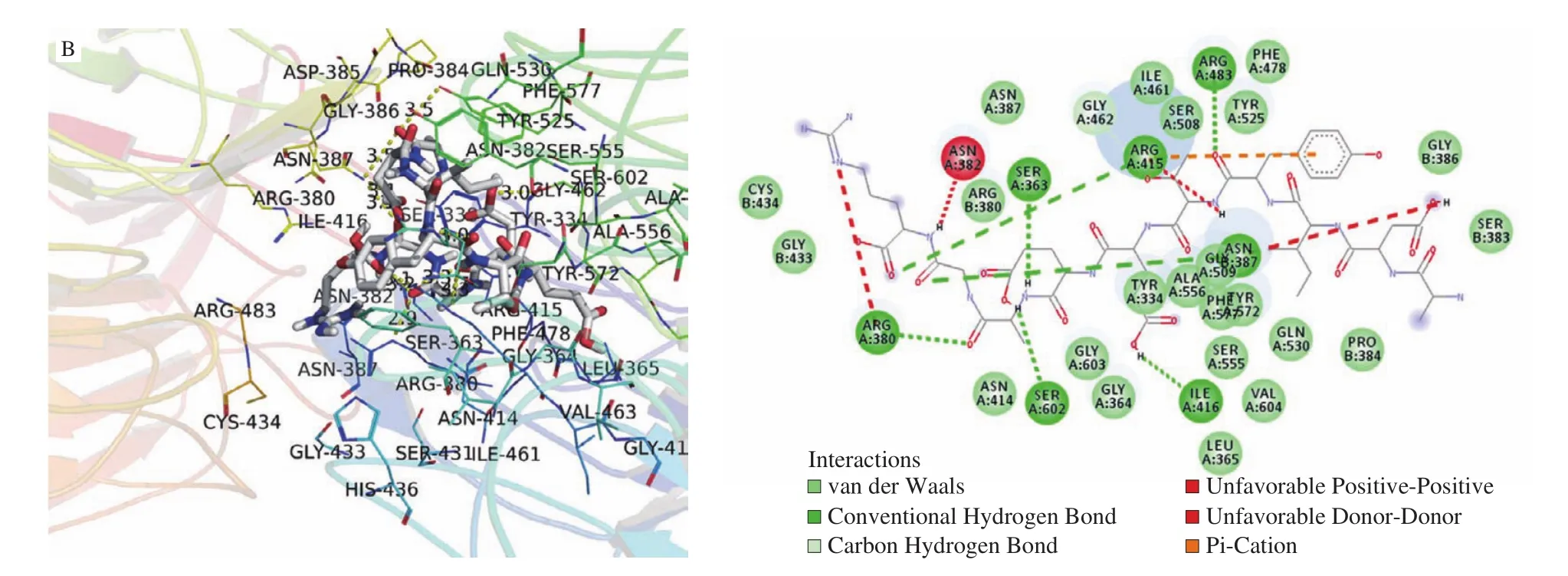
Fig. 3 (Continued)
3.5 Effects of WSREEQEREE and ADIYTEEAGR on intracellular Ca2+ concentration
It is widely accepted that glutamate-induced excitotoxicity triggers the loss of Ca2+homeostasisviathe uncontrollable production of peroxide [16,17]. In Fig. 4A, glutamate insult for 24 h remarkably increased the fluorescence intensity of intracellular [Ca2+]i((423.75 ± 5.45) mmol/L) in PC12 cells. However, treatment with cerebrolysin, WSREEQEREE and ADIYTEEAGR (0.1 mmol/L)had the obvious reverse effects on the fluorescence intensity of intracellular [Ca2+]i, which were (280.06 ± 3.44), (233.79 ± 6.40) and(240.84 ± 6.16) mmol/L, respectively.
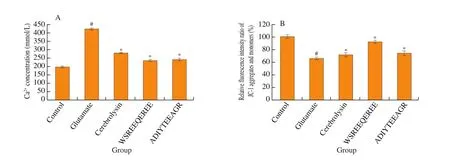
Fig. 4 Effects of WSREEQEREE and ADIYTEEAGR (0.10 mmol/L) on the (A) glutamate-induced intracellular Ca2+ influx and (B) MMP loss. The data are represented as means ± S.D. # represents P < 0.05 versus control group. * represents P < 0.05 versus glutamate group.

Table 1The forces and residues of the interaction of Keap1 with peptide.
3.6 Effects of WSREEQEREE and ADIYTEEAGR on MMP(ΔΨm)
The phenomenon of MMP collapse has been widely reported in many models of apoptosis. To investigate the effects of WSREEQEREE and ADIYTEEAGR on glutamate-induced apoptosis in PC12 cells, JC-1 staining method was used for detecting the changes of MMP. The relative fluorescence intensity ratio of JC-1 aggregates and monomers showed that glutamate insult partly led to the loss of MMP in PC12 cells. The collapse of MMP induced by glutamate was significantly reversed by treating with cerebrolysin, WSREEQEREE and ADIYTEEAGR, which were (71.66 ± 3.34)%, (92.61 ± 2.41)%, and (74.66 ± 3.42)%,respectively (Fig. 4B).
3.7 Effects of WSREEQEREE and ADIYTEEAGR on the protein expression of Bcl-2 and Bax
Western blotting is a commonly used method to evaluate the level of protein expression. In our study, it was used to assess the protective effects of WSREEQEREE and ADIYTEEAGR on glutamate-induced apoptosis by measuring the changes in expression of apoptosis-related proteins. As shown in Figs. 5A, B, C, compared with the control group, glutamate insult up-regulated the expression of Bax and down-regulated the expression of Bcl-2 (P< 0.05). However, cerebrolysin,WSREEQEREE and ADIYTEEAGR had obvious recovery effects on the expression levels of Bax and Bcl-2 (P< 0.05). As glutamate insult, the Bax/Bcl-2 ratio is 1.27 times that of the control group,incubating with cerebrolysin, WSREEQEREE and ADIYTEEAGR could alter the Bax/Bcl-2 ratio to (120.41 ± 6.11)%, (83.21 ± 9.50)% and (54.71 ± 7.15)%, respectively (Fig. 5D).

Fig. 5 Effects of WSREEQEREE and ADIYTEEAGR (0.10 mmol/L) on the expression of Bax and Bcl-2 in PC12 cells. (A) Western blotting analysis of Bax. (B)Western blotting of Bcl-2. (C) Quantitative analysis of Bax and Bcl-2 expression. (D) Bcl-2/Bax. The data are represented as means ± S.D. # represents P < 0.05 versus control group. * represents P < 0.05 versus glutamate group.
3.8 Degradation of WSREEQEREE and ADIYTEEAGR during in vitro GI digestion
It is widely accepted that peptides might be further digested before absorbing by GI tract, thereby causing changes in the bioactivities of peptides. In order to investigate the digestive stabilities of WSREEQEREE and ADIYTEEAGR, the two peptides were subjected to simulated GI digestionin vitro. Results showed that WSREEQEREE and ADIYTEEAGR were almost completely digested into several peptide fragments (Fig. 6). Notably, REE, EEQ and WS were the main derivative peptides in the digesta of WSREEQEREE,and their peak areas were almost higher than 1.0 × 106(Table 2). On the other hand, although nine fragments were found in the digesta of ADIYTEEAGR, the peak areas of fragments (TEEAGR, TEEAG,ADIYT, YTEE, ADIY, YTE and GR) were below 8.0 × 105, which was much lower than that of ADI (2.3 × 106) and TEE (1.2 × 106)(Table 3). It indicated that most of ADIYTEEAGR were digested into ADI and TEE after the simulated GI process.

Fig. 6 Characterization of peptides and their in vitro digestion on UPLC-MS/MS profile. (A) WSREEQEREE and (B) ADIYTEEAGR. The area indicates the peak area of the corresponding peptide.

Table 2Characterization of WSREEQEREE and its in vitro digestion on UPLC-MS/MS profile.
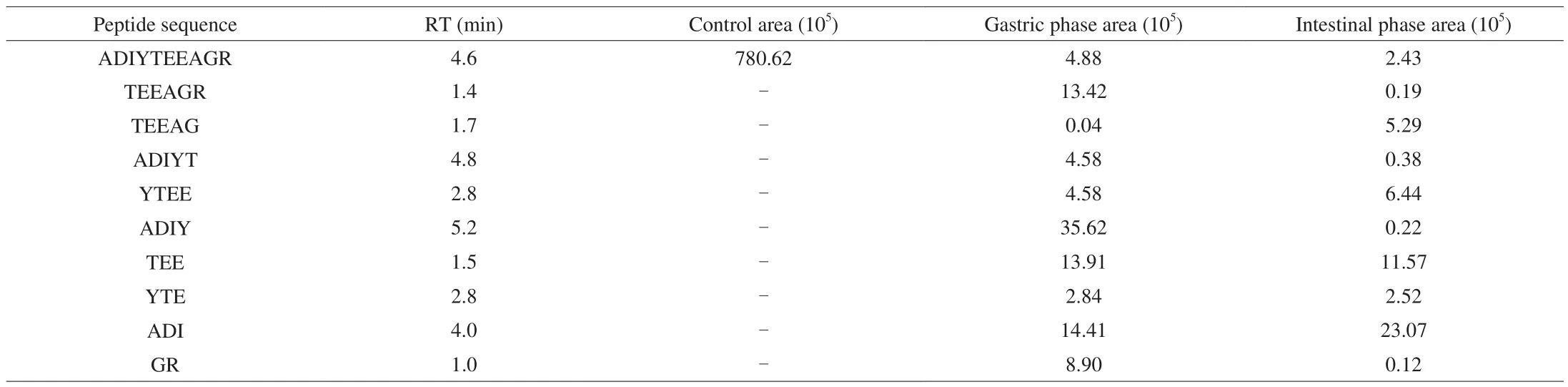
Table 3Characterization of ADIYTEEAGR and its in vitro digestion on UPLC-MS/MS profile.
4. Discussion
In the present study, MTT assay results showed a remarkable degree of recovery after incubating with DWMH (WSREEQ,WSREEQE, WSREEQEREE, ADIYTE, ADIYTEEAG and ADIYTEEAGR). As we all know, molecular weight, amino acid compositions and sequences of peptides are all pivotal factors to determine their bioactivities. The reverse effects presented in this study could be partly due to the free radical scavenging activities of Trp and Tyr in these peptides [18,19]. As reported in our previous study, these peptides showed strong radical scavenging activity in oxygen radical absorbance capacity (ORAC) assay. The reactions of free radical chain can be slow or even be terminated by Tyr and Trp through giving hydrogen atoms to free radicals. Additionally,the intermediates like benzene oxygen free radical and indole free radical formed in this process can be stabilized by resonance [20].Interestingly, the cell viability of PC12 cells after treatment with WSREEQEREE and ADIYTEEAGR increased significantly compared with that treated with WSREEQE and ADIYTEEAG, respectively,indicating that the Arg or Glu residue might play an important role in improving the neuroprotective effects. However, WSREEQ and WSREEQE almost had similar protection effects, indicating that the addition of Glu had no effects on the protective effects of WSREEQE.As a biosynthesis precursor, Arg can be transformed to nitric oxides(NO). According to report [21], NO has the ability to modulate learning and memory in cognitive performance. Paul et al. [22]found that administration of Arg exhibited strong inhibitory activity on learning and memory impairment caused by picrotoxin [22].Therefore, the addition of Arg residue in WSREEQEREE and ADIYTEEAGR might be the potential reason for their stronger protective effects. Additionally, there was no improvement in the reverse effect of ADIYTEEAG compared with ADIYTE. It indicated that the hydrophobic amino acids Gly and Ala had no neuroprotective effect on glutamate-induced oxidative injury in PC12 cells, although they were reported to be capable of improving the antioxidative activities [23,24]. Therefore, the most active peptides WSREEQEREE and ADIYTEEAGR were chosen to further study the underlying molecular mechanisms of protective effects mediated by oxidative stress and related apoptosis pathways in PC12 cells.
As the main excitatory neurotransmitter in central nervous system(CNS), glutamate plays a vital role in the conduction of signals.However, excessive cluster of extracellular glutamate in CNS can promote the neuronal disorders [25]. Two primary pathways have been proposed on glutamate toxicity. The first one, which is caused by over-activation of glutamate receptors (N-methyl-D-aspartic acid(NMDA) receptors) along with large amount of extracellular Ca2+influx, was called excitotoxicity [26]. As for the other one called oxidative glutamate toxicity, it can competitively inhibit cysteine/glutamate antiporter system, and further to deplete intracellular glutathione content, as well as increase NADPH oxidase-dependent hydrogen peroxide accumulation and ROS production. Additionally,mitochondrial dysfunction and apoptosis-related death are also involved in this process [27,28].
Overproduction of ROS is the main feature in glutamate-induced cell excitotoxicity. ROS cluster could accelerate the cell death and apoptosis mediated by oxidative injury through DNA damage,mitochondrial dysfunction, protein de ficit and lipid peroxidation [29].It has been reported that many antioxidants could reduce or delay cell apoptosis induced by oxidative stress by increasing the levels of endogenous antioxidant defense systems, such as glutathione peroxidase [30]. Compared with the PC12 cells treated by glutamate alone, incubating with WSREEQEREE or ADIYTEEAGR could obviously reduce the production of ROS in cells (Fig. 2A).Additionally, glutamate-induced oxidative toxicity caused a series of detrimental changes in intracellular SOD activity, GSH-Px activity and MDA level [31]. Glutamate insult evidently decreased the activities of SOD and GSH-Px and increased the level of MDA in PC12 cells. Simultaneously, the obvious reverse effects by incubating with WSREEQEREE and ADIYTEEAGR were observed, indicating that WSREEQEREE and ADIYTEEAGR could be regarded as antioxidants, thereby protecting PC12 cells from oxidative damage.Two peptides (Phe-Tyr-Tyr and Asp-Trp) derived fromBenthosema pterotumwere reported to be capable of attenuating H2O2-induced oxidative apoptosis by activating intracellular antioxidant defence including catalase, SOD and GSH-Px activities in SH-SY5Y cells [7].Our results supported the assumption that reducing the production of ROS through activating endogenous antioxidants could improve cell viability [32]. Additionally, it is widely accepted that Keap1-Nrf2 pathway plays a pivotal role in regulating cellular antioxidant system. As a transcription factor, Nrf2 is dissociated from Keap1 to enter in the nucleus and mediates the transcription of genes encoding antioxidant enzymes [33]. Peptides (DDK and DWW) were capable of promoting and upregulating the activity of antioxidant defense enzymes, which were mainly due to the interference with Keap1 through hydrogen bond [34]. Thus, we speculated that WSREEQEREE and ADIYTEEAGR might be the potential Nrf2 activators to inhibit Keap1-Nrf2 interaction, which were recognized by molecular docking. Docking analysis showed that there existed strong conventional hydrogen bond, carbon–hydrogen bond and pi–pi interaction between peptides (WSREEQEREE and ADIYTEEAGR)and Keap1. The results of molecular docking were consistent with the activity of antioxidant enzymes in our study, indicating that WSREEQEREE and ADIYTEEAGR might upregulate the antioxidant enzymes through interference with Keap1.
It has been demonstrated that glutamate-induced excitotoxicity mediated by interacting with NMDA receptors would result in an excessive increase of ROS and intracellular calcium influx [26].Therefore, the injury appears in neuronal cells might be associated with the over-activation of glutamate receptors followed by uncontrollable influx of calcium into neurons [35]. In this study,overproduction of ROS and Ca2+influx were observed after treating with glutamate alone in PC12 cells as reported by other researchers.However, treatment with WSREEQEREE, ADIYTEEAGR and cerebrolysin could significantly prevent the influx of Ca2+and the overproduction of ROS (P< 0.05). Additionally, calcium overload in cells could also lead to the over-stimulation of proteolytic enzymes,generating of free-radical and lipid peroxidation. In our research,treatment with WSREEQEREE and ADIYTEEAGR were capable of decreasing the intracellular Ca2+influx and increase in MDA content connected with oxidative stress in glutamate-treated PC12 cells(P< 0.05). Therefore, the amelioration of oxidative stress in glutamate-induced PC12 cells may partially account for the inhibitory effects of WSREEQEREE and ADIYTEEAGR on Ca2+influx, even the activation of NMDA receptors.
Mitochondria is the center of intracellular energy metabolism, and calcium overload can negatively affect the function of mitochondria such as the disruption of MMP. Furthermore, the collapse of MMP triggers the enhanced ROS production, nuclear condensation and eventually results in cellular apoptosis [36]. Previous research demonstrated the key function of apoptosis in glutamate-induced cascade responses. It was reported that a series of genes and proteins involved in mitochondrial pathway play important roles in the course of cellular apoptosis [37,38]. The Bcl-2 family, as the main regulators of apoptosis-related proteins, was involved in cellular apoptosis. Commonly, Bcl-2 family was divided into anti-apoptotic proteins (Bcl-2, Bcl-xL) and pro-apoptotic proteins (Bax, Bad). The abnormal expression of pro-apoptotic and anti-apoptotic proteins can be regarded as the dominant factors in cell apoptosis [39]. Our study showed that WSREEQEREE and ADIYTEEAGR exhibited obvious reverse effects on MMP loss and further up- and down-regulated the expression of Bax and Bcl-2 proteins attacked by glutamate insult in PC12 cells. Thus, WSREEQEREE and ADIYTEEAGR might exert their neuroprotective effects on glutamate-induced apoptosis via amelioration of mitochondrial apoptotic pathway.
The prerequisite for bioactive peptides to exert physiological effectsin vivois that they can survive in the digestion of the GI tract, and then absorb to reach their target sites. Accordingly,comprehensively consideration of the bioavailabilities and bioactivities of food-derived peptides are necessary. It was demonstrated that digestive sensibility of peptide was determined by its molecular weight, charge property and amino acid composition [40].The obtained results exhibited that WSREEQEREE and ADIYTEEAGR were completely degraded duringin vitroGI digestion, indicating that they were sensitive to the enzymes in GI tract. It might be due to the fact that the long sequences provided more potential binding sites between digestive enzymes and peptides. Based on our study on digestive stability, encapsulation of WSREEQEREE and ADIYTEEAGR are required to ensure integrality during digestion and absorption processes. Similar results were reported by Ruiz et al. [41], who found that large peptides were fragile to the digestive enzymes during simulated GI digestion, while small peptides remained intact.
5. Conclusion
In conclusion, the reason why the cell viability of PC12 cells exposed to glutamate increased significantly after treatment with WSREEQEREE and ADIYTEEAGR compared with other four peptides might be due to the addition of Arg residue. All data suggested that WSREEQEREE and ADIYTEEAGR from DWMH exhibited strong neuroprotective effects on glutamate-induced toxicity in PC12 cells by activating of intracellular antioxidant enzymes (SOD and GSH-Px) through Keap1 inhibition, inhibiting ROS production,Ca2+influx and MMP collapse as well as regulating the expression of apoptosis-related proteins. Additionally, WSREEQEREE and ADIYTEEAGR were found to be hydrolyzed completely duringin vitroGI digestion, and encapsulation of them should be included in the further research. Therefore, WSREEQEREE and ADIYTEEAGR may be considered as potential neuroprotective peptides against age-related cognition and memory deficits. Moreover, whether the occurrence of Arg in other peptides could also enhance the neuroprotective effects remains to be verified.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Taishan Industry Leading Talent Project, Guangdong Provincial Key R&D Program(2020B020226005), the Specific Fund Program for Basic and Applied Basic Research of Guangdong Province (2019A1515011952), the Fundamental Research Funds for the Central Universities (No.x2skD2192510), the Natural Science Foundation of Guangdong for Basic and Applied Basic Research (2020A1515010659) and Special Support Project of Guangxi Province for Innovation driven Development (Guangdong Huapeptides Biotechnology Co., Ltd.,AA17204075).
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

