Effects of Siraitia grosvenorii extracts on high fat diet-induced obese mice:a comparison with artificial sweetener aspartame
Ke Lü, Xiown Song, Peng Zhng, Wei Zho, Ning Zhng, Fn Yng,Wenqing Gun, Jinfu Liu, He Hung, Chi-Tng Ho, Rong Di*, Hui Zho,*
a Tianjin Key Laboratory of Food and Biotechnology, School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
b Department of Biochemical Engineering, School of Chemical Engineering and Technology, Key Laboratory of Systems Bioengineering, Ministry of Education,Tianjin University, Tianjin 300072, China
c Department of Food Science, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA
d Department of Plant Biology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA
Keywords:
Siraitia grosvenorii
High fat diet
Obese
In flammation
Gut microbiota
A B S T R A C T
Long-term artificial sweetener intake is linked to increased risk of obesity. In the present study, supplement of natural sweetener from Siraitia grosvenorii (SG) (or Momordica grosvenorii) fruit, compared with the artificial sweetener aspartame (ASM), was evaluated for anti-obesity effects on mice fed with high fat diet (HFD). We found that, in contrary to ASM, SG extracts prevented body weight gain, the insulin resistance and fat mass accumulation in HFD mice. SG extracts treatment inhibited the inflltration of inflammatory macrophages and lowered the levels of the fat inflammatory cytokines (leptin, macrophage chemoattractant protein 1 (MCP-1)and tumor necrosis factor-α (TNF-α)) in adipose tissues. In addition, SG extracts supplement counteracted the remodeling of gut microbiota resulted from HFD. However, ASM supplement aggravated the HFD-induced obese performances, fat inflammation and dysregulation of gut microbiota. Taken together, our results indicate that supplement of SG extracts may represent a promising alternation of artificial sweeteners in preventing metabolic diseases.
1. Introduction
According to the report from World Health Organization in 2014, more than 1.9 billion adults were overweight and over 600 million were obese worldwide [1]. Obesity has been an important risk factor contributing to many chronic non-communicable diseases,especially to type 2 diabetes, cardiovascular diseases, sleep apnea syndrome and a variety of cancers [2]. The rapid increase in the prevalence of obesity worldwide has been partially attributed to the overconsumption of added sugars or sweeteners [3]. Therefore,limiting the intake of sugar or sweeteners is considered to prevent the diseases related to obesity.
Siraitia grosvenorii(SG) as an emerging natural source of sweetener has been well used as a traditional Chinese medicine for treating the colds, throats and congestion [4,5]. Mogrosides are the representative bioactives of this plant with the portraits of high sweetness resembling aspartame (around 200–300 times sweeter than sucrose) [6,7],low calories and nontoxic [5,6]. Mogrosides possess a wide range of health-promoting properties including antitumor [8,9]antiinflammation [10]and immunoregulation [11]. It has also been shown that SG extracts target hyperglycemic involved in the maltase in the small intestinal epithelium and exert anti-arteriosclerosis potential through lowering low-density lipoprotein (LDL) oxidation [12].
Obesity is tightly linked with inflammation. The infiltrated inflammatory macrophages play an important role in initiating and dampening obesity development [13]. In particular, accumulated evidence suggests that the microbiota shape the gut metabolites and modulate the obesity-associated inflammation (including macrophage ontogeny) and insulin resistance [14]. Given the health benefits of SG, we investigated whether SG extracts could improve high fat diet (HFD)-induced obesity in C57BL/6J mice and elucidated its mechanisms.
2. Materials and methods
2.1 SG and its extracts
The dried SG fruit was gifted by Dr. Rankun Zhang from the Affiliated Hospital of Guilin Medical University. The water extracts from SG were obtained as described previously with some modifications [15]. Brie fly, 20 g fruit powder was boiled three times in water (1 : 20,m/m) at 100 °C for 1 h each time which were then centrifuged at 5 000 ×gfor 15 min and the supernatant was then dried at room temperature to obtain the extract powder. All extracts were prepared in water and filtered through a 0.22 μm nylon filter before analysis.
2.2 Ultra performance liquid chromatography (UPLC) analysis
The major components in SG extracts were analyzed on a Waters Acquity UPLC system (Agilent Technologies, USA), with Agilent Poroshell 120 EC-C18column (130 Å, 2.1 mm × 100 mm, 2.7 μm,Agilent Technologies, USA). Acetonitrile/water (21 : 79) was used as a mobile phase at a flow rate of 0.2 mL/min and extended 30 min.Major peaks were selected based on UV absorbance at 203 nm. Then,mogroside I (S-I), mogroside III (M-III), mogroside IV (M-IV) and mogroside V (M-V) (purity ≥ 98%) were used as the standards to calculate the standard curves of mogrosides, respectively.
2.3 Animals and food
C57BL/6J mice (6 weeks of age) were purchased from HFK Biotechnology Co., Ltd. (Beijing, China) and the mouse protocol was approved by the Animal Use Committee at Tianjin University of Commerce. Animals were housed in cages and allowed to adapt the environment for 7 days under 12-h light and dark cycle at (22 ± 2) °C before the experiments.
SG extracts dissolved in the aseptic water at the concentration of 10 mg/mL was selected for subsequent administration due to its sweetness close to aspartame (0.5 mg/mL) solution assessed by sensory analysis (supplementary materials). Mice were assigned to 4 groups at random: (1) control mice were fed with the standard diet,(2) mice were fed with high fat diet to induce obese (HFD group),(3) mice were fed with high fat diet receiving daily drinking water containing 10 mg/mL SG extracts (SG group), and (4) mice were fed with high fat diet receiving daily drinking water containing 0.5 mg/mL aspartame (ASM group). Mice were observed for 18 weeks until the experiment ended.
2.4 Glucose tolerance tests (GTT) and insulin tolerance tests (ITT)
GTT was implemented 16 weeks after the commencement of the experiment. Mice were fasted for 12 h and were intraperitoneally injected with 25% glucose solution at 1.5 g/kg. For ITT, 17 weeks after the start of the experiment, mice were fasted for 4 h and were administered with intraperitoneal insulin solution at 0.1 U/mL and 0.75 U/kg. Blood was drawn from the tail vein 15, 30, 60, 90 and 120 min after glucose and insulin injections. The blood glucose levels were measured using the glucometer. Areas under the curve (AUC)were analyzed with GraphPad Prism 7.0 (San Diego, CA).
2.5 Fasting glucose and fasting insulin test
Fasting glucose was measured in mice using the glucometer after overnight fasting from the tail vein. Fasting Insulin levels in plasma were determined using an enzyme linked immunosorbent assay(ELISA) kit, according to the manufacturer’s instructions.
2.6 Analysis of serum
Triglycerides (TG), total cholesterol (TC), alanine transaminase(ALT) and aspartate amino-transferase (AST) were quantified using the ELISA kits following the instructions of the manufacturer.
2.7 Hematoxylin and eosin (H&E) staining
Mouse body adipose tissue was collected and fixed in 4% Paraform-Aldehyde. Fixed epididymal adipose tissue was embedded in paraffin (4 μm thick) and then stained with hematoxylin and eosin. Photomicrographs were taken with a LUCPLFLN microscope(Olympus, Japan). The cell diameter of adipose tissue was quantified using ImageJ software.
2.8 Immunohistochemical (IHC) staining
Sections were deparaffinized in xylene and rehydrated in graded alcohol. The endogenous peroxidase was quenched with 3% H2O2for 20 min and the nonspecific binding was interdicted with normal goat serum dissolved in 3% BSA for 1 h at room temperature. Sections were incubated with mouse F4/80 monoclonal antibody overnight at 4 °C and then incubated with SPlink Detection Kits and stained with DAB Kit according to manufacturer’s protocol.
2.9 Real-time polymerase chain reaction (RT-PCR)
Total RNA in adipose tissues was extracted using Trizol reagent according to the manufacturer’s instruction to determine mRNA expression levels. The RNAs were reverse-transcribed using the Transcriptor First Strand cDNA Synthesis Kit according to the manufacturer’s instructions. PCR was performed with a LightCycler®FastStart DNA Master SYBR Green Kit. The primers used were: Ucp1, forward: CACGGGGACCTACAATGCTT,reverse: ACAGTAAATGGCAGGGGACG;Mcp1,forward:CCCCAAGAAGGAATGGGTCC,reverse:GGTTGTGGAAAAGGTAGTGG;TNF-α,forward:ACGGCATGGATCTCAAAGAC,reverse:AGATAGCAAATCGGCTGACG;F4/80:forward:CTTTGGCTATGGGCTTCCAGTC,reverse:GCAAGGAGGACAGAGTTTATCGTG;CD11c:forward:CTGGATAGCCTTTCTTCTGCTG,reverse:GCACACTGTGTCCGAACTC;Leptin:forward:TGAGTTTGTCCAAGATGGACC,reverse:GCCATCCAGGCTCTCTGG;β-actin:forward:CACCCCAGCCATGTACGT,reverse:GTCCAGACGCAGGATGGC.
2.10 16S RNA sequencing
Total genomic DNA from samples was extracted using CTAB/SDS method and was diluted to 1 ng/μL using sterile water. Distinct regions of 16S rRNA/18S rRNA/ITS genes (16S V3-V4) were amplified using specific primer with the barcode. All PCR reactions were carried out with Phusion® High-Fidelity PCR Master Mix. The PCR products were electrophoresed on 2% agarose gel. Samples with the bright main bands at 400–450 bp were chosen for further analysis. The mixed PCR products were purified with the Qiagen Gel Extraction Kit. Sequencing libraries were generated usingTruSeq®DNA PCR-Free Sample Preparation Kit following the manufacturer’s instructions with index codes added. The library quality was assessed on the Qubit@2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system. At last, the library was sequenced on an Illumina HiSeq2500 platform, and 250 bp paired-end reads were generated.
2.11 Statistical analysis
Data were described as the mean ± standard deviation (SD) and analyzed by GraphPad Prism 7.0 software (San Diego, CA, USA).P< 0.05 was regarded as significantly different.
3. Results and discussion
3.1 The main mogroside components of SG extracts
Four sweet mogrosides M-V, S-I, M-IV, and M-III in SG extracts were analyzed according to previous reports [5,16]. The results indicated the richest morgroside component of our sample was M-V which is with the retention time between 7.17 min and 7.49 min,constituting approximately 2.2% of the total mogrosides (Fig. 1).

Fig. 1 The UPLC profiles of the major mogrosides from S. grosvenorii. (a) Mogroside mixed standarad. (b) Mogroside extract sample.
3.2 Intake of SG extracts decreased mouse body weight gain
As shown in Fig. 2a, the body weight gain in the control mice was leveled starting at week 8, while mice fed with HFD, HFD + SG and HFD + ASM continued to gain weight throughout 18 weeks.However, the weight gain rate of HFD + SG-fed mice became lower compared to the HFD- and HFD + ASM-fed mice around week 10. At the end of week 18, SG extracts treatment led to lower body weight compared to ASM treatment (Fig. 2a,P< 0.05). Furthermore, the body weight gain in the SG groups (13.94 ± 1.11) were clearly lower than the ASM groups (18.84 ± 3.21,P< 0.05) (Fig. 2b). To exclude the food over-intake intervening in weight gain, we assessed the food intake (Fig. 2c) versus the weight gain to calculate the food efficiency ratio (FER = body weight gain/food intake) (Fig. 2d). Our results showed that there were no significant differences of food intake among the groups received HFD feeding, however the higher FER index values were observed in the ASM groups (5.64 ± 0.94) than that in the SG group(4.17 ± 0.38,P< 0.05). These findings indicate that the more storage or less consumption of energy intake might contribute to the difference between SG and ASM treatments.

Fig. 2 Effects of water extracts interventions from Siraitia grosvenorii on HFD-induced obesity in C57BL/6J mice. The control mice were fed with the standard diet. SG water extracts was orally administered daily with HFD diets, 10 mg/mL SG or 0.5 mg/mL ASM for 18 weeks. (a) Body weight throughout 18 weeks.(b) Body weight gain at the end of the experiment. (c) Food intake at the end of the experiment. (d) Food efficiency ratio (body weight gain/food intake). Mice were randomly assigned to five groups. Body weight gain and food intake were recorded every week. Data are means ± SD (n = 5).
3.3 SG intake improved HFD-induced metabolic abnormalities
Published data from both animal models and humans suggest that high-fat diet and artificial sweeteners such as ASM exacerbate obesity and metabolic syndrome epidemic [17]. In this study, we investigated the biomarkers of metabolic syndrome in mouse serum to assess the effects of SG and ASM treatments. Our data showed that,in comparison with ASM, SG extracts appeared to lower the levels of liver enzymes AST and ALT, and mildly improve TG and TC in mice (Supplementary data). Notably, SG extracts counteracted, but ASM aggravated, the rise of fasting blood glucose, fasting insulin and homeostatic model assessment of insulin resistance (HOMA-IR)resulted from HFD (Figs. 3a-3c). Furthermore, the glucose tolerance (GTT) and insulin resistance (ITT) in SG group displayed better performances than those in ASM group and HFD group(Figs. 3d-3g). These findings suggest that SG extracts supplement improved the metabolic biomarkers induced by HFD, and that,opposite to SG extracts supplement, addition of artificial sweetener ASM failed in rescuing and rather worsened HFD-induced abnormalities of blood glucose and insulin.
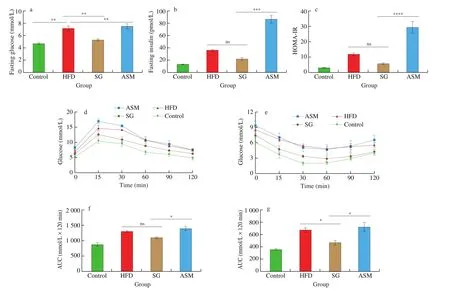
Fig. 3 Effects of SG intake on biomarkers of metabolic syndrome in serum. (a, b) Fasting blood glucose and fasting insulin in 4 groups of mice at week 18,Control, HFD, SG (10 mg/mL) and ASM (0.5 mg/mL). (c) HMOA-IR (HMOA-IR = fasting blood glucose × fasting insulin/22.5). (d-g) GTT, ITT and AUC at week 16 in the mice. Fasting glucose was measured after overnight fasting from the tail vein at 15, 30, 60, 90, and 120 min. Data are presented as the mean ± SD (n = 5).
3.4 SG extracts decreased the adipose deposition induced by HFD
Given that hypertrophy of white adipocytes is associated with systemic metabolic changes, such as hyperglycemia, insulin resistance and dyslipidemia, we weighted mouse body adipose tissues to evaluate the effect of SG extracts on the adipose deposition. Our data showed that the SG groups displayed less adipose mass in both the epididymal and perirenal adipose tissues compared to the HFD groups and the ASM groups (Figs. 4a, 4b). The histological analysis also showed the diameters of perirenal adipocytes in mice of ASM or HFD group was significantly larger compared to the SG group (Figs. 4c, 4d).
In addition to inhibition of hypertrophy of white adipocytes,activation of brown/beige adipocytes, which are usually described as uncoupling protein 1 (UCP1) -positive adipocytes, contributes to energy expenditure and thermogenesis, improves insulin sensitivity and reduces obesity [18]. Our observation showed that there was lower FER in the SG treatment group than in the HFD group and the ASM treatment group, suggesting that browning of adipocytes might take part in modulating the pathogenesis of HFD-induced obesity.We then asked whether the increase of UCP1 gene expression in adipocytes was associated with the effect of SG extracts on HFD-induced obesity. As predicted, the fat tissue UCP1 was induced to a significantly higher level in the SG treatment group than that in the ASM treatment and the HFD groups (Fig. 4e). Therefore, the anti-obesity effects of SG extracts might be related to inhibiting hypertrophy of white adipocytes and stimulating the browning of adipocytes.

Fig. 4 H&E-stained adipose tissues sections (200 ×). (a) Epididymal fat weight (g). (b) Perirenal fat weight (g). (c) H&E-stained adipose tissues sections (200 ×).(d) The cell diameter (μm). (e) Ucp-1 mRNA levels were measured by RT-PCR in mice. Data are presented as the mean ± SD.
3.5 SG extracts inhibited inflamed adipose tissue
Besides fat mass increase, compelling evidence suggested that obesity is characterized by the interaction of inflltrated macrophageenlarged adipocyte in obese individuals [19,20]. Inflamed macrophages in fat tissue predominantly produce inflammatory cytokines such as macrophage chemoattractant protein 1 (MCP-1)and tumor necrosis factor alpha (TNF-α) to further aggravate hypertrophy of adipocytes and loss of insulin sensitivity [20,21].Interestingly, we noticed that ASM treatment was in contrary to SG extracts treatment in response to HFD-induced increased inflltration of inflammatory cells in adipose tissues (Fig. 4c), indicating that attenuation of macrophage inflammation might contribute to the efficacious mechanism of SG extracts in obesity. Thus, we investigated the markers of infiltrated macrophages and found that SG supplement reduced F4/80 and CD11c in fat tissue, whereas ASM treatment led to more serious macrophage inflltration than HFD(Figs. 5a-5b, 5f-5g). Furthermore, fat inflammatory cytokines leptin,TNF-α and MCP-1 were greatly induced by 2-5-folds in HFD and ASM groups compared to the SG treatment (Figs. 5c-5e). Taken together, these findings indicated that SG extracts counteracted HFD-induced recruitment of inflamed macrophages, leading to the reduction of dysregulated secretion of pro-inflammatory cytokines which sequentially exacerbated the insulin resistance.

Fig. 5 Prolonged SG-feeding decreased the adipose tissue inflammation in mice. (a, b) Macrophage marker F4/80 and CD11c in adipose tissue. F4/80 and CD11c were increased after HFD or ASM induction for 18 weeks, while SG decreased this trend. (c-e) mRNA expression levels of Leptin, TNF-α,Mcp-1. (f-g) IHC for F4/80. All data were expressed as mean ± SD.
3.6 Effects of SG extracts on gut microbiota
Mounting evidence indicates that there is a strong association between the gut microbiota and obesity [22]. Artificial sweeteners such as ASM have been reported to interact with gut microbial communities and contribute to obesity [23]. As the representative SG sweetener compound, mogroside V is 250 times sweeter than sucrose [24].We hence analyzed the effect of SG extracts on alteration of HFD-induced gut microbiome composition.
As shown in Fig. 6a, alpha diversity analysis in gut microbiota of the mice revealed that bacterial richness was higher in SG group than that in either the HFD-induced obese group or ASM group, indicating that unlike the artificial sweetener ASM, SG extracts promoted the gut bacterial diversity, in contrary to HFD which induced the decline of gut microbiota. In addition, when we compared the operational taxonomic units (OTUs) shared between lean mice and HFD or ASM treated mice, data from the Venn diagram showed that 588 and 544 OTUs were shared between lean mice and HFD/ASM groups respectively, whereas 650 OTUs were shared between lean mice and SG group, suggesting that SG extracts authentically enhanced the diversity of gut microbiota (Fig. 6b). Interestingly, 460 OTUs were present in the 4 compared microbiomes, showing that the presence of these bacterial species was not affected by the changes in diet. As shown in Fig. 6c, microbiota compositions showed that the genres of the most abundant phyla including Bacteroidetes, Proteobacteria,Firmicutes, Deferribacteres, Actinobacteria, and Tenericutes were convergent in all groups. However, as shown in Fig. 7, the richness of some gut bacteria was significantly different in SG group compared to that in ASM or HFD group in addition to Bacteroidetes,Firmicutes, Proteobacteria, and Bacteroidales_S24-7_group which tended to be consistent in all groups. Exactly, gut bacteria such asLactobacillus, Lachnospiraceae, and Christensenellaceae_R-7_group in SG group were more markedly close to those in the control group compared to the ASM or HFD group. On the contrary, the richness ofBifidobacterium, anaerobic commensal of the intestinal tract, in ASM or HFD group is lower than that in SG group. Moreover, among the observed groups (HFD, SG, and ASM group), some gut bacteria such asEnterococcusandBlautiawere divergent but some bacteria such as Prevotellaceae andAlistipeswere convergent, as might represent the complexity of gut modulation upon various dietary stimuli. Taken together, these findings indicated that SG extracts could correct the remodeling of gut microbiota resulted from HFD, which might be one of its anti-obesity mechanisms.
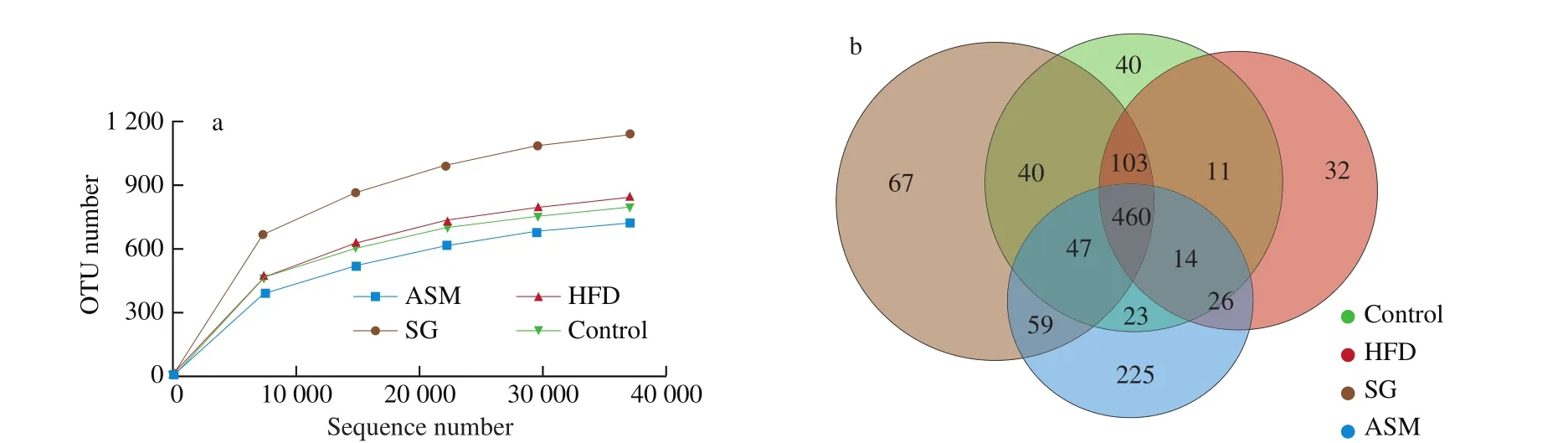
Fig. 6 Alpha diversity analysis in gut microbiota of the mice. (a) SG consumption increased the quantity of gut microbiota more significantly compared to the HFD and ASM groups. (b) Venn diagram of bacterial OUTs. (c) OUT analysis in the phylum level.
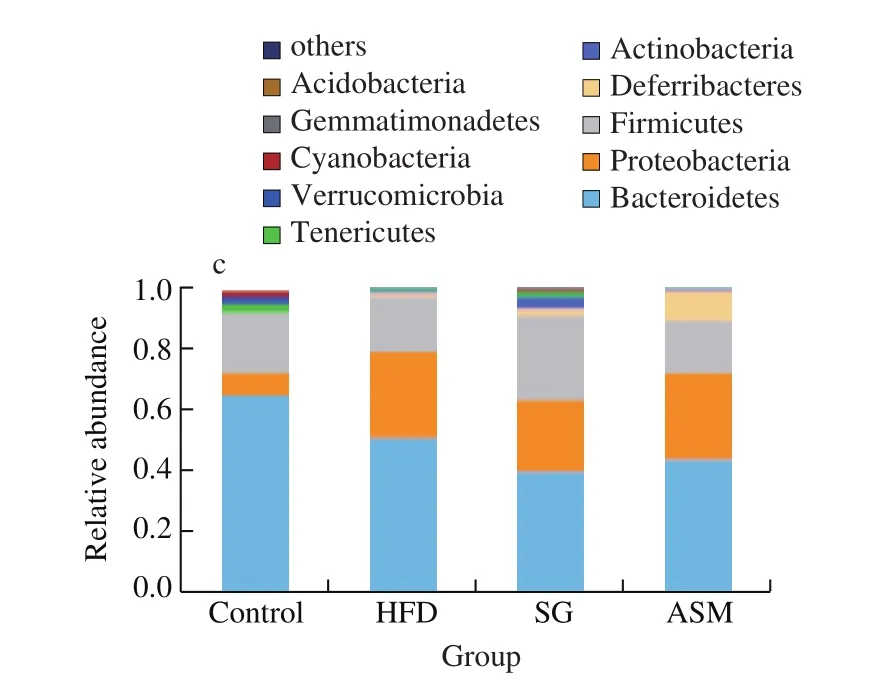
Fig. 6 (Continued)
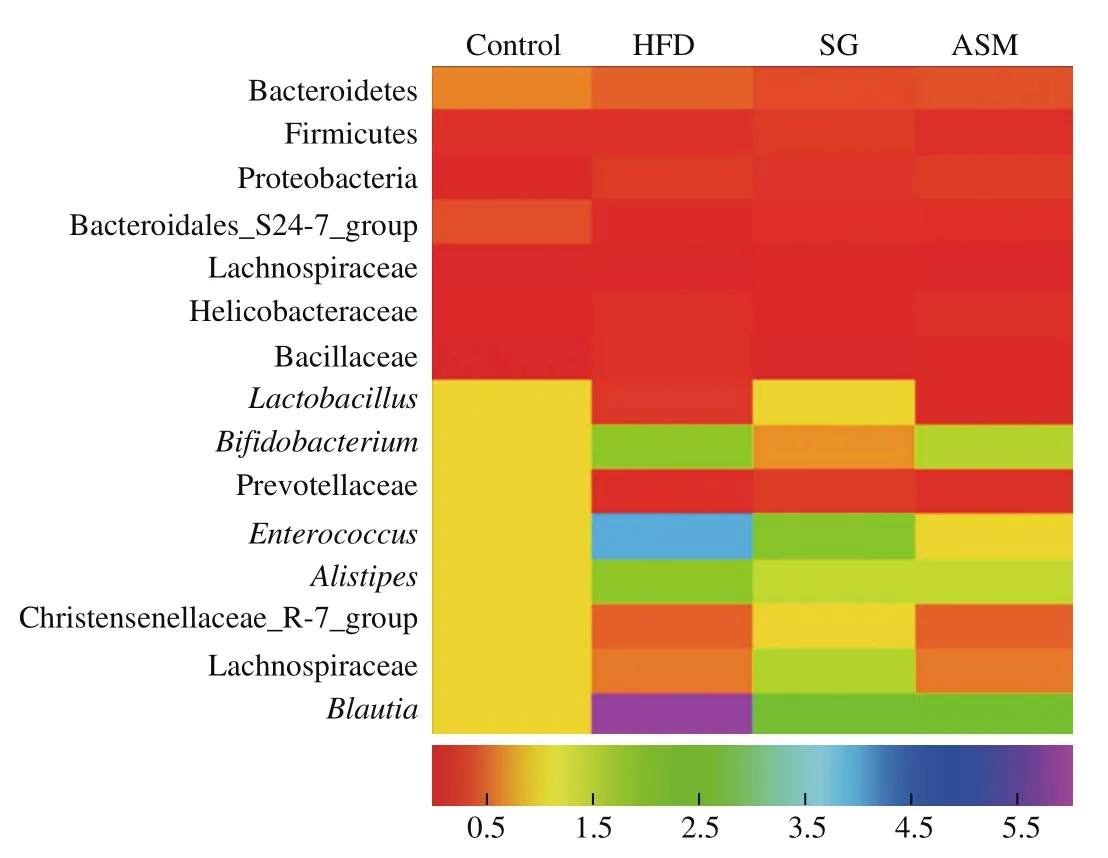
Fig. 7 The diversity of gut microbiome in difference groups. The data of microbiome were presented as fold changes.
4. Discussion
Strategies aiming at curbing overweight and obesity are basically rooted in inhibiting energy intake and/or increasing food consumption.There are few satisfactory approaches applicable to large majority of obese/overweight people at the present. For example, the current antiobesity drugs including sibutramine, orlistat, and rimonabant were not fully used due to serious side effects or expensive prices [25].As for the risk factors contributing to obesity, mounting evidence from epidemic investigations and experimental researches indicates potential obesogenic risks of artificial sweeteners although the exact relationship between artificial sweeteners and abnormal metabolic population is still a controversial topic [17,26-28]. However, seeking sweetness is the nature of human being. Therefore, finding and developing healthy substitutes of artificial sweeteners represents a promising avenue. Notably, extracts or compounds from naturally sweet plants have roused public interesting recent decades due to their health effects particularly on obesity. Among the developing natural sweeteners, mogrosides, almost exclusively existing in SG, have been regarded highly for obesity due to their superior effects on regulation of glucose and lipid. Here, we showed that orally administrated SG extracts performed better than the artificial sweetener ASM in counteracting the body weight gain and adipose deposition induced by HFD without observable side effects for over 4 months.
Traditionally, the fruit of SG has been used in a manner of drinks for the treatment of digestive diseases and respiratory diseases.The sweetness of SG fruit mainly boils down to the components of triterpenoid glycosides mogrosides, especially mogroside V which has the estimated sweetness of hundreds of times than sucrose [10,29].Consistent with the extracts from the fruit, most mogrosides including mogroside V are documented to possess various activities against inflammation [10], diabetes, and oxidative stress [10,30-31]. Based on the health effects of SG, our present study revealed that oral intake of SG extracts dramatically alleviated HFD-induced adverse effects including body weight gain, fasting glucose and insulin sensitivity in mice. On the contrary, the artificial sweetener ASM failed at improving HFD-induced obesity. Furthermore, our investigation indicated that ASM treatment did not improve obesity rather it deteriorated HFD-induced obesity pathogenesis.
Although the obesity mechanisms are complicated, there is some generally accepted consensus including the hypertrophy of white adipocytes, adipose tissue inflammation and dysregulation of the gut microbiota [32]. Targeting these known mechanisms has served as a valid path to improve obesity. The classic example is the Mediterranean diet that is rich in natural phytochemicals to prevent obesity related to modulation of the gut microbiota and low-grade fat inflammation [33-35]. In line with this example, our findings indicated that the natural triterpenoid glycoside mogroside V is abundant in SG extracts, and SG extracts supplement counteracted the HFD-induced inflammation in fat tissues and gut microbial dysbiosis. Meanwhile,the effects of ASM on obese mice is far worse than SG extracts supplement although it is similarly sweet as mogroside V. Plenty of evidence from humans and rodents suggested that chronic aspartame intake could launch a number of crucial obesogenic factors including persistent low-grade inflammatory responses and dysbiosis of gut microbiota [36,37]. Moreover, these adverse effects of ASM will pass down to offsprings without direct ASM exposure [38]. Our present observation also indicated that ASM worsened HFD-induced obese performances in mice including increased inflltration of inflammatory macrophages in fat tissues, release of proinflammatory cytokines and aggravated dysbiosis of gut microbiota. Given the fruits of SG with high sweetener mogrosides, our findings indicate that mogrosides can be a viable substitute of artificial sweeteners.
In summary, our data suggest that supplement of SG extracts attenuates, but ASM aggravates, HFD-induced obesity in mice through modulating fat tissue inflammation and gut microbiota.Obesity caused by commercial artificial sweeteners and Western diet is becoming a serious public health issue. This study not only contributes to further development of natural plant-derived sweeteners such as mogrosides from SG, but also presents a feasible and alternative source of sweeteners in addition to commercial artificial sweeteners.
Conflicts of interest
None of the authors declare a conflict of interest.
Acknowledgement
This work was supported by grants from the National Key Research and Development Program of China (No.2019YFA0905600), Tianjin Innovative Team Project (TD13-5087),and Shangrao Crucial Research and Development Project (19A005).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.03.009.
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

