Glyoxal induced advanced glycation end products formation in chicken meat emulsion instead of oxidation
Rui Fng, Zongshui Zhu*, Anthony Pius Bssey Iftikhr Ali Khn, Ming Hungd
a College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b Center Testing International Pinbiao (Jiangsu), Certification Technology Co. Ltd., Nanjing 210046, China
c Shenzhen Key Laboratory of Marine Microbiome Engineering, Institute for Advanced Study, Shenzhen University, Shenzhen 518060, China
d Nanjing Huangjiaoshou Food Science and Technology Co. Ltd., Jiangsu Research Center for Livestock and Poultry Products Processing Engineering Technology, Nanjing 211200, China
Keywords:
Glyoxal
Emulsion
Oxidation
Advanced glycation end products
Chicken meat
A B S T R A C T
Advanced glycation end products (AGE) are potential harmful substances formed in the advanced Maillard reaction and increasingly investigated in muscle foods. However, the contribution of oxidation to the AGE formation is controversial. Moreover, reports on glyoxal (GO) induced AGE formation in chicken meat emulsion (CME) are limited. Thus, the effects of GO on emulsifying properties, rheological behavior and AGE formation in CME were investigated. Our findings exhibited that levels of Nε-carboxymethyllysine(CML) and Nε-carboxyethyllysine (CEL) were associated with lipid oxidation but not significantly (P > 0.05).Levels of AGE peaked when GO concentration ranged from 5 mmol/L (CML) to 10 mmol/L (CEL). The droplets’ aggregation associated with the disul fide bond when the concentration of GO was at 0.5-30 mmol/L while non-disul fide bond association occurred at 30-50 mmol/L GO concentration. In conclusion, compared to the effect of oxidation, GO exhibited the main role in the AGE formation of CME. This study will provide theoretical significance for further understanding and controlling the formation of AGE in CME.
1. Introduction
Ad vanced glycation end products (AGE) are harmful compounds formed at the advanced stage of Maillard reaction and lipid oxidation [1].As two typical AGE,Nε-carboxymethyllysine (CML) andNε-carboxyethyllysine (CEL) are increasingly investigated in muscle foods [2]. To note, although series of researches about AGE formation have been ongoing for years, the mechanism is still controversial,especially in thermal meat processing [3]. Also, reports from relevant studies have supported our findings that both oxidation and di-carbonyl participate in different roles and levels to induce AGE formation [1,3-6]. In the process of thermal meat processing, some dicarbonyl compounds formed via Maillard reaction and lipid oxidation [7].Thes e compounds are two adjacent carbonyl groups represented by glyoxal(GO), methylglyoxal (MGO) and malondialdehyde (MDA), etc [8].These di-carbonyl compounds combined with lysine or arginine,resulting in AGE largely generated in meat products [9-11]. However,CML and CEL are not only formed by lysine and arginine glycation but also through their combinations with di-carbonyl compounds [11].
To n ote, thermal processing is considered to be the main inducer of AGE formation in muscle food. For non-thermal processing condition, such as emulsion, reports on the relationship of emulsifying properties, rheological behavior and AGE formation under different concentrations of di-c arbonyl compounds in chicken meat emulsion(CME) are limited. According to our recent study, we added different concentrations of MGO and MDA (0-50 mmol/L) as inducers to CME to observe the behavioral characteristics of the emulsion, the relationship between oxidation and AGE. It was found that protein oxidation was significantly (P< 0.05) positive with CML and CEL formation in MGO modified CME [12]. In contrast, lipid oxidation also showed a positive significance (P< 0.05) with CML and CEL formation when CME were induced by different concentrations of MDA [13]. As another important di-carbonyl compounds, GO has been proved that it was an important precursor of CML and CEL [14],However, the relationship between oxidation and AGE formation in different GO concentrations of CME is still unclear.
Following the above limitations, we therefore set different GO concentrations in CME. The purpose of this study was therefore to:(1) measure and compare the emulsion characteristics induced by GO concentrations, and (2) analyze GO on AGE levels, oxidation of lipid and protein in emulsion as well as their relationships. Our effort,nonetheless, would provide a baseline for a better understanding of AGE formation mechanism in meat protein emulsion.
2. Materials and methods
2.1 Collection of study materials
Fresh yellow feather broiler breasts (24 h postmortem) with an average weight of (5.0 ± 0.1) kg were purchased from New Hope Liuhe Food Co., Ltd. (Shandong, China). The visible sarcolemma(in the basement membrane and cytoskeleton), connective tissue and fat tissues in broiler breasts were aseptically discarded and samples were cut using a ceramic knife into small pieces of 1 cm × 1 cm ×1 cm, followed by crushing using a tissue crusher (HM100, Beijing Greatman Instrument Co., Ltd.). Soybean oil was bought from Nanjing Suguo Supermarket (Nanjing, China). To chemical reagent,GO (ID: G810357-500 mL) was purchased from Macklin Shanghai Biochemical Co., Ltd. (Shanghai, China). Nile blue, Nile red,phosphate buffer saline (PBS, pH 7.2–7.4), 1,1,3,3-tetrathoxypropane and bicinchoninic acid (BCA) protein quantification kits were obtained from Nanjing Ruiyi Biological Technology Co., Ltd.(Nanjing, China). Other reagents were analytical purity.
2.2 Preparation of protein solution
To separate the protein solution from minced chicken meat,sample was mixed with extraction buffer in a ratio of 1:4 (m:V).Protein extraction solution was using low concentration salt solution.Briefly, 80 mmol/L KCl, 2 mmol/L MgCl2and 2 mmol/L ethylene glycol tetraacetic acid (EGTA) were added into the PBS (pH 7.2).Full protein solution was separated by homogenization (Ultra-Turrax,IKA, Germany) at 8 000 r/min for 3 min following centrifugation(Allegra 64R, Beckman Coulter Co., Ltd., USA) at 12 000 r/min at 4 °C for 12 min. Three layers of gauze were used to filter the supernatant thereby removing lipid and meat foam. Finally, the concentration of protein solution was measured by BCA kits using microplate reader (Spectral Max M2e; Molecular Devices, California,USA), and protein concentration was adjusted to 17 mg/mL with PBS(pH 7.2).
2.3 Preparation of CME induced by GO
To prepare the emulsifying system, the method according to Zhou et al. [15]was used. Briefly, 20% soybean oil (m/m, %) dispersed in 17 mg/mL protein solution in the preparation of oil-in-water (O/W) coarse emulsion. The coarse emulsion was thoroughly homogenized with a high-speed blender (Ultra-Turrax, IKA, Germany) at 10 000 r/min for 3 min. Then, GO standard solution was diluted with 50 mmol/L phosphate buffer (pH = 7.0) to reach the final concentration of 100 mmol/L named GO prepared solution before use. Finally, GO prepared solution was dispersed in the prepared emulsion then mixed at 12 000 r/min (Ultra-Turrax, IKA, Germany)and the final GO concentrations were allotted in different levels(GO1 = 0 mmol/L, GO2 = 0.5 mmol/L, GO3 = 5 mmol/L, GO4 =10 mmol/L, GO5 = 30 mmol/L, and GO6 = 50 mmol/L), respectively.
2.4 Emulsifying properties of CME
The method adopted in the research of Li et al. [16]was used to determine the emulsifying properties at different levels of GO.Brie fly, the prepared emulsion was homogenized with a high-speed blender (Ultra-Turrax, IKA, Germany) at 8 000 r/min for 3 min to make the GO was dispersed. The dispersed emulsion was then left at room temperature (25 °C) for 10 min. 50 mL of the emulsion was then pipetted into 5 mL of 0.1% sodium dodecyl sulfate (SDS), rigorously mixed and measured at 500 nm absorbance (Spectral Max M2e;Molecular Devices, California, USA) after vibration using a vibrator(Scilogex MX-S, SCILOGEX, USA). The emulsifying stability index(ESI) and emulsion activity index (EAI) were calculated using the following equation:

WhereAis the absorbance at 500 nm;Dis the dilution multiples(100);Lis the optical path (0.01 cm);T0andT10are the absorbance at 500 nm after 0 min and 10 min respectively;φis the oil volume fraction (20%);cis the protein concentration (17 mg/mL); andtis the resting time (10 min). To avoid the minus value of ESI,T10/T0was represented byk. Thus,kvalue is positively correlated with ESI.
2.5 Appearance observation and confocal laser scanning microscopy (CLSM)
To determine this parameter, prepared emulsions were transferred into a glass bottle of 20 mL immediately. The appearance of the emulsions at first and 10 min after, were captured using a phone camera (iPhone 6s, Apple, USA) while microscopic observation of emulsions was conducted using CLSM (TCS SP8, Leica Microsystems, Germany) [17]. Briefly, 200 μL of 0.1% (m/V) Nile Blue ( fluorescence, at 633 nm) and 200 μL of 0.02% (m/Vin ethanol)Nile Red ( fluorescence, at 488 nm) were added to 4 mL of samples and mixed thoroughly with the observations carried out at room temperature with 40 × oil lenses.
2.6 Measurement of the particle size distribution (PSD)
Zetasizer Nano ZS 90 (Malvern Instruments Ltd., UK) was also used to determine the particle size distribution (PSD) as outlined by Chen et al. [18]with slight modifications. The emulsion (1 mg/mL)containing different levels of GO was placed in a 1 cm pathlength quartz cuvette and detected by dynamic light scattering(DLS) method. Z-average diameter value was exhibited from the autocorrelation function according to the Cumulants method. To determine the size distribution of the emulsion, polydispersity index(PDI) value was measured.
2.7 Zeta potential measurement
The procedure described by Chen et al. [18]was followed for this measurement using Zetasizer Nano ZS 90 (Malvern Instrument Ltd.,UK). The laser doppler electrophoresis mobility was measured by the direction and velocity of polymer movement in an electric field under the M3 Mode (Malvern Instruments Ltd., UK) while Smoulokowski model was adopted to determine the zeta potential value.
2.8 Measurement of dynamic rheological
The method of Chen et al. [19]was adopted to measure the dynamic rheology with a slight modification. Physica MCR 301 rheometer (Anton Paar, Graz, Austria) was used to measure the temperature ramp tests and steady shear. The protein samples(17 mg/mL) containing different levels of GO were loaded on the sensor plate for 2 min. To avoid dehydration, all the samples were covered with liquid paraffin.
Prior to the parallel plate geometry (50-mm diameter),temperature ramp tests were carried out with a gap distance of 0.5 mm applied under oscillatory mode. The GO emulsion was applied to the temperature sweep program (heating from 20 °C to 90 °C at a temperature step of 2 °C/min). The storage modulus (G’)and phase angle (tanδ) were obtained within the linear viscoelastic regime at a frequency of 1 Hz and a strain of 5% per 0.5 min. Steady shears were also conducted at GO emulsion groups before the parallel plate geometry (50-mm diameter), with a gap distance of 0.5 mm applied. Shear rate range of 0.1–1 000 s-1was used to measure the shear viscosity (apparent viscosity) of the samples.
2.9 Infrared spectroscopy analysis
Fourier-transform infrared spectroscopy (FT-IR) was used to analyse the secondary structure of each protein sample determined according to the method described by Li et al. [20]with slight modifications. Brie fly, protein samples were analyzed by NICOLET IS10 FT-IR (Thermo Fisher Scientific Inc., USA) with a resolution of 4 cm-1. 2 mg freeze-dried protein powder and 200 mg KBr were thoroughly ground and pressed into the flake. After background and baseline correction, FT-IR spectra were analyzed at a scanning speed of 2.8 mm/s with the results automatically recognized at the wavenumber of 4 000–400 cm-1.
2.10 SDS-polyacrylamide gel electrophoresis (PAGE)analysis
The procedure described by Zhou et al. [21]was used for SDS-PAGE analysis with few modifications with slight modifications. Briefly, emulsions with different levels of GO treatments were homogenized with sample buffer with and without 5%β-mercaptoethanol (β-ME) to obtain a concentration of 2 mg/mL protein followed by incubation at 98 °C for 5 min. 10 μL of the sample was then loaded to a pre-gel (20% running gel and 4% stacking gel, 10 holes, Bio-Rad Laboratories, Benicia, CA, USA)before centrifugation at 10 000 ×gfor 5 min. Finally, the gel was automatically dyed and decolorized by being placed into a protein staining device (eStain L1 Protein Staining Device, Genscript. Co.,Ltd). For visual representation of the gel, a gel imager was used(Universal Hood II, Bio-Rad Co., Ltd., USA).
2.11 Measurement of thiobarbituric acid reactive substance(TBARs) and carbonyl
Carbonyl and TBARs measurements were performed using the procedures described in our previous study with slight modification [4].Briefly, the values of TBARs were calculated by using the MDA standard curve (prepared by 1,1,3,3-tetrathoxypropane). A 0.5 mL of protein extraction (contained 8% trichloroacetic acid (TCA)) and 0.5 mL of thiobarbituric acid (TBA, 0.02 mol/L) was added to 2 mL polyethylene (PE) tube for reaction at 95 °C for 30 min. After samples were cooled to room temperature, the absorbance was read at 532 nm using a microplate reader (Spectral Max M2e; Molecular Devices,California, USA). The result was expressed as mg MDA/kg.
Carbonyl was measured by using 2,4-dinitrophenylhydrazine(DNPH). Here, 1 mL of sample solution was sucked into a 10 mL PE tube, and 10 mmol/L DNPH solution was added (hydrochloric acid concentration was 2 mol/L). After repeated centrifugation at 10 000 ×gfor 10 min (Avanti J-26S XP; Beckman Coulter, Brea,CA, USA), the supernatant was determined by a microplate reader at 370 nm (Spectral Max M2e; Molecular Devices, California, USA).Carbonyl was expressed as nmol/mg protein.
2.12 Measurement of CML and CEL
Enzyme-linked immunosorbent assay (ELISA) kit (Nanjing Maibo biotechnology Co. Ltd., China) was used to measure the CML and CEL in chicken as adopted in our previous study [4]. Samples with high concentrations were diluted to fall within the linear range of the standard curve (10–320 ng/mL CML,R2= 0.993 2; 0.25–8 ng/mL CEL,R2= 0.996 6). The content was expressed as mg/kg.
2.13 Statistical analysis
Every data (three independent trials for each sample) was statistically analyzed using SAS analysis software (SAS software research institute, USA, version 8.1). One-way analysis of variance method was used for the analysis of variance while Duncan’s multiple range test was used to compare the differences between mean values atP< 0.05. Function images were drawn by Excel analysis software(version 2010; Microsoft) while SPSS analysis software (Version 16;IBM Corp. Armonk, NY) was used to determine Pearson’s correlation.
3. Results and discussion
3.1 Characterization of CME
3.1.1 Changes in EAI and ESI appearance observation
Emulsifying properties are associated with emulsion aggregates or dispersion behavior. In most studies, EAI and ESI are used as indicators to estimate the ability of protein sorption behavior and stability at the interface of oil/water [22]. As shown in Fig. 1A, the trend of ESI (kvalue) and EAI was different. Compared with the control group (GO1), values of ESI and EAI progressively decreased when GO was added. The value of ESI increased first and further declined. It was noticed that when the 30 mmol/L of GO was added,a peak value of ESI was observed. Conversely, the value of EAI decreased with the increase of GO concentration. As observed, EAI decline could be attributed to protein migration and adsorption on the interface of water/oil [23]. Some studies have indicated that the emulsifying properties of protein are influenced by the covalent binding property, especially in the glycation environment. Because when the GO was added, it was easy to react with proteins even at room temperature resulting in protein glycation [24]. More so, protein glycation will strengthen the covalent binding of proteins as well as increase the hydrophilicity of CMC, making the surface-active at the oil/water interface. In addition, changes in emulsifying properties attributed with the presence of non-protein constituents (such as enzymes and glycosylation products, etc.) and non-protein covalent binding interaction illustrates another phenomenon associating ESI with GO concentration [25].

Fig. 1 Appearance and microscopic observation. (A) EAI and ESI of emulsions. (B) Zeta potential of emulsions. Appearance of emulsions at (C) 0 and (D)10 min. (E) Microscopic observation at 30 min. The GO concentration from left to right is GO1 = 0 mmol/L, GO2 = 0.5 mmol/L, GO3 = 5mmol/L,GO4 = 10 mmol/L, GO5 = 30 mmol/L, GO6 = 50 mmol/L.
3.1.2 Changes in zeta potential and PSD
Zeta potential of control (GO1) and GO treated groups were presented in Fig. 1B. Results showed that the absolute value of zeta potential was significantly (P< 0.05) higher in control than respective GO groups with the least value observed in GO2. Higher zeta potential is associated with the changes of protein unfolding resulting in more charged groups exposed around the particle surface [26]. However,our results indicated that the protein structure were changed by GO in the emulsion system, resulting in protein folding and decrease of dispersion. Also, the results in Table 1 con firmed this phenomenon as a progressive increase in Z-average and PDI was observed following the addition of GO. Additionally, the PSD increase with GO addition could be attributed to the protein folding for the glycation. GO was also observed to increase the PSD and aggregation behavior in the emulsion system, which could be explained by the adsorption effect.Further observation indicated that GO changed the adsorption ability of glycated protein at the surface of oil/water, preventing new droplets formation because of its steric and size repulsion [22].

Table 1Level of Z-average and PDI of emulsions with different levels of GO.
3.2 Appearance and microscopic observation of emulsion
As shown in Figs. 1C and 1D, emulsion appearance was applied to monitor the emulsion stability. In Fig. 1C, we observed that all emulsion groups showed no distinct aqueous phase and homogeneity at 0 min. However, a distinct contrast amongst respective GO groups was noticed in Fig. 1D after storage 10 min at room temperature.We also noticed higher polymer formation at GO5 and GO6 which resulted in a stickier emulsion.
To effectively evaluate the emulsions and analyze emulsifying properties, microstructural droplets were conducted in Fig. 1E. Higher separation and flocculation of droplets were observed in GO2 and GO3 than control (GO1). This is evident in droplet linking, a typical phenomenon associated with bridging flocculation [27]. Also, larger protein that could be easily stained by Nile Red was observed as GO concentration increased from GO3 to GO4. This progression is associated with an increase in GO-induced aggregates.
Furthermore, phase separation and protein aggregation were observed in GO5 to GO6 due to a larger size of oil droplets caused by the coating of dispersed oil with protein [15]. This phase separation or “oil leak” was observed to be attributed to the covalent bonding formed by protein-protein aggregation as enhanced by protein absorption at the surface of oil/water [28].
3.3 Characterization of emulsion rheology
3.3.1 Effects of thermal condensation characteristics
As shown in Fig. 2A, a typical thermal condensation characteristic was exhibited at the GO1 (control group) sample from a tacky-protein sol to a visco-elastic gel network. An identical trend was observed for all GO groups at 20 to 50 °C. Also, a progressive change was noticed from 60 °C to 90 °C with GO1 recording the highestG’ value at 70 °C before declining. This change, resulting from elastic component formation, was attributed to protein aggregation. This influence head-to-head interaction with increasing temperature is attributed to the denaturation of myosin S1 sub-fragment and unfolding [19].In addition, lower GO level (GO2) expressed similar analogous characteristics as GO1. However, a rapid decline was observed in GO1 while higher GO levels (GO3 to GO6) recorded an increasing trend in typical rheological profile as temperature progressed. This phenomenon elucidated that an increase in GO concentration not only ensured emulsion system stability, but also enhanced net structure connected by covalent bonds.
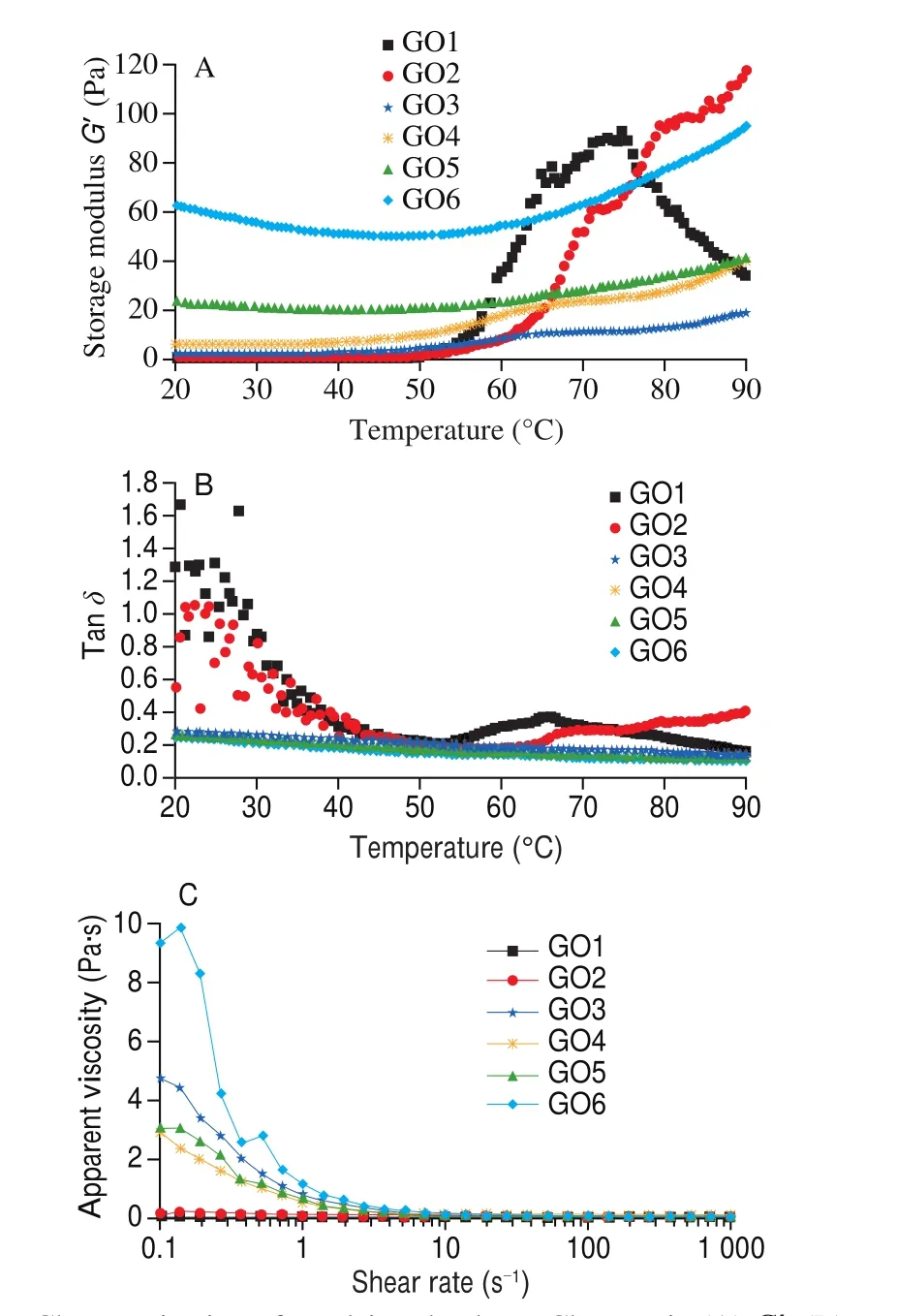
Fig. 2 Characterization of emulsion rheology. Changes in (A) G’, (B) tan δ and (C) flow curve (apparent viscosity as a function of shear rate) of emulsions with different levels of GO. GO1 = 0 mmol/L, GO2 = 0.5 mmol/L, GO3 =5 mmol/L, GO4 = 10 mmol/L, GO5 = 30 mmol/L, GO6 = 50 mmol/L.
Furthermore, the protein aggregation induced by di-carbonyl compound can also influence the digestibility and protein bioavailability. Wang et al. [29]found that MGO induced strong aggregation of glutenin after heating. Moreover, carbonyl oxidation induced by MGO led to intermolecular cross-linking of glutenin that increasingly masked or even destroyed cleavage sites, further decreased digestibility. Xu et al. [30]investigated the degradation of peptide-bound Maillard reaction products of GO-glycated casein by human colonic microbiota, and found that a higher depletion rate of CML was exhibited in the low molecular weight (< 1 kDa) fraction than high molecular weight (> 10 kDa).
Tanδ, which represents the ratio ofG”/G’ is shown in Fig. 2B.The curve demonstrates the transition information between viscous and elastic by monitoring tanδ[19]. The trends of GO1 and GO2 along the typical curve were similar to the findings of Zhao et al. [31].Hayakawa et al. [32]also reported that a 90° ofδrepresented a fully viscous material while a 0° ofδwas considered a fully elastic material. As shown, the results observed in Fig. 2B were similar to the results in Fig. 2A. Therefore, we summarized that at a low level of GO concentration (0.5 mmol/L), GO induced the unfolding and denaturation of protein while proteins folding and cluster were induced at high concentrations (5–50 mmol/L).
3.3.2 Effects offluidity
Flow properties of CME were measured (Fig. 2C) with viscosity changes (Pa·s) versus shear rate (s-1) used to evaluate the flow behavior [19]. Our results showed that all the viscosity of emulsion samples (GO treated) gradually decreased with increase of shear rate except for GO1 and GO2 groups. This phenomenon, known as shear thinning behavior [33]is an important index to evaluate the interaction between proteins [19]. In our study, it was observed that protein interaction was strongest in GO6 while a rapid change in viscosity was exhibited from 0.1 s-1to 10 s-1. This result further proved that the protein was modified by GO, hence its influence in CPEs changes.
3.4 Characterization of SDS-PAGE
To analyze the binding types of proteins in different levels of GO treated groups, SDS-PAGE was applied for analysis. As shown in Fig. 3, each sample expressed two conditions: a no-reducing condition (–β-ME) as shown in Fig. 3A and a reducing condition(+βME) reported in Fig. 3B. From both results, it was found that there were some myofibrillar protein (MPs) compositions in CME.These MPs compositions included troponin T (TnT), tropomyosin(Tm), myosin heavy chain (MHC) andα-actin playing a pivotal role in the formation and cross-linking of covalent bonds [15]in three distinct observations. Firstly, with the increase in GO concentration,the bands’ intensities of some proteins (Fig. 3A) progressively decreased and faded. Secondly some high molecular weights polymers(HMWPs) accumulated the gel top but were impermeable because of their weight (Fig. 3B). Thirdly, most of the protein bands could not be recovered even with the presence ofβ-ME. These observed results indicated that HMWPs was not only generated by disul fide bonds but also by non-disul fide covalent bonds. These findings were similar to the investigations of Zhou et al. [34].

Fig. 3 The results of (A) -β ME SDS-PAGE, (B) +β ME SDS-PAGE and(C) FT-IR analysis of emulsions with different levels of GO. Lanes: M means markers; GO1 = 0 mmol/L, GO2 = 0.5 mmol/L, GO3 = 5 mmol/L,GO4 = 10 mmol/L, GO5 = 30 mmol/L, GO6 = 50 mmol/L.
3.5 Characterization of protein secondary structure
To elucidate the relationship between changes in protein structure induced by GO, secondary structure of protein was analyzed. Li et al. [20]reported that the relative proportion ofα-helix,β-sheet,β-turn and random structure representing GO-induced protein conformation could be assessed by analyzing the amide I band (1 600–1 700 cm-1).As shown in Fig. 3C, the peak position of amide I increased from 1 648.43 cm-1(GO1) to 1 654.69 cm-1(GO4) then became stable from GO5 (1 655.18 cm-1) to GO6 (1 656.14 cm-1). It was reported that an increase in wave number revealed that part of theβ-sheet turned intoα-helix or random coil [35]. Thus, the changes in the structural compositions observed in this study con firmed that GO reacted with protein resulting in structural modification.
3.6 Characterization of oxidation, CML and CEL
As shown in Figs. 4A and 4B, lipid oxidation is reflected by TBARs while protein oxidation is presented as carbonyl value in the emulsion system. It was noticed that the oxidation degree of the CME increased with increasing GO concentration (0.5-5 mmol/L).However, when the GO concentration exceeded 10 mmol/L, the oxidation content was stable, while CML and CEL gradually decreased, indicating that lipid and protein oxidations are not so closely related to AGE at high GO levels.

Fig. 4 The results of (A) TBARs, (B) carbonyl, (C) CML and (D) CEL content of emulsions with different levels of GO. Different letters indicate significant differences (P < 0.05).
Results of CML and CEL were presented in Figs. 4C and 4D.Similar trends were exhibited in both results with GO3 and GO4 showing the highest content of CML and CEL respectively. It was reported that CML and CEL formations are not only induced by Maillard reaction but also promoted by oxidation [36]. In addition,the di-carbonyl compound (GO and MGO, etc.) could also react with lysine and arginine to form large CML and CEL by connecting with a disul fide bond [5].
3.7 Correlation analysis
As discussed in CML and CEL formation, and oxidation levels of the CME, questions were raised if GO induced lipid and protein oxidation as well as the correlation between CML, CEL, TBARs, and carbonyl. To this effect, a Pearson’s correlation was analyzed (Fig. 5).The result showed that correlation values of TBARs with CML and CEL were more positive than those of carbonyl, indicating that CML and CEL are more sensitive to lipid oxidation induced by GO. This result agreed with the finding that AGE is also named advanced lipid oxidation products (ALPs) [37]. Furthermore, it was noticed that the formation of CML and CEL were not synchronous at different GO levels as earlier reported in Figs. 4C and D. Thus, we created a correlation function model based on the CML/CEL with TBARs(y= 0.447x2– 3.036x+ 35.626) and carbonyl (y= –2.521x2+36.699x– 106.660) to elucidate the disparity. We noticed that the value of CML/CEL did not show a significant change with the increase of TBARs (R2= 0.254 6) and carbonyl (R2= 0.430 3), thus indicating that GO combination with proteins and lipids induced the CML and CEL formation while oxidation just played a catalytic role in this process.

Fig. 5 Correlation analysis between emulsifying properties, rheological behavior and AGE formation. (A) Correlation heating map.(B) Correlation value. (C) Mechanism hypothesis.
3.8 Mechanism hypothesis
Based on the above results and analyses, we found an interesting phenomenon that the properties of the CME and oxidation level changed with the addition of different GO levels. Thus, our hypothesis was concluded that addition of different GO concentrations not only influenced the emulsion properties but also induced the CML and CEL formation by changing some cross-linking of protein reaction with oxidation. This detailed description is shown in Fig. 5C.Firstly, at 0.5-5 mmol/L GO, emulsion droplets aggregated under mild oxidation, both CML and CEL started to form a connection by disul fide bond as induced by GO combination with lysine in protein.Secondly, at 5–30 mmol/L GO, large oil droplets were formed due to emulsion droplets combined at the surface of oil/water, and HMWPs were increasingly formed due to lipid oxidation. To note, some of the disul fide bonds were masked and broken out leading to CML and CEL reduction. Thirdly, under 30–50 mmol/L GO concentrations,larger oil droplets at oil/water surface became more stable and protein-protein linking was changed to the non-disul fide bond (GOGO interaction). At this stage, the HMWPs were fully formed and disulfide bond was also fully broken, resulting in a stable level of CML and CEL.
4. Conclusions
In conclusion, the changes of EAI, ESI, PSD, zeta potential and rheological behavior and AGE formation in CME were associated with different levels of GO. Appearance and microscopic observation of emulsion indicated bridging flocculation at 0.5–5 mmol/L GO while the large size of oil droplets, big polymer and “oil leak” were observed at 30–50 mmol/L GO respectively. Also, the correlation between oxidation and AGE revealed that oxidation was did not influence CML and CEL formation (P> 0.05) as GO was attributed as the primary reason for protein cross-linking at the force of lipid oxidation. To sum up, GO not only changed emulsifying behavior but also induced CML and CEL formation in CME.
Conflicts of interest
The authors declare no competing conflicting interest.
Acknowledgments
This study was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0579) and the China Scholarship Council (No. 202006850022). Also, this work was supported by Agriculture Research System of China(CARS-41-Z) and Science and Technology Project of Nanjing City(No. 202002040).
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

