Description of two new species of Pseudoaliinostoc(Nostocales, Cyanobacteria) from China based on the polyphasic approach*
Fangfang CAI , Gongliang YU , Renhui LI
1 Hubei Key Laboratory of Animal Nutrition and Feed Science, Hubei Collaborative Innovation Center for Animal Nutrition and Feed Safety, Wuhan Polytechnic University, Wuhan 430023, China
2 Key Laboratory of Algal Biology, State Key Laboratory of Freshwater Ecology and Biotechnology of China, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
3 School of Life and Environmental Sciences, Wenzhou University, Wenzhou 325035, China
Abstract Two cyanobacterial strains CHAB5870 and CHAB5871 morphologically identified as Nostoclike species were isolated from different habitats in China, and they were phylogenetically and taxonomically characterized based on a polyphasic approach combining morphological, ecological, and molecular data. In the 16S rRNA gene phylogeny inferred using maximum likelihood, maximum-parismony, and bayesian inference methods, these two strains clustered within the Pseudoaliinostoc clade. The 16S rRNA gene sequences of these two strains displayed ≥95.5% and ≤98% similarity to Pseudoaliinostoc species, which indicated them to represent new species of the genus Pseudoaliinostoc. Furthermore, the unique pattern of D1-D1′ and Box-B helix of the 16S-23S rRNA internal transcribed spacer (ITS) secondary structure also revealed that two strains represented novel species. These results supported the establishment of two new Pseudoaliinostoc species with the name P. jiangxiense sp. nov. and P. yunnanense sp. nov.
Keyword: 16S rRNA gene; 16S-23S ITS; new species; morphology; polyphasic approach; taxonomy
1 INTRODUCTION
Since cyanobacteria are diverse in morphology, the traditionally classification of cyanobacteria was mainly based on morphological characteristics for a long time (Komárek, 2003; Taton et al., 2003, 2006;Johansen and Casamatta, 2005; Turicchia et al., 2009;Genuário et al., 2013, and many others), but morphology alone seems to be an inadequate tool in the modern taxonomy. With the progress and development of modern science, the introduction of modern methods such as molecular biology and electron microscopy is necessary for taxonomic classification of cyanobacteria (Komárek, 2016). In recent years, with the application of polyphasic approach to cyanobacteria, the taxonomy of cyanobacteria has undergone extensive reconstruction and revision (Komárek et al., 2014). Cyanobacteria currently include eight orders: Gloeobacteriales,Spirulinales, Synechococcales, Chroococcales,Oscillatoriales, Pleurocapsales, Chroococcidiopsidales,and Nostocales (Komárek et al., 2014). Since 2000,more than 140 new genera have been defined, mainly based on genetic criteria. However, there still exist several unsolved problems in this newly introduced method (Komárek, 2020). Under the circumstances,Komárek (2020) demonstrated that modern classification must be applied consistently the polyphasic approach based on phylogenetic classification, but it is necessary to combine and add all cytomorphological and ecological important data,and proper use of nomenclature prescriptions.
The genusNostocin the family Nostocaceae has repeatedly been shown to be genetically heterogeneous and polyphyletic (Hrouzek et al., 2005, 2013;Rajaniemi et al., 2005; Papaefthimiou et al., 2008;Lukešová et al., 2009; Silva et al., 2014). SeveralNostoc-like genera have been split out from ‘Nostocsensustricto’ clade in an attempt to achieve monophyly in this genus, includingMojavia(Řeháková et al., 2007),Desmonostoc(Hrouzek et al., 2013),Halotia(Genuário et al., 2015),Aliinostoc(Bagchi et al., 2017),Komarekiella(Hentschke et al.,2017),Desikacharya(Saraf et al., 2019),Minunostoc(Cai et al., 2019a),Compactonostoc(Cai et al.,2019b),Purpureonostoc(Cai and Li, 2020; Cai et al.,2020b), andVioletonostoc(Cai et al., 2020a).
The genusAliinostocBagchi, Dubey & Singh(Bagchi et al., 2017) was one example separating fromNostoc, morphologically similar toNostoc,while the phylogenetic location was in a distant group.The speciesAliinostocmorphoplasticum, as the type species, was isolated from eutrophic pond in India.Up to now, six species,A.morphoplasticum,A.catenatum,A.constrictum,A.magnakinetifex,A.soli, andA.tiwarii, have been reported under this genus. However, Cai et al. (2020b) have found that the definition ofAliinostocwas too broad, and this genus has been shown to be polyphyletic in their analyses, resulting that someAliinostocspecies need to be revisited. In accordance with Cai et al. (2020b),Lee et al. (2021) further verified this suggestion, and the members of the genusAliinostochave been clustered into two phylogenetically distant nodes.Base on their observation, Lee et al. (2021) convinced that the studied KoreanAliinostoc-like strains,includingA.constrictum,A.soli, andA.tiwarii, should be separated into a new genus. As a consequence,Pseudoaliinostocgen. nov. has been described from the separation ofAliinostoc, withP.solicomb. nov.as the type species. FourPseudoaliinostocspecies,namely, type speciesP.soli,P.constrictum,P.tiwarii,andP.sejongenshave been taxonomically evaluated and described at present.
In the present study, we isolated twoNostoc-like strains from two different sampling areas in China,such as moist soil in Jiangxi province (CHAB5870)and water sample in Dali city, Yunnan province(CHAB5871). Since it is very difficult to differentiate theNostoc-like group only according to morphological characteristics, phylogenetic studies must therefore be carried out to confirm the correct taxonomic status of all strains. A polyphasic approach was employed to intensively study these two strains. Morphologically,we concluded that both areNostoc-related morphotypes and the molecular analyses confirmed their position in thePseudoaliinostocclade. Our aim in this study is to present the phylogenetic position of these two strains, distinguish them from otherPseudoaliinostocspecies and demonstrate that they belong to novel species.Pseudoaliinostocjiangxiensesp. nov. andPseudoaliinostocyunnanensesp. nov. are here taxonomically described, in accordance with the International Code of nomenclature for algae, fungi,and plants.
2 MATERIAL AND METHOD
2.1 Sampling, isolation, and culturing of strains
The cyanobacterial samples presented in this study were collected from two different localities of China. The strain CHAB5870 was collected from moist soil in Jiangxi Province, China (25°85.09′N,114°94.02′E), on March 25, 2017. The strain CHAB5871 was isolated from a water sample in Dali city, Yunnan Province, China (26°11.11′N,99°95.08′E), on August 6, 2016. Under microscope(Olympus C31, Japan), single filaments from the cyanobacterial samples were separated by using labmade pasteur pipette washing method and then cultured in spiral cap tubes containing 5 mL of CT medium (Watanabe and Ichimura, 1977). All individual isolates were subsequently maintained at 25 °C, 12 h∶12 h in a light-dark cycle with a photon flux density of 40 μmol/(m2·s) under white fluorescent light. The living cyanobacterial strains were cultured in the Chinese Harmful Algae Biology(CHAB) culture collection of the Institute of Hydrobiology (IHB), Chinese Academy of Sciences(CAS). Dry materials of strains were freeze-dried at-50 °C and stored in the Freshwater Algal Herbarium(HBI), IHB, CAS.
2.2 Morphological characterization
Morphological characterization of the strains CHAB5870 and CHAB5781 was performed using Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan)using its differential interference contrast microscopy.The micrographs were taking using Nikon software NIS-Elements 3.2D (Nikon, Tokyo, Japan). The Nikon Eclipse 80i microscope equipped with a Nikon DS-Ri1 digital camera was used to observed and described the shape and sizes of vegetative cells,heterocytes, and akinetes (n≥50), as well as the presence or absence of sheaths. The sizes are presented by “min-average-max”.
2.3 DNA extraction and polymerase chain reaction
Total genomic DNA was isolated from 25-day old liquid cultures of unialgal cyanobacterial strains using Clarke’s method (Clarke, 2009). Amplification of 16S rRNA gene was performed using primer pA(5′-AGAGTTTGATCCTGGCTCAG-3′) and primer B23S (5′-CTTCGCCTCTGTGTGCCTAGGT-3′)(Edwards et al., 1989; Gkelis et al., 2005). Primers 322 and 340 (Iteman et al., 2000) were used for amplification of the 16S-23S ITS region. The PCR reaction was carried out in 20-μL reaction mixture containing 1 μL of genomic DNA (100 ng/μL), 0.5 μL of each primer (10 μmol/L), 10 μL of 2× PCR mix with Taq polymerase (Cat TSE001, Beijing Tsingke Biotech Co. Ltd., Beijing, China), and 8 μL of sterile water. The PCR was conducted on an MJ Mini Personal Thermal Cycler (Bio-Rad, Hercules,California USA), and the PCR cycle had initial denaturation at 95 °C for 3 min, followed by 33 cycles of 95 °C for 30 s, 58 °C for 30 s (30 s at 55 °C for ITS), 72 °C for 1 min (30 s for ITS), and a final 5-min elongation step at 72 °C. The resulting PCR products were purified using TSINGKE DNA Gel Extraction Kit (Cat GE0101-200, Beijing Tsingke Biotech Co.Ltd., Beijing, China) and subsequently cloned into pMDTM18-T vector (TaKaRa, Japan) using the procedure of Sambrook and Russell (2001). All sequencing was performed by the ABI 3730 Automated Sequencer (PerkinElmer, Waltham, MA,USA). The 16S rRNA and 16S-23S ITS gene sequences of the cyanobacterial strains CHAB5870 and CHAB5871 were deposited in the NCBI (National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/) GenBank database under accession numbers: MW829373, MW829374, MW829375,MW829376, and MW829377.
2.4 Phylogenetic analysis
16S rRNA gene sequences obtained in the present study and those representing main groups of heterocytous cyanobacteria retrieved from GenBank were used for phylogenetic analyses. All downloaded sequences were edited by using MAFFT v7.312(Katoh and Standley, 2013) with auto-selected strategy FFT-NS-I (with default parameters) after multiple sequence alignment, and visually checked in MEGA v.7.0.14 (Kumar et al., 2016). The 16S rRNA gene phylogenetic trees were inferred by maximumparismony (MP), maximum likelihood (ML), and bayesian inference (BI) methods. The MP analysis with 1 000 repeated heuristic searches was performed by MEGA software X (Kumar et al., 2018). The bestfit models, GTR+I+G, were selected for the ML, MP,and BI analysis under the Akaike Information Criterion (AIC) in ModelFinder (Kalyaanamoorthy et al., 2017). The BI was calculated with MrBayes v3.2.6 (Ronquist et al., 2012) in the CIPRES Science Gateway V.3.3 (Miller et al., 2015, http://www.phylo.org/). In the BI analyses, two runs of eight Markov chains were run for 8 million generations, sampling every 100 generations, with 25% of the sampled trees discarded as burn-in, the final average standard deviation of splitting frequency was <0.01. In the ML analyses, 10 000 bootstrap replicates were performed to evaluate the relative support of the branches by using ultrafast bootstrap on IQ-TREE web server(Trifinopoulos et al., 2016). The obtained consensus phylogenetic trees were visualized in FigTree, v1.4.3(Rambaut, 2016) withChroococcidiopsisthermalisPCC7203 as the outgroup. Thep-distance was calculated with pairwise deletion of gaps by MEGA software v.7.0.14 (Kumar et al., 2016) and used to calculate sequence identity [100×(1-p)] for 16S rRNA data.
2.5 Analyses of 16S-23S internal transcribed spacer (ITS)
The ITS secondary structures of D1-D1′ and Box-B helix of the two studied strains and other closely species were determined using software RNAstructure ver. 5.6 (Mathews Lab, 2013).
3 RESULT
3.1 Morphology
PseudoaliinostocjiangxienseF. Cai et R. Li sp.nov. (Fig.1)
Description: Growing as a soft macroscopic mat on wet soils, with visible blue green color. Filaments loosely or densely entangled, sometimes straight.Sheath colorless, thin, or slightly thick, distinct or gelatinized. Trichomes isopolar, straightly or curved,blue-green, distinctly constricted at the cross-walls,attenuated at the ends. Vegetative cells barrel-shaped to spherical or cylindrical, 3.20-3.41-4.95-μm long,3.40-3.62-4.78-μm wide. Heterocytes usually intercalary, rarely terminal, spherical or barrelshaped, 3.53-3.84-4.77-μm long, 3.20-3.59-4.15-μm wide. Akinetes intercalary, elliptical, 5.01-5.85-6.72-μm long, 2.24-3.24-3.50-μm wide.
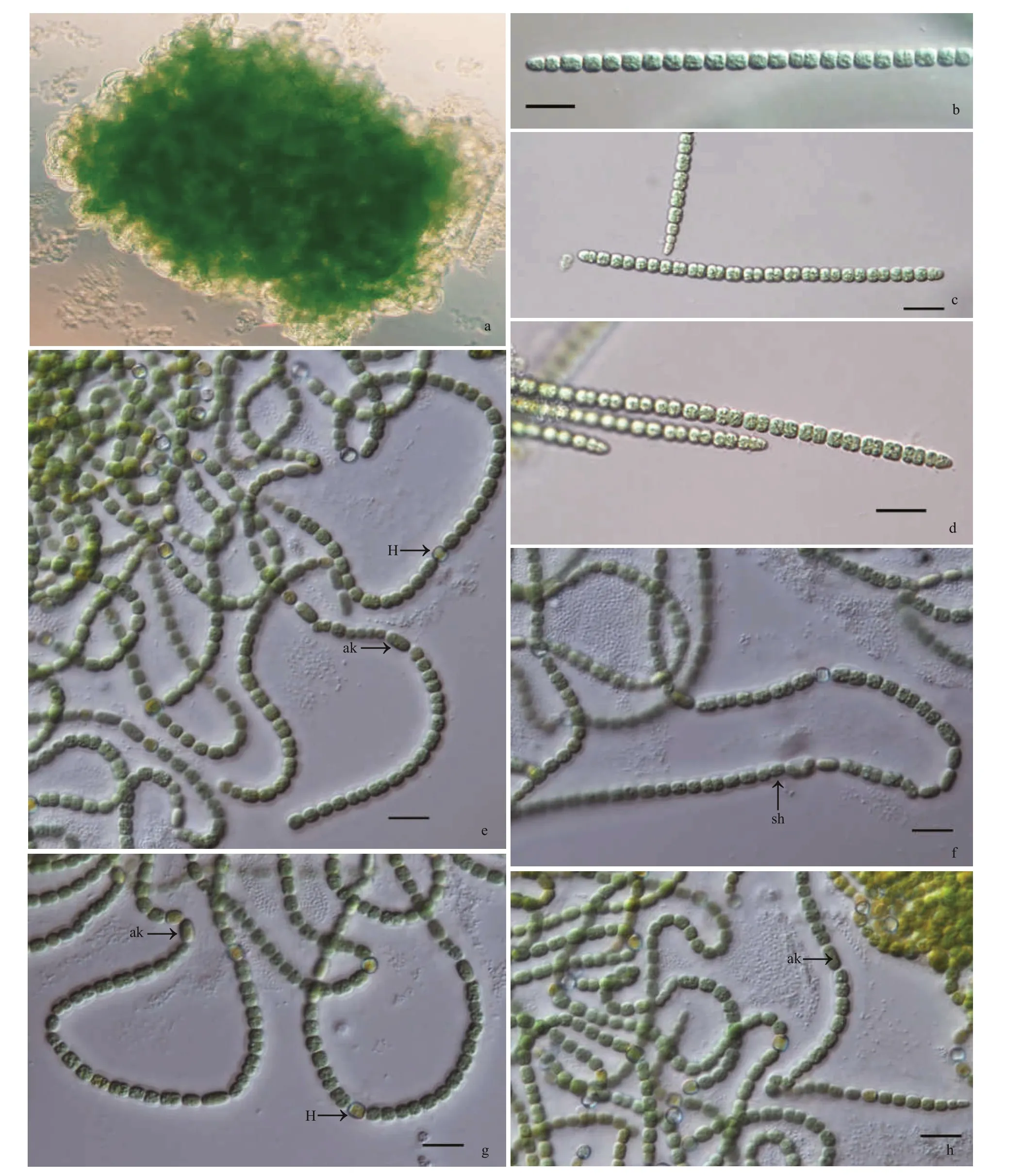
Fig.1 Micrographs of Pseudoaliinostoc jiangxiense under the light microscopy (LM)
Holotype here designated: Dry material of the strain CHAB5870 was stored at the Freshwater Algal Herbarium (HBI), Institute of Hydrobiology, Chinese Academy of Science, Wuhan, China, as specimen No.JXGJ201701.
Habitat: wet soils.
Type locality: Isolated from wet soils in Jiangxi province, China (25°85.09′N, 114°94.02′E).

Fig.2 Micrographs of Pseudoaliinostoc yunnanense under the light microscopy (LM)
Reference strain: The living culture was deposited in Collection of Harmful Algae Biology (CHAB),Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, Hubei Province, China as strain CHAB5870.
Etymology:jiangxienserefers to Jiangxi province where the strain was isolated, transliterated into Latin.
PseudoaliinostocyunnanenseF. Cai et R. Li sp.nov. (Fig.2)
Description: Filaments straight, loosely or densely entangled. Sheath colorless, thin, usually invisible.Trichomes isopolar, straightly or curved, blue-green,distinctly constricted at the cross-walls. Vegetative cells spherical to cylindrical, 2.15-3.79-5.21-μm long,2.05-2.25-4.89-μm wide. Heterocytes usually intercalary, rarely terminal, 3.30-3.59-4.25-μm long,2.90-3.05-4.40-μm wide. Akinetes intercalary, oval,5.19-5.72-6.00-μm long, 3.68-3.87-4.18-μm wide.
Holotype here designated: Dry material of the strain CHAB5871 was stored at the Freshwater AlgalHerbarium (HBI), Institute of Hydrobiology, Chinese Academy of Science, Wuhan, China, as specimen No.YNDL201601.
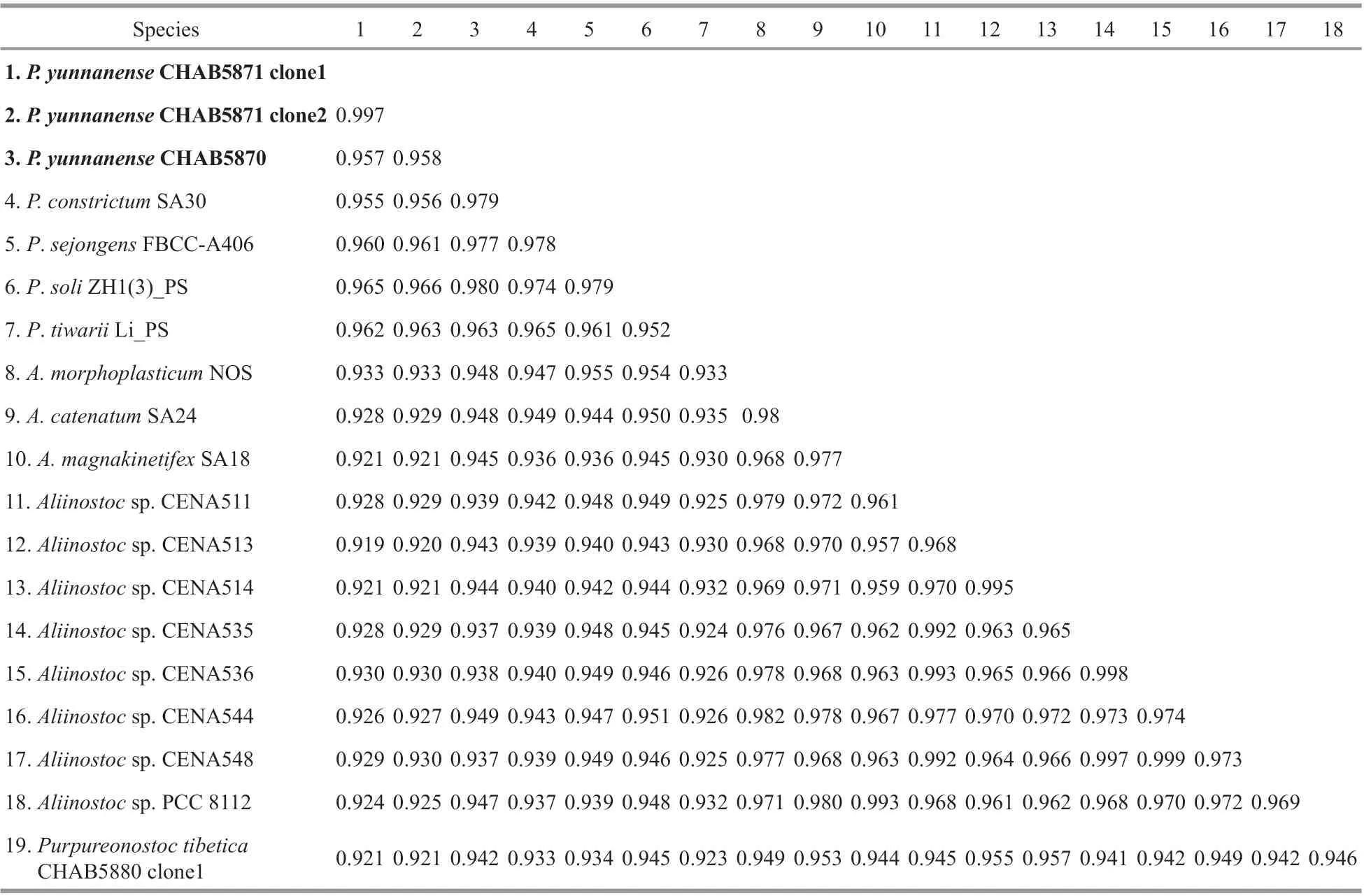
Table 1 Comparison of the 16S rRNA gene sequence similarity among Pseudoaliinostoc species and their related taxa
Habitat: Free-living in water.
Type locality: Isolated from a water sample in Yunnan Province, China (26°11.11′N, 99°95.08′E).
Reference strains: The living culture was deposited in Collection of Harmful Algae Biology(CHAB), Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, Hubei Province, China as strain CHAB5871.
Etymology:yunnanenserefers to Yunnan province where the strain was isolated, transliterated into Latin.
3.2 Molecular and phylogenetic analyses
The 16S rRNA gene sequences of the studied strains were obtained and evaluated with BLAST analyses in NCBI. The 16S rRNA gene sequence of the strain CHAB5870 showed 93.7%-94.8%similarity with existingAliinostocspecies, 96.3%-98.0% similarity with existingPseudoaliinostocspecies, and 94.2% similarity withPurpureonostocspecies. The strain CHAB5871 containing two clones shared 99.7% similarity with each other, 91.9%-93.3% similarity withAliinostocspecies, 95.5%-96.6% similarity withPseudoaliinostocspecies, and 92.1% similarity withPurpureonostocspecies. The sequence similarity of the two strains described in this study were also compared and presented with percent identity of 95.8% (Table 1).
Accession numbers for GenBank sequences that were used for phylogenetic analyses were listed as a supplementary file (Supplementary Table S1). The16S rRNA gene phylogenetic trees, from 164 cyanobacterial groups including the studied strains,were constructed by the MP, ML, and BI methods.The Bayesian tree revealed that the studiedPseudoaliinostocstrains, together with otherPseudoaliinostocspecies, formed a single and unique cluster, and this distinctive clade was supported by MP, ML, and BI approaches with high bootstrap values of 100%, 100%, and 1.00, respectively (Fig.3).
3.3 ITS secondary structures
The ITS secondary structure of D1-D1′ of the strains CHAB5870 and CHAB5871 were compared amongst themselves and with the four existingPseudoaliinostocspecies. For Box-B helix, the secondary structures were obtained for two studied strains,P.constrictumSA30 andP.sejongensFBCC-A406, but except forP.soliZH1(3)_PS andP.tiwariiLi_PS. Several species in this genus lack V3 helix (Saraf et al., 2018;Kabirnataj et al., 2020), so this structure has not been presented in the present study.
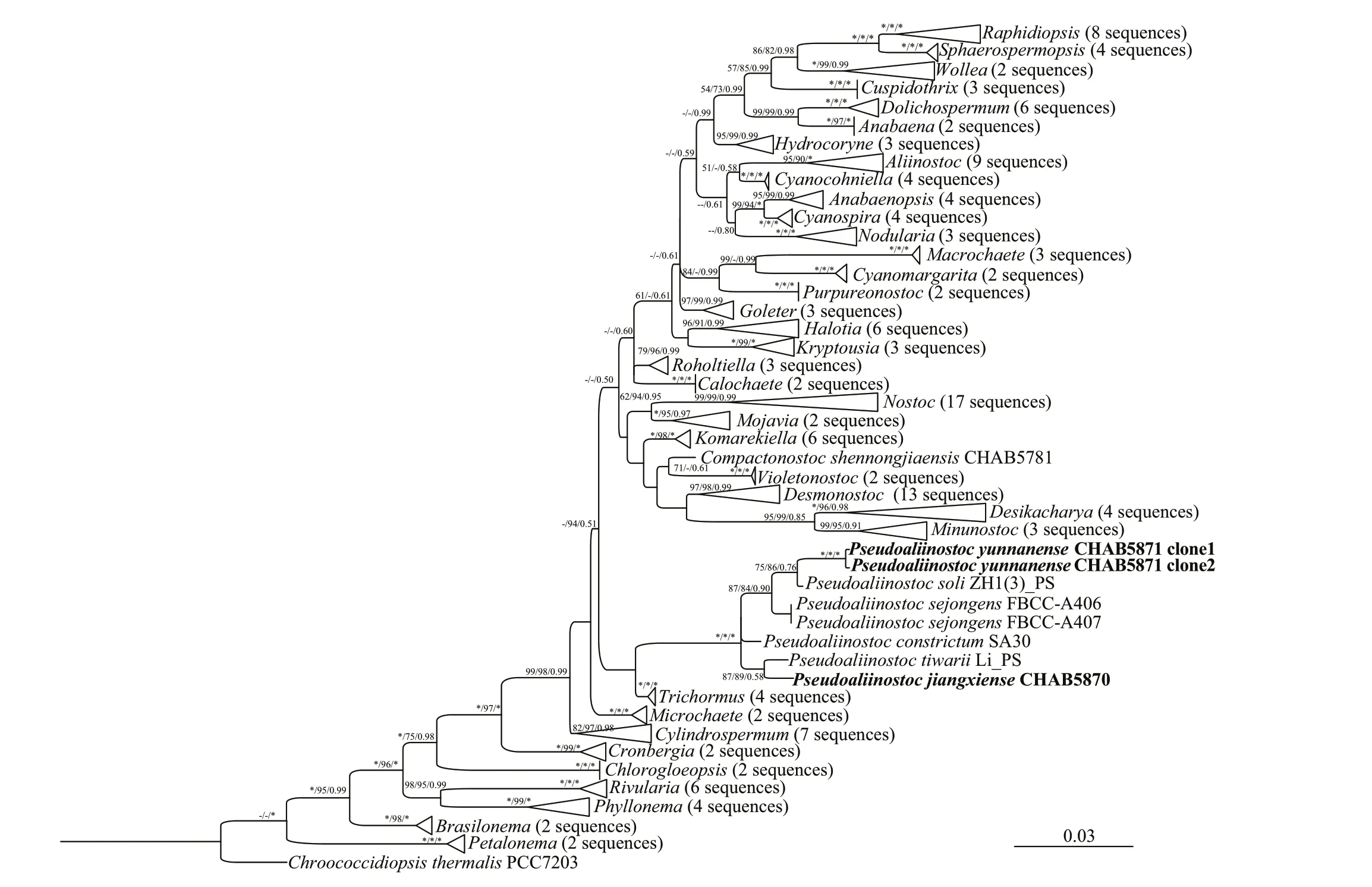
Fig.3 Bayesian inference (BI) phylogenetic tree based on 16S rDNA sequences (1 157 bp) of the studied strains and other cyanobacterial strains
Analyses of D1-D1′ helix (Fig.4) of the two studied strains showed unique structure and different nucleotides in comparison with otherPseudoaliinostocspecies. SixPseudoaliinostocstrains were divided into six types. The D1-D1′ helix structure of the strain CHAB5870 showed that the basal stem consisted of a 6-bp helix, followed by a 1∶7-bp bilateral bulge, a 1∶1-base bilateral bulge (A∶A), the bilateral bulge was connected by a side loop with a single base on the 5′side, following this side loop was one bilateral bulge of 4∶5-bases, the terminal loop contained 5-bp bases.The base stem of the D1-D1′ helix of the strain CHAB5871 consisted of 5-bp helix, followed by a big side loop with 7-bp bases on the 3′ side (other closely species do not possess this unique structure), and then further followed by three bilateral bulges of 3∶2-, 2∶1-,and 4∶5-bases, the terminal loop contained 5-bp bases.
Shown in Fig.5, the Box-B helix of the strains CHAB5870 and CHAB5871 presented unique structure when compared with other taxa. The Box-B helix of the strain CHAB5870 was comprised of a 5-bp helix, followed by a 1∶2-bp bilateral bulge, an 8-bp stem with on side loop with a single base on the 5′ side, and the terminal loop with 5 bases (AGAGA).The strain CHAB5871 consisted of 5-bp helix in the base of the stem, followed by a 1∶2 base bilateral bulge, the terminal loop contained 8-bp bases(AGAUAAUA).
4 DISCUSSION
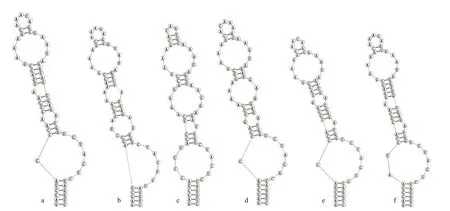
Fig.4 Secondary structure of D1-D1′ helix
In recent years, with the development of phylogenetic analysis methods based on molecular sequence data, the classification system of cyanobacteria has been extensively revised and reconstructed. It is universally recognized that ideal genera and species of cyanobacteria should be monophyletic in the modern cyanobacterial taxonomy(Komárek et al., 2014; Miscoe et al., 2016; Komárek,2018). As one of the most problematic taxonomic group, Family Nostocaceae with more or less complicated filaments has received significantly studies, and several genera of this family have been shown to be polyphyletic (Rajaniemi et al., 2005;Kozhevnikov and Kozhevnikova, 2011; Zapomělová et al., 2013; Komárek et al., 2014; Choi et al., 2018).Recent efforts have been made to reassess the genusNostoc(the type genus of the family Nostocaceae) by identifyingNostocsensustricto, a clade including the type speciesNostoccommune, and excluding the species that fall outside of this clade withNostocname, but cannot be classified into that genus if monophyly must be achieved at the genus level.Building these novel generaMojavia(Řeháková et al., 2007),Desmonostoc(Hrouzek et al., 2013),Halotia(Genuário et al., 2015),Aliinostoc(Bagchi et al., 2017),Komarekiella(Hentschke et al., 2017) andDesikacharya(Saraf et al., 2019),Minunostoc(Cai et al., 2019a),Compactonostoc(Cai et al., 2019b),Purpureonostoc(Cai and Li, 2020; Cai et al., 2020b),andVioletonostoc(Cai et al., 2020a) by separating from theNostocsensustrictoclade was significant example representing the effort to makeNostocmonophyletic.
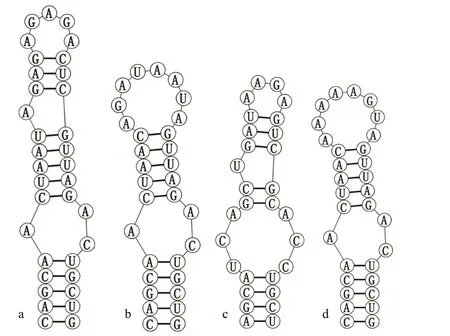
Fig.5 Secondary structure of Box-B helix
The newly described genusAliinostoc, with six species at present, has been shown to be genetically heterogeneous and polyphyletic, and members of this genus have been divided into two distinct clades (Cai et al., 2020b; Lee et al., 2021). In this case,A.constrictum,A.soli, andA.tiwariihas been separated fromAliinostocto form a new genusPseudoaliinostoc(Lee et al., 2021) in an attempt to makeAliinostocmonophyly. In this study, two strains CHAB5870 and CHAB5871 isolated from two different ecology were characterized by the polyphasic approach. The preliminary morphological characteristics of the strains indicated that they were members of the complexNostoc-like group. In the 16S rRNA gene phylogenetic tree, the strainsCHAB5870 and CHAB5871, along withPseudoaliinostocsoliZH1(3)_PS,PseudoaliinostoctiwariiLi_PS,PseudoaliinostocconstrictumSA30,andPseudoaliinostocsejongensFBCC-A406 formed a distinct clade, namely the newly describedPseudoaliinostocclade. In addition, the strong bootstrap support for the wholePseudoaliinostocclade indicated that the strains CHAB5870 and CHAB5871 were members of the genusPseudoaliinostoc.
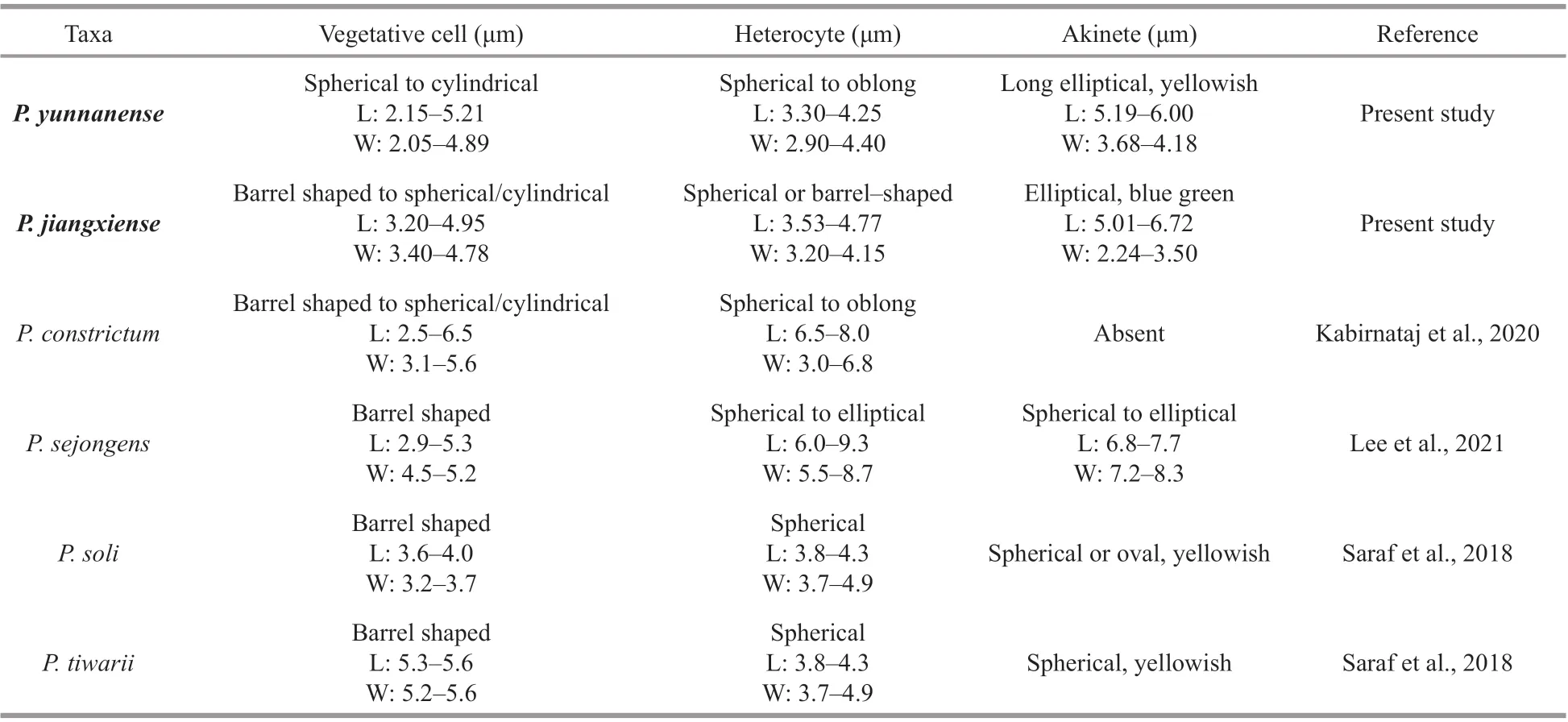
Table 2 Morphological comparison of species of the genus Pseudoaliinostoc
Based on the evolutionary distance matrix, the 16S rRNA gene sequences of the strains CHAB5870 and CHAB5871 showed ≤98% similarity with existingPseudoaliinostocspecies. In bacteriology, it has been recommended by some researchers to use percentage identities of less than 97.5% 16S rRNA gene sequences to distinguish species (Stackebrandt and Goebel, 1994), however, based on extensive comparison of more strains, Stackebrandt and Ebers(2006) recommended 98.7%-99.0% of the 16S rRNA gene sequence similarity threshold as species separation. Other research suggested that through twofold cross-validation statistical test, 98.65% of the 16S rRNA gene sequence similarity could be a threshold to differentiate two species (Kim et al.,2014). Therefore, in combination with the 16S rRNA gene sequence similarity value recently proposed, the strains CHAB5870 and CHAB5871 shared ≤98%similarity with the existingPseudoaliinostocspecies,indicating that they are new members of the genusPseudoaliinostoc.
The analysis of 16S-23S internal transcribed spacer (ITS), such as secondary structure, is an effective tool for species separation and has been demonstrated in a plenty of studies (Johansen et al.,2011; Vaccarino and Johansen, 2012; Osorio-Santos et al., 2014; Bohunická et al., 2015; Sciuto and Moro,2016; Mai et al., 2018, and many others). In this study,the ITS secondary structures of D1-D1′ and Box-B helix of the two strains were quite different investigated in comparison with otherPseudoaliinostocspecies, which enabled our strains to be distinguished from otherPseudoaliinostocspecies.
The morphological characters of the two studied strains were also compared with the species ofPseudoaliinostoc(Table 2). The strain CHAB 5870 differs fromP.sejongensin having narrower akinetes and heterocytes. Vegetative cells in CHAB5870 have smaller maximum dimensions than cells inP.tiwarii.CHAB5870 andP.solidiffers in the shape and color of the akinetes. Compared toP.tiwarii, CHAB5871 has smaller width vegetative cells. The akinetes and heterocytes ofP.sejongenshave width lager than CHAB5871. CHAB5871 andP.solidiffers in the shape and size of the akinetes. The difference between the two studied strains in this study is that CHAB5871 has wider akinetes than CHAB5870. The morphological difference amongP.constrictum,CHAB5870 and CHAB5871 was whether akinete could be observed present or absent: present in CHAB5870 and CHAB5871 and absent inP.constrictum. Minor morphological differences were observed, which also indicated the two studied strains to be novel species of the genusPseudoaliinostoc.
5 CONCLUSION
By comparing polyphasic taxonomy of twoNostoclike strains, i.e., CHAB5870 and CHAB5871, and analyzing their morphology, 16S rRNA phylogeny,and ITS secondary structures, we strongly believed that the strains represent two novel species of the genusPseudoaliinostoc:Pseudoaliinostocjiangxiensesp. nov. andPseudoaliinostocyunnanensesp. nov.,for which morphological descriptions are given. This is the first report of the two newPseudoaliinostocspecies from China. This report is helpful to expand the knowledge about the species diversity and geographical distribution of theNostoc-like cyanobacteria in China.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of the study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Typhoon-induced wind waves in the northern East China Sea during two typhoon events: the impact of wind field and wave-current interaction*
- Effect of subsea dispersant application on deepwater oil spill in the South China Sea*
- Geochemical characteristics of cold-seep carbonates in Shenhu area, South China Sea*
- Examination of seasonal variation of the equatorial undercurrent termination in the Eastern Pacific diagnosed by ECCO2*
- Deviation of the Lagrangian particle tracing method in the evaluation of the Southern Hemisphere annual subduction rate*
- Immunostimulatory effect of quaternary degree and acetyl group of quaternized chitosan on macrophages RAW 264.7*
