Characterization of Amphidinium (Amphidiniales,Dinophyceae) species from the China Sea based on morphological, molecular, and pigment data*
Zhaohe LUO , Hua ZHANG , Qun LI , Lei WANG , Hala F MOHAMED ,4,Songhui LÜ ,5,**, Haifeng GU ,6,**
1 Third Institute of Oceanography, Ministry of Natural Resources, Xiamen 361005, China
2 Research Center of Harmful Algae and Marine Biology, College of Life Science and Technology, Jinan University, Guangzhou 510362, China
3 Shenzhen Academy of Environmental Science, Shenzhen 510008, China
4 Botany & Microbiology Department, Faculty of Science, Al-Azhar University (Girls Branch), Cairo 11751, Egypt
5 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China
6 Observation and Research Station of Coastal Wetland Ecosystem in Beibu Gulf, Ministry of Natural Resources, Beihai 536015, China
Abstract Amphidinium species are amongst the most abundant benthic dinoflagellates in marine intertidal sandy ecosystems. Some of them are able to produce a variety of bioactive compounds that can have both harmful effects and pharmaceutical potentials. The diversity of Amphidinium in shallow waters along the Chinese coast was investigated by isolating single cells from sand, coral, and macroalgal samples collected from 2012 to 2020. Their morphologies were subjected to examination using light microscopy(LM) and scanning electron microscopy (SEM). A total of 74 Amphidinium strains were morphologically identified, belonging to 11 species: A. carterae, A. gibbosum, A. operculatum, A. massartii, A. cf. massartii,A. fijiensis, A. pseudomassartii, A. steinii, A. thermaeum, A. theodori, A. tomasii, as well as an undefined species. The last seven species have not been previously reported in Chinese waters. Amphidinium carterae subclades I, Ⅱ, and Ⅳ were found in the South China Sea, while subclade Ⅲ was only found in the Yellow Sea. Threadlike body scales were observed on the surface of subclades Ⅲ and Ⅳ, supporting the idea that A. carterae might contain several different species. Large subunit ribosomal RNA (LSU rRNA) sequencesbased phylogeny revealed two groups (Groups I and Ⅱ) within A mphidinium, which is consistent with the relative position of sulcus (in touch with cingulum or not). In addition, large differences in morphology and molecular phylogeny between A. operculatum (the type species of Amphidinium) and other species,suggest that a subdivision of Amphidinium might be needed. The pigment profiles of all available strains were analyzed by high performance liquid chromatography (HPLC). Eleven pigments, including peridinin,diadinoxanthin, diatoxanthin, pheophorbide (and pheophorbide a), antheraxanthin, β-carotene, and four different chlorophylls were detected. The high pheophorbide / pheophorbide a ratio in Amphidinium implies that it may be a good candidate as a natural source of photosensitizers, a well-known anticancer drug.
Keyword: harmful algae; benthic-epiphytic dinoflagellate; phylogeny; geographic distribution;pheophorbide; photosensitizers
1 INTRODUCTION
Dinoflagellates are an integral component of marine ecosystems. Some of them are also responsible for harmful algal blooms in the coastal zone, which have adverse effects on the marine ecosystem and public health. About 10% of the 2 500 known dinoflagellate species show benthic-epiphytic behavior through their life cycles (Hoppenrath et al.,2014). The genusAmphidiniumClaparède &Lachmann is one of the most abundant members of the benthic dinoflagellates in marine intertidal and neritic sandy ecosystems (Dodge, 1982; Daugbjerg et al., 2000; Murray and Patterson, 2002). SomeAmphidiniumspecies are a source ofichthyotoxins in shoal reefs and form harmful blooms (Kobayashi et al., 1991; Lee et al., 2003; Murray et al., 2015; Gárate-Lizárraga et al., 2019). On the other hand, someAmphidiniumspecies are able to produce a variety of bioactive compounds that exhibit antifungal or antimicrobial properties (Bauer et al., 1995; Echigoya et al., 2005; Kong et al., 2013; Nuzzo et al., 2014).MostAmphidiniumspecies grow easily in culture,and are therefore candidates for the mass production of bioactive compounds.
Amphidiniumwas traditionally defined by its small epicone size (Claparède and Lachmann, 1859), which does not exceed one-third of the total cell length(Kofoid and Swezy, 1921; Steidinger and Tangen,1997). About 200 marine and freshwater species have been described, which are either autotrophic,mixotrophic, or heterotrophic in terms of their nutrition modes, and can be pelagic or benthic (Taylor, 1971;Calado et al., 1998; Guiry and Guiry, 2021). It has long been suspected that the definition does not mirror its phylogeny (Daugbjerg et al., 2000; Saldarriaga et al., 2001), but a later emendation of the genus definition was performed after reinvestigation ofA.operculatumClaparède & Lachmann, the type species ofAmphidinium, as well as putative relatives of dozens ofAmphidiniumtaxa units (Jørgensen et al., 2004;Murray et al., 2004). The genusAmphidiniumsensu stricto now only includes those athecate benthic or endosymbiotic dinoflagellates with minute irregular triangular- or crescent-shaped epicones that are deflected towards the left (Jørgensen et al., 2004).Species that do not fit the criteria for the genus as redefined (Jørgensen et al., 2004; Murray et al., 2004),but have not yet been investigated to determine the generic affinities, are classified asAmphidiniumsensu lato (Hoppenrath et al., 2014). A variety of morphological features have been used to identifyAmphidiniumspecies, including the cell size and shape, the nucleus location and shape, the presence,location, and number of pusules, pyrenoids,chloroplasts, eyespots, scales, as well as life cycle stages (Claparède and Lachmann, 1859; Taylor, 1971;Maranda and Shimizu, 1996; Steidinger and Tangen,1997; Sekida et al., 2003). However, there are no unambiguous features that can be used to differentiateAmphidiniumspecies, and some characters can even overlap among species (Murray and Patterson, 2002;Jørgensen et al., 2004; Murray et al., 2004). Therefore,a combination of characters seems to be the best approach to differentiateAmphidiniumspecies(Karafas et al., 2017). The genus ofAmphidiniumsensu stricto now harbor two major clades, i.e., the Herdmanii and Operculatum clades, which are sisters to one another in large subunit ribosomal RNA (LSU rRNA) sequences-based phylogeny (Jørgensen et al.,2004; Karafas et al., 2017), although this is not reflected by their morphology. For example, within the Operculatum clade,A.operculatumhas a distinctively large cell size, with a longitudinal flagellum inserted in the lower third of the cell (Claparède and Lachmann,1859; Murray et al., 2004), whileA.gibbosum(Maranda & Shimizu) Flø Jørgensen & Murray andA.trullaMurray, Rhodes & Flø Jørgensen also have a large cell size but with a longitudinal flagellum inserted in the anterior third of the cell (Maranda and Shimizu, 1996; Murray et al., 2004). The rest of the Operculatum clade have a small cell size and a longitudinal flagellum inserted in the middle third of the cell (Jørgensen et al., 2004; Karafas et al., 2017).
There are several reports ofAmphidiniumsensu stricto along the Chinese coast.Amphidiniumstrains isolated from Lingshui Bay, Hainan, have been reported to produce polyhydroxyl compounds, with strong cytotoxic activity (Huang et al., 2004a, b), but their taxonomic identity has not been determined. The toluene extract of a strain ofA.carteraeHulburt isolated from Sanya, Hainan, was found to be toxic to pearl oysters (Wu et al., 2005). FourAmphidiniumspecies, i.e.,A.carterae,A.massartiiBiecheler,A.gibbosum, andA.operculatumwere recorded in the subtidal zone during a year-long survey around Hainan, China (Zhang, 2015), and their distribution pattern were well discussed. To fully understand the diversity of epibenthicAmphidiniumsensu stricto species in the China Sea, we collected samples from tropical to temperate areas and isolated single cells to establish strains. Their morphology was examined in detail using light microscopy (LM) and scanning electron microscopy (SEM). Moreover, both LSU rRNA and internal transcribed spacer (ITS, including ITS1, 5.8S rRNA, and ITS2) sequences were obtained.The molecular phylogeny was inferred based on ITS and LSU rRNA sequences and pigment profiles were determined from available strains.
2 MATERIAL AND METHOD
2.1 Sample collection and treatment
A total of 21 sites were sampled from the China Sea between 2012 and 2020 (Supplementary Fig.S1;Table 1). The macroalgal, seagrass, dead coral reef and upper centimeter of sandy sediments were collected from the seabed by scuba divers, and deposited into a 1-L plastic bottles containing seawater collected at the same location. The samples were vigorously stirred to detach the epibenthic cells and the suspension settled in a composite settling chamber. The settled materials were subsequently sieved through 120-μm and 10-μm filters. The 10-120-μm fractions were rinsed with filtered seawater and transferred into a polycarbonate bottle andbrought back to laboratory. Single cells were isolated from this material with a micropipette with an inverted microscope Eclipse TS100 (Nikon, Tokyo, Japan)into a 96-well tissue culture plate containing 330-μL f/2-Si (Guillard and Ryther, 1962) or L1 medium(Guillard and Hargraves, 1993). The plate was placed at 20 °C or 25 °C, 90 μmol photons/(m2·s) from coolwhite tubes, and under a light∶dark cycle of 12 h∶12 h and examined daily with the inverted microscope.The cultures were then transferred to 50-mL polystyrene tissue culture flasks.
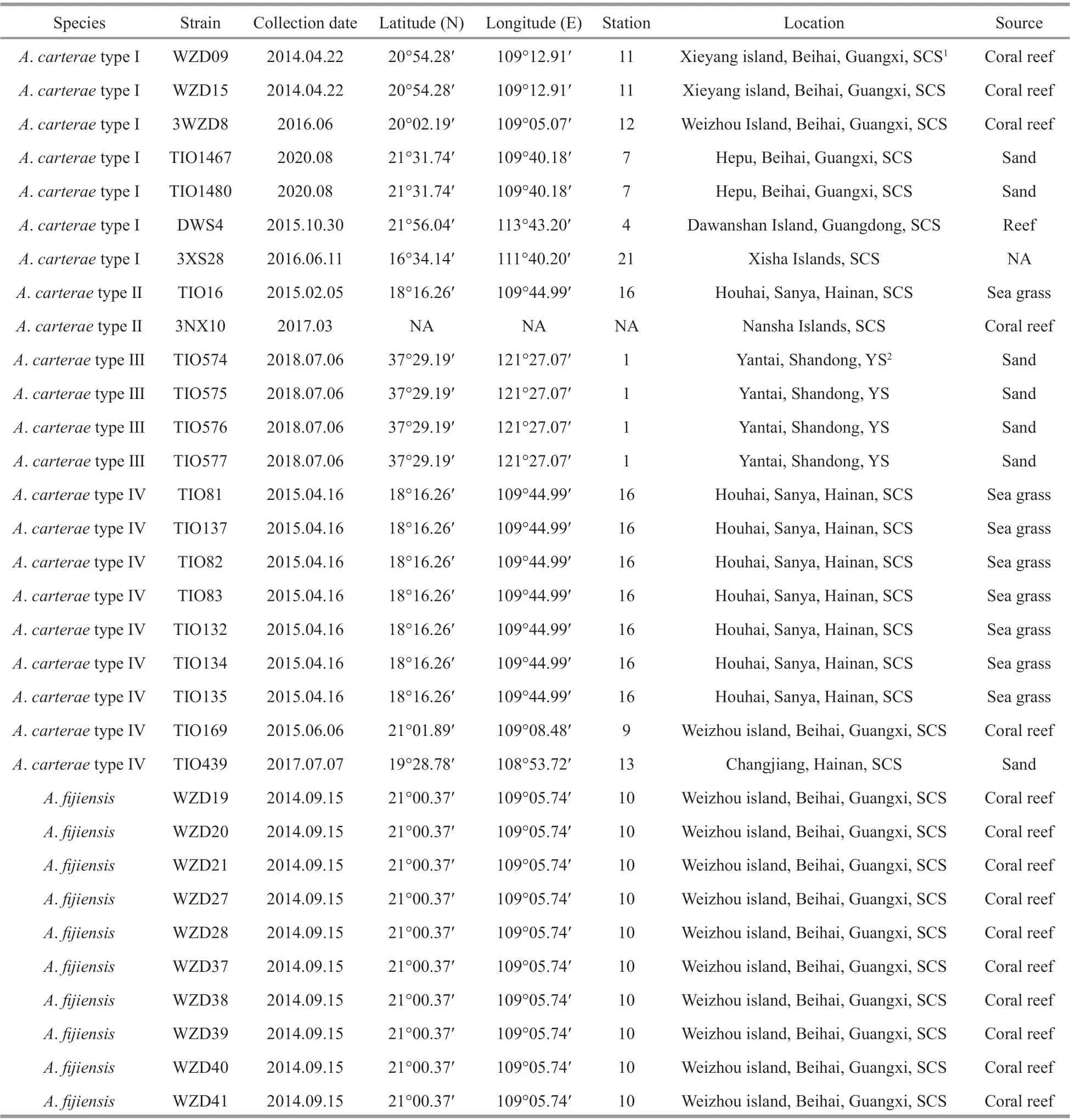
Table 1 Strains of Amphidinium examined in the present study, including collection data, locations, and sample sources
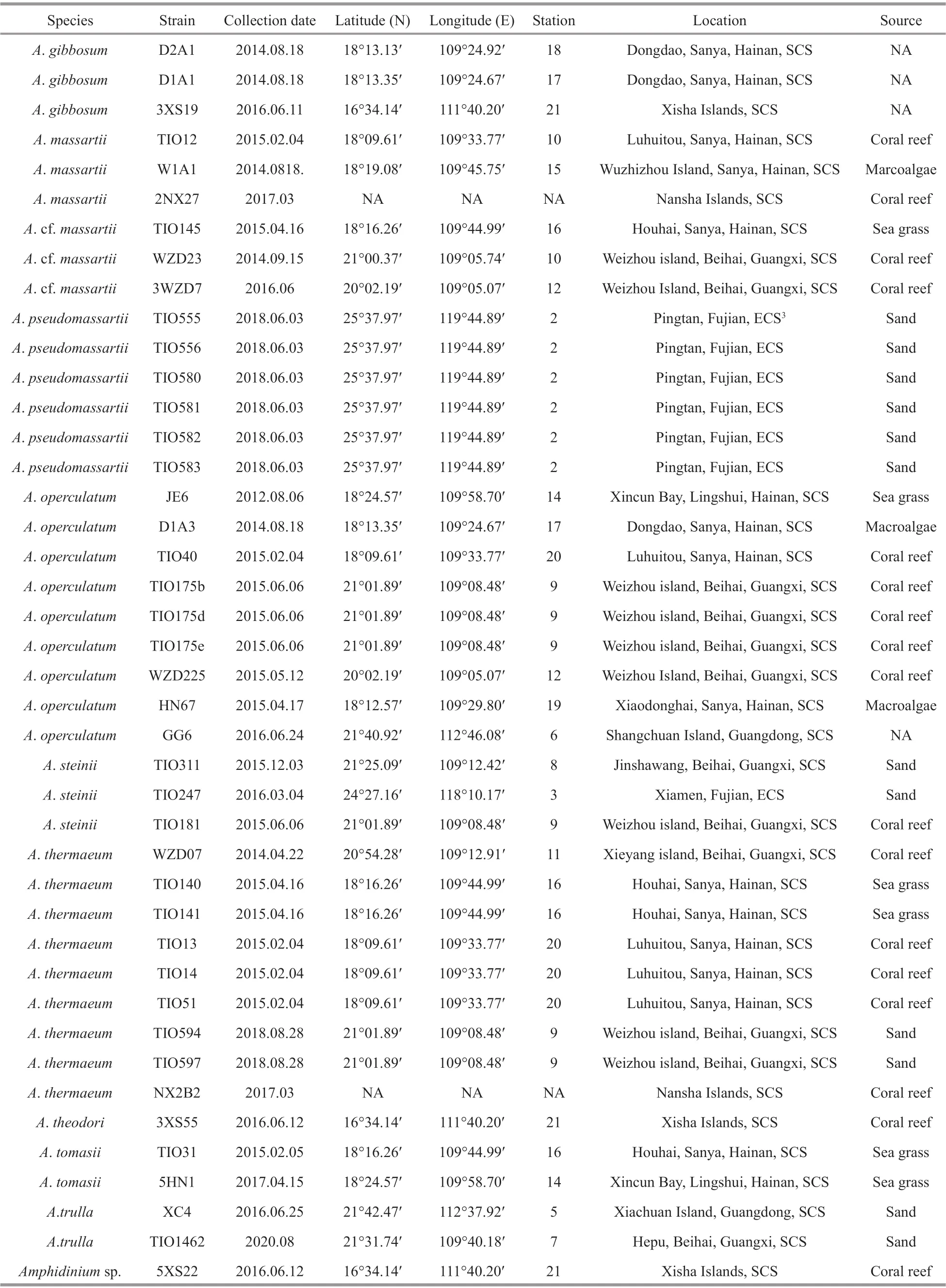
Table 1 Continued
2.2 Light microscopy (LM)
Living cells were examined and photographed using a Zeiss Axio Imager microscope (Carl Zeiss,Göttingen, Germany) equipped with a Zeiss Axiocam HRc digital camera. More than 30 cells were measured using Axiovision (4.8.2 version). To observe the shape and location of the nucleus, cells were stained with 1∶100 000 Sybr Green (Sigma Aldrich, St. Louis,USA) for 1 min, and photographed using the Zeiss fluorescence microscope equipped with a Zeiss-38 filter set (excitation BP 470/40, beam splitter FT 495,emission BP 525/50). Chloroplast auto-fluorescence microscopy was carried out on live cells using a Leica DM6000B fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with a G filter cube (emission filter BP495/15, dichromatic mirror 510, suppression filter BP530/30), and digitally photographed using a Leica DFC300 FX digital camera.
2.3 Scanning electron microscopy (SEM)
Mid-exponential batch cultures were fixed overnight at 4 °C with 1% OsO4made up with 0.2-μm filtered seawater. The supernatant was removed and the cell pellet was allowed to adhere to a coverslip coated with poly-L-lysine (molecular weight 70 000-150 000). Subsequently, cells were washed in Milli-Q water for 10 min and dehydrated through a graded ethanol series (10%, 30%, 50%, 70%, 90% and 3 times in 100%) for 10 min at each step. The samples were then critical point dried in a K850 Critical Point Dryer (Quorum/Emitech, West Sussex, UK), sputtercoated with gold, and examined with a Zeiss Sigma FE (Carl Zeiss Inc., Oberkochen, Germany) scanning electron microscope. Images were presented on a black background using Photopea online program(https://www.photopea.com/). The standard terminology proposed by Jørgensen et al. (2004) was applied for the description of morphological features,and cell orientation.
2.4 Molecular analysis
Total genomic DNA was extracted from 15 mL of exponentially growing cultures using a MiniBEST Universal DNA Extraction Kit (TaKaRa, Japan)according to the manufacturer’s protocol. PCR amplifications were carried out using 1×PCR buffer,50-μmol/L dNTP mixture, 0.2 μmol/L of each primer,10 ng of template genomic DNA, and 1 U of ExTaq DNA Polymerase (TaKaRa, Japan) in 50-μL total volume reactions. The total ITS1-5.8S-ITS2 was amplified using ITSA/ITSB (Adachi et al., 1996)primers. The LSU rRNA was amplified using the primers of D1R/28-1483R (Daugbjerg et al., 2000).The thermal cycle procedure was 4 min at 94 °C,followed by 30 cycles of 1 min at 94 °C, 1 min at 47 °C, 1 min at 72 °C, and a final extension of 7 min at 72 °C with a Mastercycler (Eppendorf, Hamburg,Germany). The PCR product was purified using a DNA purification kit (Sangon Biotech, Shanghai,China) and sequenced directly in both directions on an ABI PRISM 3730XL (Applied Biosystems, Foster City, CA) following the manufacturer’s instructions.
2.5 Sequence alignment and phylogenetic analyses
Newly obtained LSU rRNA (D1-D6) and ITS region sequences were incorporated into those ofAmphidiniumsensu stricto available in GenBank(Supplementary Table S1 & S2). Sequences were aligned using MAFFT v7.110 (Katoh and Standley,2013) online program (http://mafft.cbrc.jp/alignment/server/) under L-INS-I (Carroll et al., 2007).Alignments were manually checked with BioEdit v.7.2.5 (Hall, 1999). Completed alignments of ITS sequences were saved as NEXUS files and imported into PAUP*4b10 software (Swofford, 2002) to estimate divergence rates using simple uncorrected pairwise (p) distance matrices.Karlodiniumarmigerwere used as the outgroup in both ITS and LSU rRNA sequences-based phylogeny. For Bayesian inference(BI), the program jModelTest 2 v2. 1. 4 (Darriba et al., 2012) was used to select the most appropriate model of molecular evolution with Akaike Information Criterion (AIC). Bayesian reconstruction of the data matrix was performed using MrBayes 3.2 (Ronquist et al., 2012) with the best-fitting substitution model(GTR+G for LSU, TVM+G for ITS). Four Markov chain Monte Carlo (MCMC) chains ran for 5 000 000 generations, sampling every 100 generations.Convergence diagnostics were graphically estimated using Tracer ver. 1.7.1 (http://tree.bio.ed.ac.uk/software/tracer/) and the first 10% of burn-in trees were discarded. A majority rule consensus tree was created in order to examine the posterior probabilities of each clade. Maximum likelihood (ML) analyses were conducted with RaxML v7.2.6 (Stamatakis,2006) on the T-REX web server (Boc et al., 2012)using the model GTR+G. Node support was assessed with 500 bootstrap replicates.
2.6 Pigment analysis
Five milliliters of culture were filtered onto a 25-mm diameter Whatman GF/F filter (Whatman International Ltd., Kent, UK) under gentle vacuum(<100 mmHg). The filter was then soaked in 1-mL N,N-dimethylformamide (DMF) and extracted in a freezer (-20 °C) in the dark for 1 h. Whatman GF/F filters of 13 mm diameter (Swinnex®filter holder,Millipore, Bedford, Massachusetts, USA) were used to clean the debris in the extractions. The filtrate was mixed 1∶1 with 1-mol/L ammonium acetate solution.A quarter of each mixture was injected into a Shimadzu LC20A-DAD HPLC system fitted with a 3.5-μm Eclipse XDB C8column (100 mm×4.6 mm;Agilent Technologies, Palo Alto, California, USA).The gradient elution was performed according to the standard method (Zapata et al., 2000). Pigment quantification was confirmed using standards manufactured by the Danish Hydraulic Institute Water and Environment (DHI), Hørsholm, Denmark.
3 RESULT
Totally 74Amphidiniumstrains were established from the Chinese waters. Among them, 22 strains were identified based on morphology and phylogeny asA.carterae(including four ribotypes), ten asA.fijiensis, three asA.gibbosum, three asA.massartii,three asA. cf.massartii, six asA.pseudomassartii,nine asA.operculatum, three asA.steinii, nine asA.thermaeum, one asA.theodoriTomas and Karafas,two asA.tomasii, and one strain as undefined species(Table 1; Supplementary Table S3; Supplementary Fig.S1). In addition, two strains were attributed toA.trullabased on LSU rRNA sequences only, as the morphological features could not be examined due to the loss of cultures.
3.1 Morphology
3.1.1Amphidiniumcarterae
Cells ofA.carteraestrain WZD09 (subclade I in the phylogeny) appeared as oval to elliptical shape from the ventral side and dorsoventrally slightly flattened (Fig.1a-g). Cells were 14.2-19.4-μm long(16.4±1.4 μm,n=41) and 9.4-12.9-μm wide(11.2±1.2 μm,n=41), with the length/width ratio varying from 1.3 to 1.7 (1.5±0.1,n=41). Cells were actively swimming in the water column, and asexual division was by binary fission in the motile cell(Fig.1b). A pyrenoid was visible in the center of the cell (Fig.1a-c). The nucleus was round and located in the posterior end of the cell (Fig.1c). A single and reticulate chloroplast was present (Fig.1d). No pusule could be observed by LM. The epicone was crescent shaped, left-deflecting, and 6.5±1.2-μm(n=10) long (Fig.1e). The ventral ridge was short and straight (3.6±0.7 μm,n=13), and connected the two flagella insertion points (Fig.1e). The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v” shape (Fig.1e). The longitudinal flagellum was inserted in the middle third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus (Fig.1e). The sulcus was shallow and did not reach the antapex of the cell(Fig.1e). The anterior shoulders of the hypocone were symmetrical or slightly higher in the left, and the posterior of the hypocone was rounded to elliptical (Fig.1e-g).Amphidiniumcarteraesubclade I was encountered in Guangdong, Guangxi,and Hainan (Xisha Islands), South China Sea(stations 4, 11, 12, and 21; Supplementary Fig.S1;Table 1).Amphidiniumcarteraesubclade Ⅱ (strain TIO16, Fig.1h & Supplementary Fig.S2) showed no obvious differences from subclade I, and was encountered in Hainan (including Nansha island),South China Sea (station 16; Supplementary Fig.S1;Table 1).Amphidiniumcarteraesubclade Ⅲ (strain TIO574, Fig.1i & Supplementary Fig.S3) and subclade Ⅳ (strain TIO81, Fig.1j & Supplementary Fig.S4) had obvious pentagonal or hexagonal amphiesmal vesicles, which were covered by threadlike body scales.Amphidiniumcarteraesubclade Ⅲ was encountered in Shandong, Yellow Sea (station 1; Supplementary Fig.S1; Table 1),whileA.carteraesubclade Ⅳ was encountered in Guangxi and Hainan, South China Sea (stations 9,13, and 16; Supplementary Fig.S1; Table 1).
3.1.2AmphidiniumfijiensisKarafas & Tomas
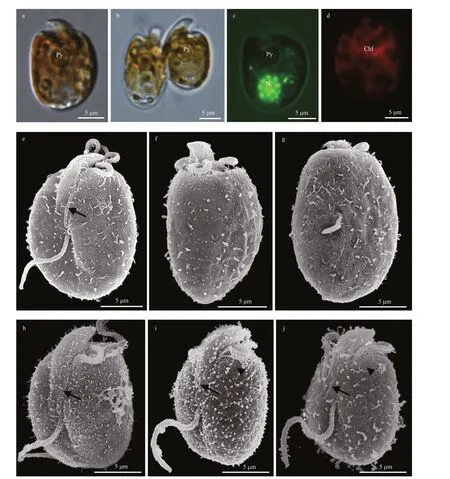
Fig.1 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium carterae
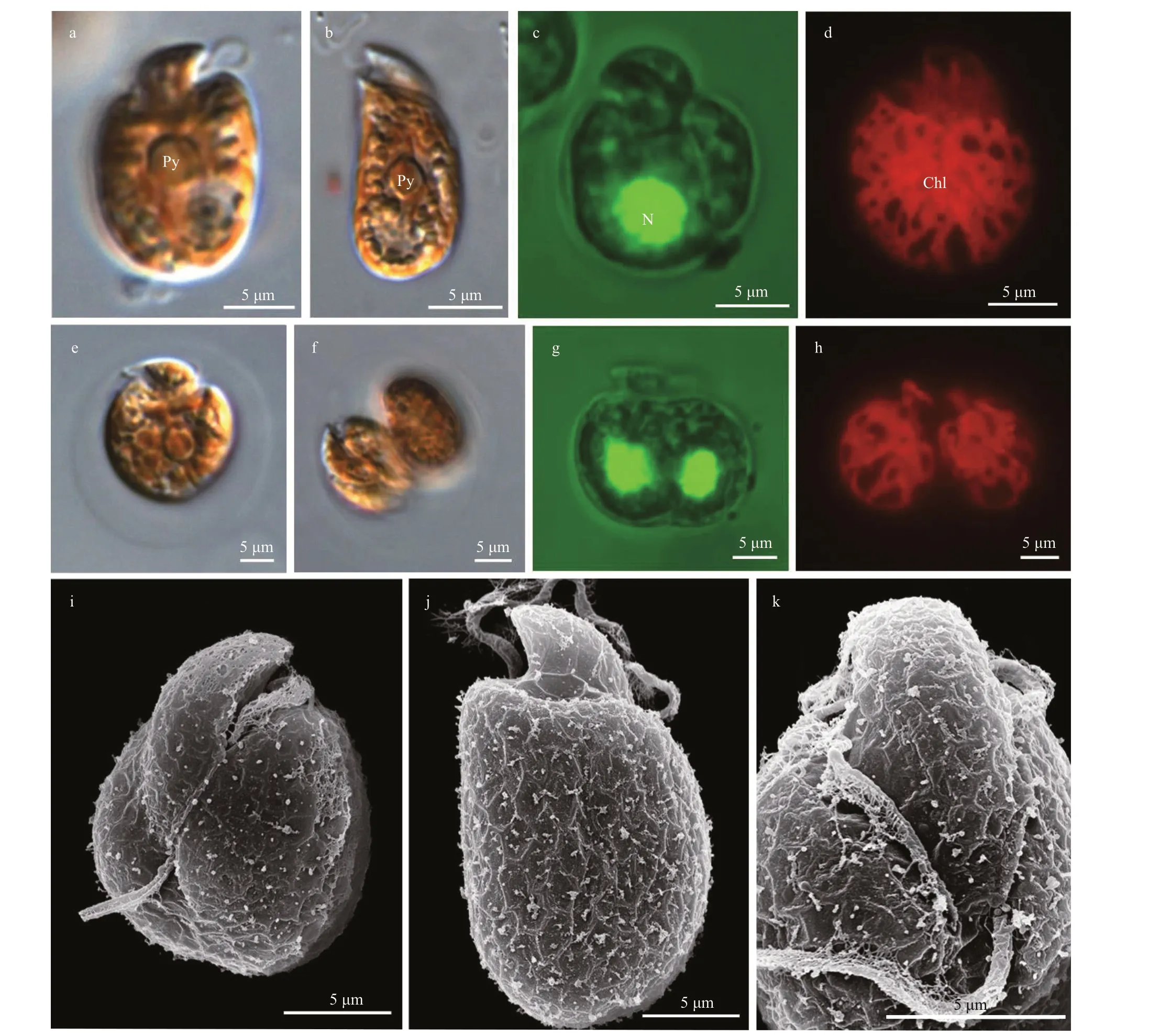
Fig.2 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium fijiensis
Cells ofA.fijiensisstrain WZD19 varied from round, oval to elliptical and dorsoventrally slightly flattened (Fig.2). Cells were 12.8-20.2-μm long(15.9±2.1 μm,n=31) and 9.4-15.7-μm wide(11.3±1.4 μm,n=31), with the length/width ratio varying from 1.0 to 2.2 (1.4±0.3,n=31). A pyrenoid was visible in the center of the cell (Fig.2a-b). The nucleus was round, located in the posterior end of the cell (Fig.2c). A single and reticulate chloroplast was present (Fig.2d). No pusule could be observed by LM. Asexual reproduction occurred within temporary hyaline cysts (Fig.2e-h). The metabolic movements were not observed. The epicone was minute, left-deflecting and 9.4±0.6-μm long (n=6),and protruded minimally on the ventral side (Fig.2i& k). Hexagonal amphiesmal vesicles were observed on the surface of cell (Fig.2j). The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v”shape (Fig.2i-k). The ventral ridge was short and straight (4.7±0.3-μm long,n=6), and connected the two flagella insertion points (Fig.2i & k). The longitudinal flagellum was inserted in the middle third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus (Fig.2i& k). The sulcus was shallow and did not reach the antapex of the cell (Fig.2i & k). The anterior shoulders of the hypocone were symmetrical or slightly higher in the left, and the posterior of the hypocone was rounded (Fig.2i-k).Amphidiniumfijiensiswas encountered in Guangxi, South China Sea (station 10; Supplementary Fig.S1; Table 1).
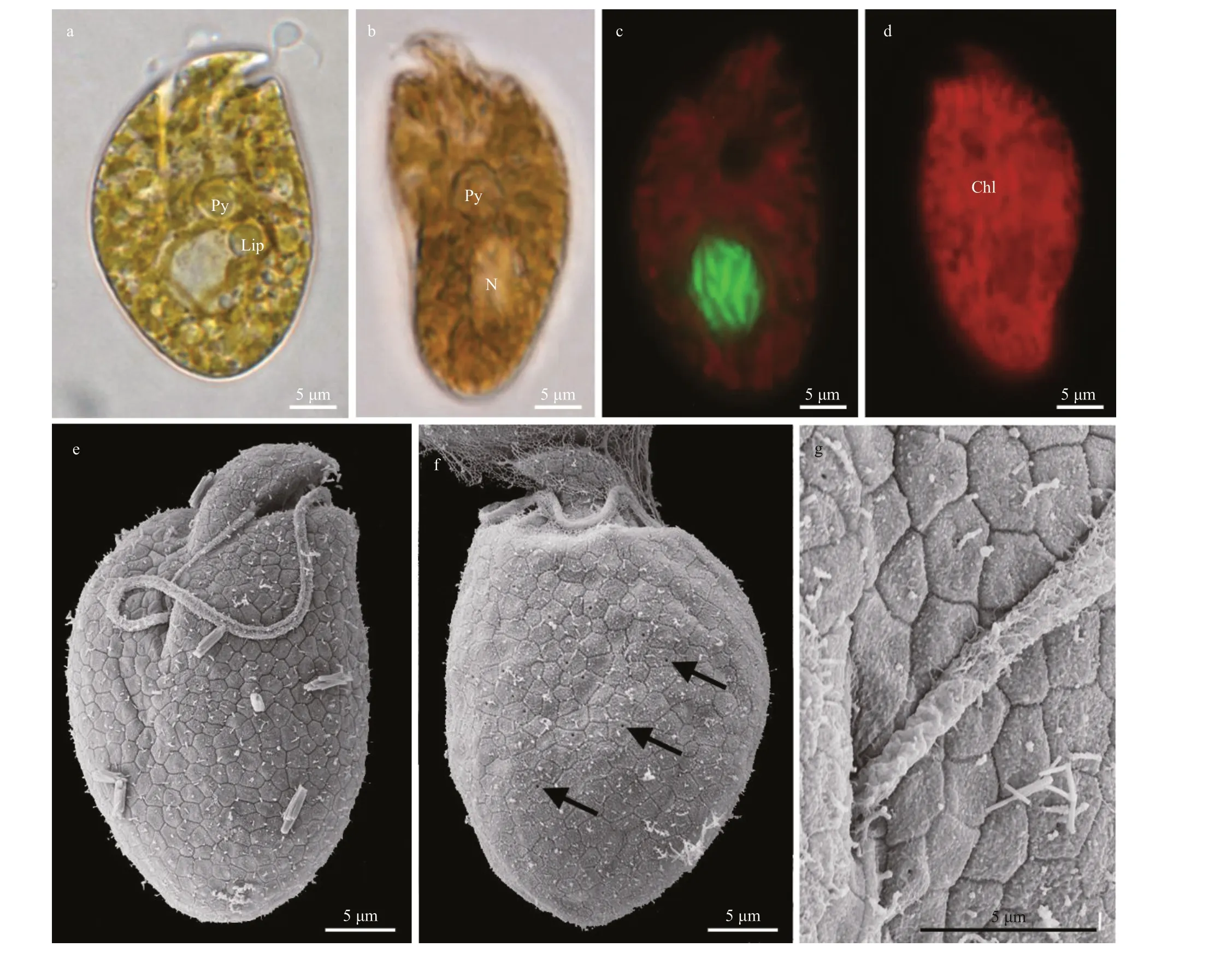
Fig.3 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium gibbosum
3.1.3Amphidiniumgibbosum
Cells ofA.gibbosumstrain 3XS19 were asymmetrically ellipsoid and dorsoventrally flattened(Fig.3). Cells were 31.8-43.9-μm (36.2±2.4 μm;n=61) long and 14.5-22.5-μm (18.8±1.5 μm;n=61)wide, with a length/width ratio of 1.6-2.6 (1.9±0.2;n=61). A pyrenoid was visible in the center of the cell(Fig.3a-b). Lipid globules were observed in the center and periphery of cells (Fig.3a). The nucleus was round,located in the posterior end of the cell (Fig.3c). The chloroplast comprised of several slender lobes (Fig.3c& d). No pusule was observed by LM. The epicone was triangle, left-deflecting and 6.0±0.9-μm long(n=3, Fig.3a & e). The hypocone varied from oval to heart shaped with a strongly convex right side (Fig.3a& e), giving it a hump-backed shape. A ventral ridge(5.3±0.2-μm long;n=5) ran between the two points of the flagellar insertion (Fig.3e). The cingulum was deeply incised and the end was displaced (Fig.3a & e).The proximal and distal ends of the cingulum nearly contacted the ventral ridge and sulcus to form a “v”shape (Fig.3a & e). The sulcus originated from just posteriorly to the cingulum. It was very shallow, short and did not run to the antapex (Fig.3e). Pentagonal or hexagonal plate-like structure was observed on the cell surface (Fig.3e-g). Small round pores were scattered randomly on the cell surface (Fig.3f-g;arrows).Amphidiniumgibbosumwas encountered in Hainan (including Xisha Islands), South China Sea(stations 17, 18, and 21; Supplementary Fig.S1;Table 1).
3.1.4Amphidiniummassartii&A. cf.massartii
Cells ofA.massartiistrain TIO12 were oval to elliptical, and dorsoventrally flattened (Fig.4a-g).Cells were 13.8-19.1-μm long (17.2±2.0 μm,n=30)and 10.8-12.6-μm wide (11.5±0.7 μm,n=30), with the length/width ratio varying from 1.3 to 1.7 (1.5±0.1,n=30). A pyrenoid was visible in the upper half of the cell (Fig.4a & c). Cells were actively swimming in the water column, and asexual divisions occurred by binary fission in the motile cells (Fig.4b). The nucleus was round, located in the posterior end of the cell(Fig.4c). A single and reticulate chloroplast was present (Fig.4d). No pusule could be observed by LM. The epicone was crescent shaped, left-deflecting and 9.4±1.5-μm long (n=7, Fig.4e-f). The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v”shape (Fig.4e-f). The ventral ridge was short and straight (4.9±0.5-μm long,n=7), and connected the two flagella insertion points (Fig.4e). The longitudinal flagellum was inserted in the middle third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus (Fig.4e). The sulcus was shallow and did not reach the antapex of the cell(Fig.4e). Round doughnut-like body scales were observed on the surface of the cell body and flagella(Fig.4g). The anterior shoulders of the hypocone were symmetrical or slightly higher in the left, and the posterior of the hypocone was rounded (Fig.4e-f).Amphidiniummassartiiwas encountered in Hainan(including Nansha islands), South China Sea (stations 10 and 15; Supplementary Fig.S1; Table 1).
Cells ofA. cf.massartiistrain WZD23 were oval to elliptical, and dorsoventrally flattened (Fig.4h-j). Cells were 12.1-18.1-μm long (15.4±1.8 μm,n=30) and 10.4-15.5-μm wide (13.1±1.8 μm,n=30), with the length/width ratio varying from 1.0 to 1.3 (1.2±0.1,n=30). The epicone was crescent shaped, leftdeflecting, and 7.3±0.8-μm long (n=7, Fig.4h). The ventral ridge was short and straight (3.8±0.4-μm long,n=7), and connected the two flagella insertion points(Fig.4h). Round doughnut-like body scales were observed on the surface of both the cell body and flagella (Fig.4j).Amphidiniumcf.massartiiwas encountered in Guangxi and Hainan, South China Sea(stations 10, 12, and 16; Supplementary Fig.S1; Table 1).
3.1.5AmphidiniumpseudomassartiiKarafas & Tomas
Cells ofA.pseudomassartiistrain TIO555 were round to elliptical (Fig.5). Cells were 17.7-23.3-μm long (20.7±1.8 μm,n=50) and 12.9-18.7-μm wide(14.7±1.9 μm,n=50), with the length/width ratio varying from 1.2 to 1.6 (1.4±0.1,n=50). A pyrenoid was visible in the center of the cell (Fig.5b). The nucleus was round, located in the posterior end of the cell (Fig.5c). A single and reticulate chloroplast was present (Fig.5d). No pusule was observed by LM. The epicone was crescent shaped, left-deflecting with a rounded tip, and 11.8±1.8-μm long (n=10, Fig.5e-f).The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v” shape (Fig.5e-f). The ventral ridge was short and straight (6.2±0.8-μm long,n=10),and connected the two flagella insertion points(Fig.5e). The longitudinal flagellum was inserted in the middle third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus(Fig.5e). The sulcus was shallow and did not reach the antapex of the cell (Fig.5e). Body scales were not observed on the surface of the cell (Fig.5g). The anterior shoulders of the hypocone were asymmetrical with the left side slightly higher, and the posterior of the hypocone was rounded (Fig.5e-f). Cells were actively swimming in the water column, and asexual division took place by binary fission in the motile cell(Fig.5h-k).Amphidiniumpseudomassartiiwas encountered in Fujian, East China Sea (station 2;Supplementary Fig.S1; Table 1).
3.1.6Amphidiniumoperculatum

Fig.4 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium massartii and A. cf. massartii
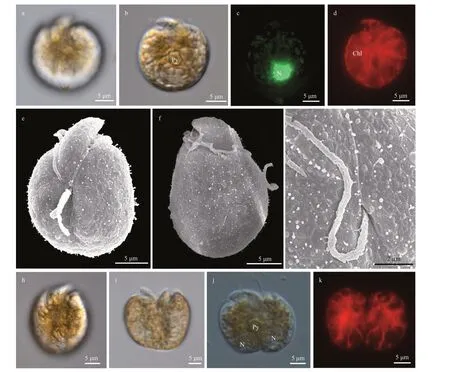
Fig.5 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium pseudomassartii
Cells ofA.operculatumstrain TIO40 were oval to elliptical (Fig.6). The right side of the hypocone was convex, whereas the left was almost straight (Fig.6a).Cells were 28.7-36.3-μm long (32.9±2.4 μm,n=50)and 20.9-25.8-μm wide (23.9±1.7 μm,n=50), with the length/width ratio varying from 1.3 to 1.5 (1.4±0.1,n=50). Many lipid globules were observed in the periphery of cells (Fig.6a), and one big “dark spot”,globular inclusions, was located in the center of the cell (Fig.6b & c). No pyrenoid was observed by LM(Fig.6b-c). The nucleus was elongated, located in the posterior end of the cell (Fig.6c). A single and reticulate chloroplast was present (Fig.6d). The epicone was quite minute compare to the hypocone,left-deflecting, and 8.7±0.7-μm long (n=6, Fig.6a-b,& e). The ventral ridge was 17.8±1.9-μm long (n=6),and connected the two flagella insertion points(Fig.6e-f). The cingulum was displaced and the proximal end is distant from the sulcus (Fig.6e). The longitudinal flagellum was inserted in the lower third of the cell and at the beginning of the sulcus (Fig.6e).The sulcus was shallow and did not reach the antapex of the cell (Fig.6e). The anterior shoulders of the hypocone were symmetrical, and the posterior of the hypocone were rounded (Fig.6a-e). Cell were actively swimming in the water column and temporary hyaline cysts were not found.Amphidiniumoperculatumwas encountered in Guangdong, Guangxi, and Hainan,South China Sea (stations 6, 9, 14, 17, 19, and 20;Supplementary Fig.S1; Table 1).
3.1.7AmphidiniumsteiniiLemmermann
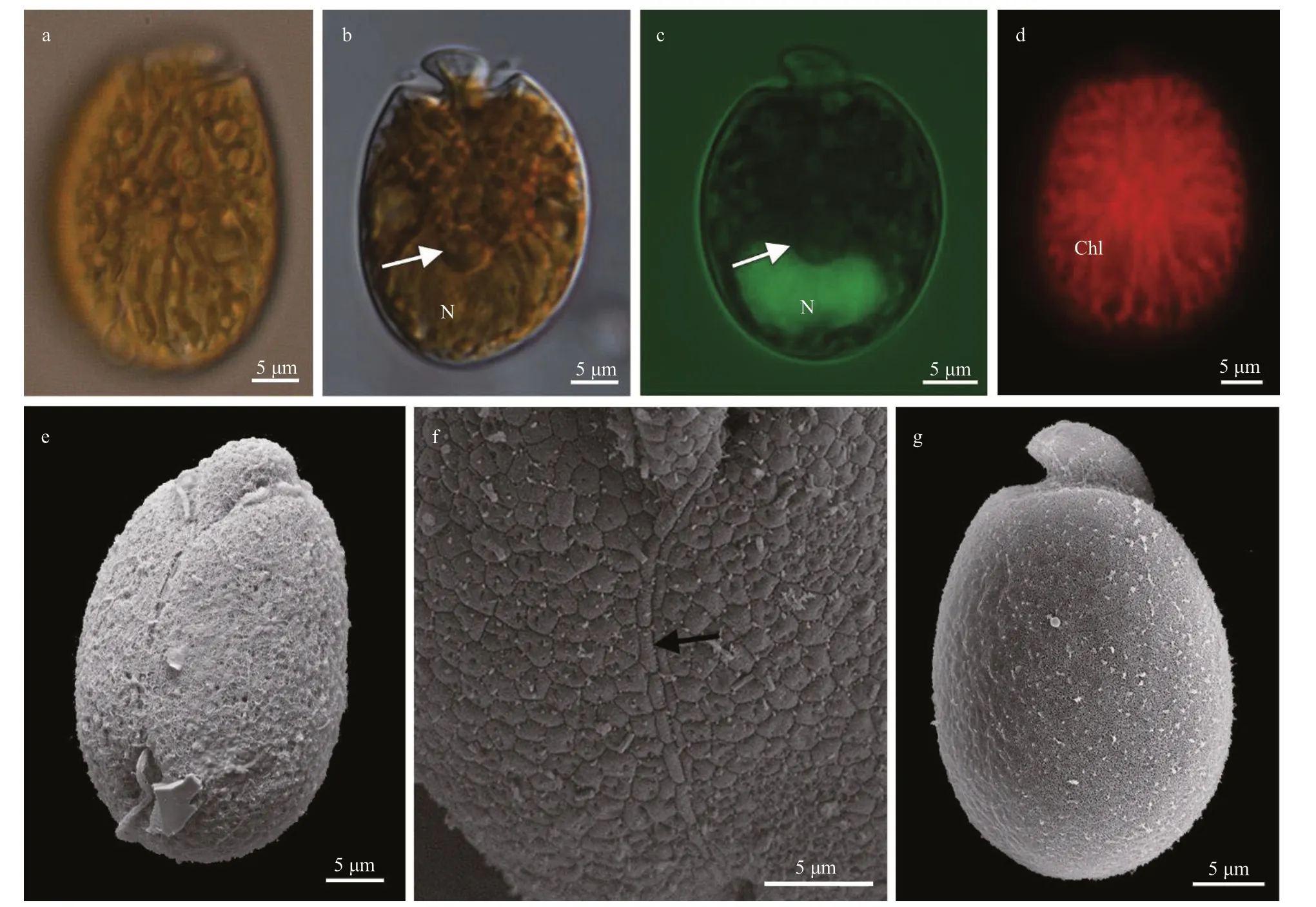
Fig.6 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium operculatum
Cells ofA.steiniistrain TIO311 appeared oval to elliptical (Fig.7). Cells were 33.3-47.7-μm long(39.1±5.3 μm,n=30) and 17.2-29.6-μm wide(23.8±3.1 μm,n=30), with the length/width ratio varying from 1.5 to 2.1 (1.7±0.2,n=30). Lipid or starch droplets were often found in cultures (Fig.7a-b),and a pyrenoid was observed under LM (Fig.7b). The nucleus was round to oval, located in the posterior part of the hypocone (Fig.7a & c). A potential pusule was observed by LM (Fig.7b & c). A single and reticulate chloroplast was present (Fig.7d). The epicone was quite minute compared to the hypocone,triangular, left-deflecting, and 13.4±1.1-μm long(n=9, Fig.7e-g). The ventral ridge was short and straight (6.9±0.6-μm long,n=9), and connected the two flagella insertion points (Fig.7e & g). The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v” shape (Fig.7e & g). The longitudinal flagellum was inserted in the upper third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus, which was narrow and short (Fig.7e & g). Hexagonal amphiesmal vesicles were present on the cell surface, but body scales were not observed (Fig.7g). The anterior shoulders of the hypocone were asymmetrical with the left side slightly higher and the posterior of the hypocone was elliptical (Fig.7e-g). Asexual divisions occurred in hyaline-covered cysts (Fig.7h-l). Metabolic movement was observed in strain TIO311.Amphidiniumsteiniiwas encountered in Fujian, East China Sea and Guangxi, South China Sea (stations 3,8, and 9; Supplementary Fig.S1; Table 1).
3.1.8AmphidiniumthermaeumDolapsakis &Economou-Amilli
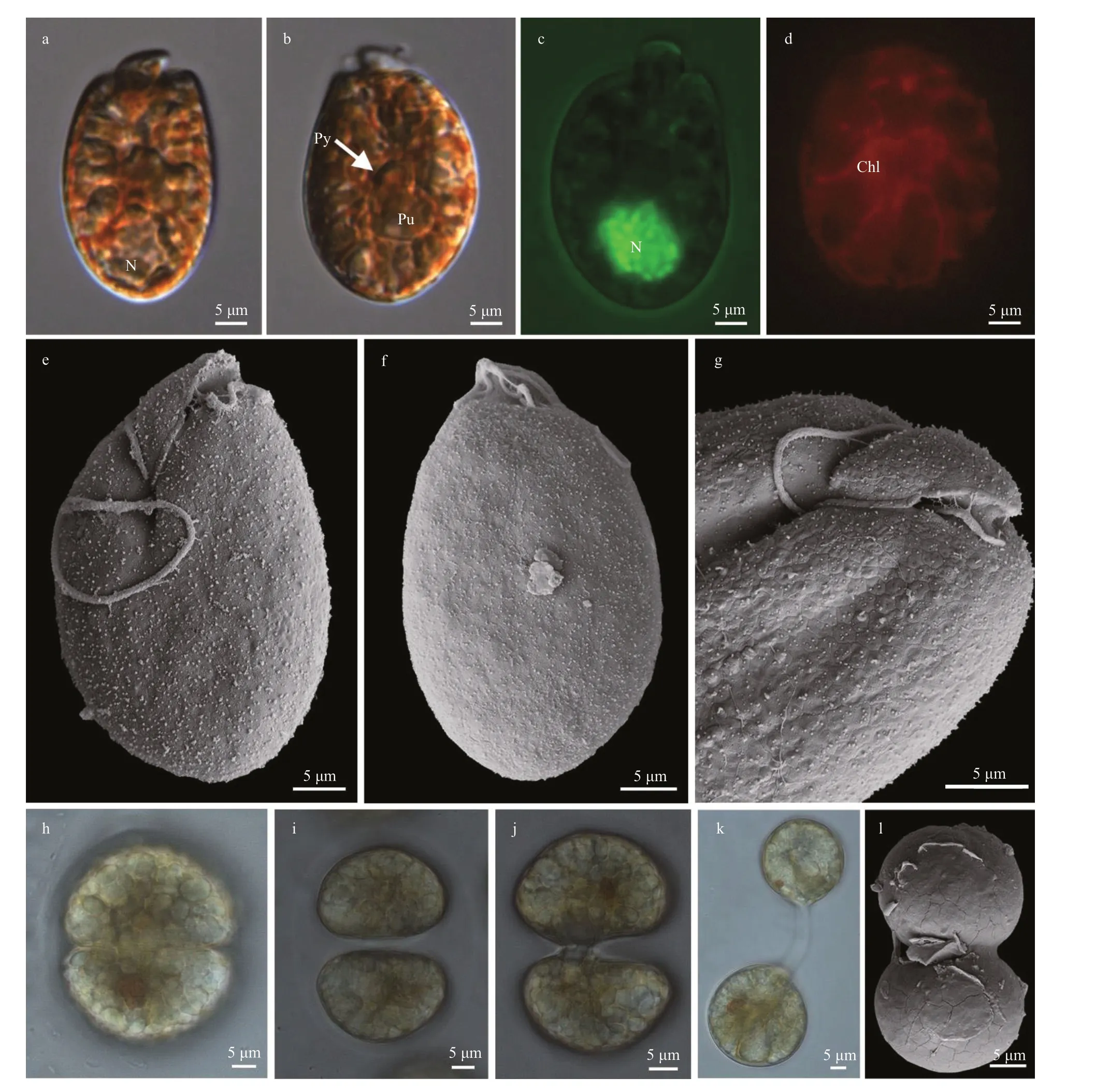
Fig.7 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium steinii
Cells ofA.thermaeumstrain WZD07 were rounded, oval to oblong, and dorsoventrally slightly flattened (Fig.8). Cells were 16.3-22.1-μm long(18.9±2.2 μm,n=30) and 14.9-18.1-μm wide(16.1±1.4 μm,n=30), with the length/width ratio varying from 1.0 to 1.3 (1.7±0.2,n=30). A pyrenoid was observed by LM (Fig.8a & c). A potential pusule with radiating vesicles was present (Fig.8b). The nucleus was round, located in the posterior end of the cell (Fig.8c). A single and reticulate chloroplast was present (Fig.8d). Metabolic movement was observed in a few cells, and cell shapes changed sharply from oblong to oval (Fig.8e-g). Asexual divisions occurred in hyaline-covered cysts (Fig.8h). The epicone was triangular, left-deflecting and 10.2±0.9-μm long (n=6,Fig.8i-k). The ventral ridge was short and straight(6.1±0.5-μm long,n=6), and connected the two flagella insertion points (Fig.8i-j). The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v”shape (Fig.8i-j). The longitudinal flagellum was inserted in the middle third of the cell just below the proximal end of the cingulum and at the beginning of the sulcus (Fig.8i-j). The sulcus was wide and shallow and reached the antapex of the cell (Fig.8i-j). The anterior shoulders of the hypocone were symmetrical or slightly higher in the left, and the posterior of the hypocone were rounded (Fig.8i & k).Amphidiniumthermaeumwas encountered in Guangxi and Hainan(including Nansha Islands), South China Sea (stations 9, 11, 16, and 20; Supplementary Fig.S1; Table 1).
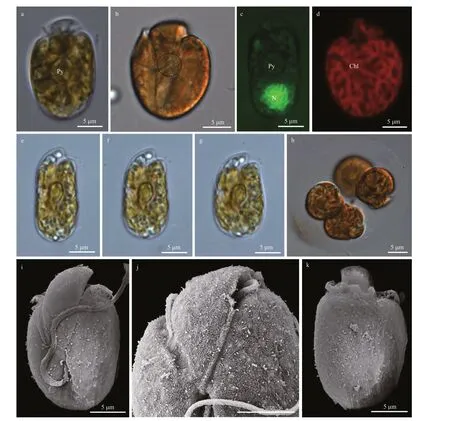
Fig.8 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium thermaeum
3.1.9AmphidiniumtheodoriTomas & Karafas
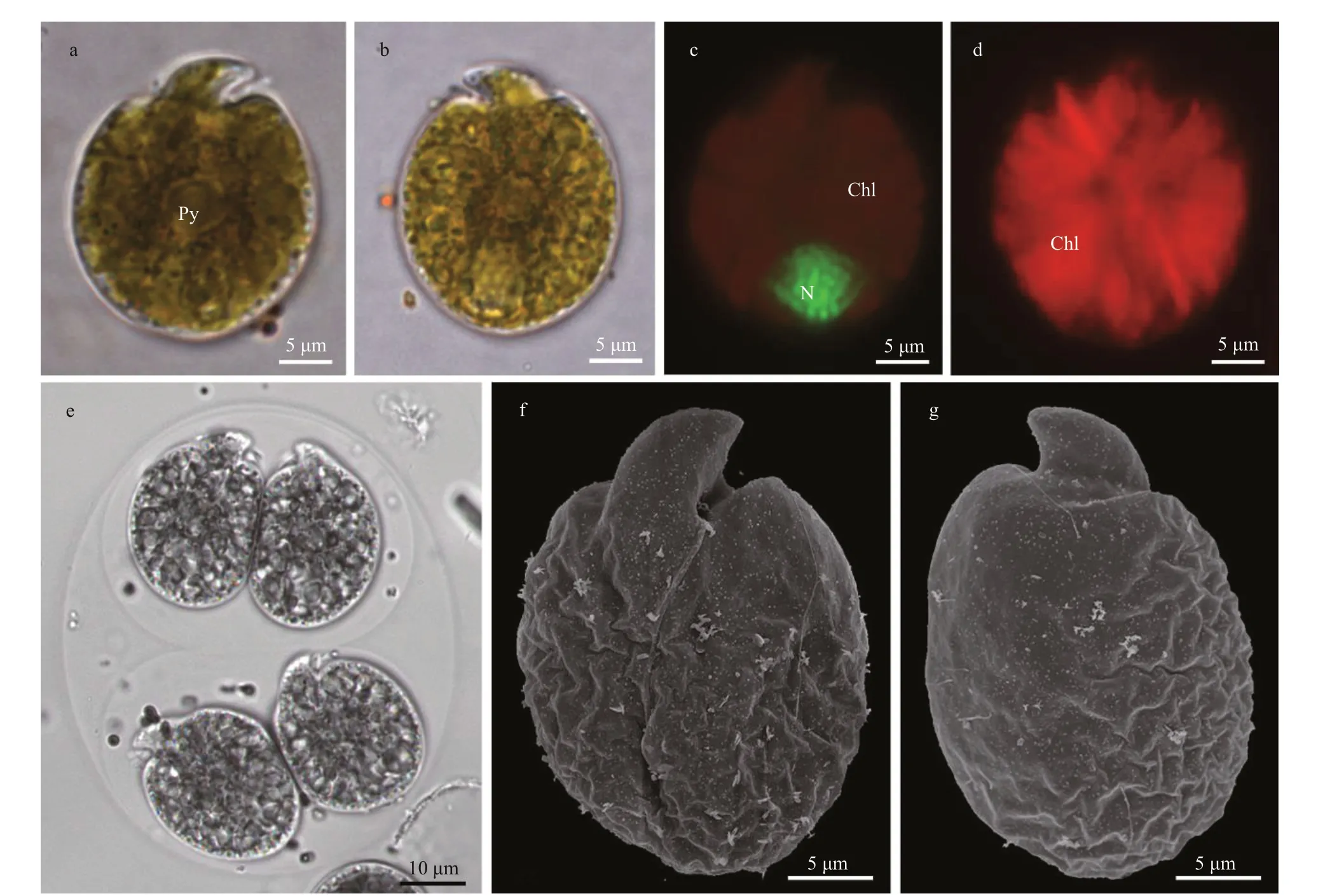
Fig.9 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium theodori
Cells ofA.theodoristrain 3XS55 appeared oval to elliptical with a range of 21.1-35.8-μm (28.0±2.5 μm;n=35) long and 19.4-33.0-μm (24.9±2.7 μm;n=35)wide (Fig.9). The length/width ratio was 1.0-1.3(1.1±0.1;n=35). A central pyrenoid was observed, but no pusule was found under LM (Fig.9a-b). The nucleus was oval and located in the posterior end of the cell (Fig.9c). Chloroplast lobes appeared to radiate from the central pyrenoid and spread to the periphery of the cell (Fig.9d). Asexual divisions occurred in hyaline-covered cysts (Fig.9e). The epicone was triangular, left-deflecting, and the ventral ridge(6.6±0.4-μm long;n=4) was straight (Fig.9f). The anterior shoulders of the hypocone were symmetrical or slightly higher at the left, and the posterior of the hypocone was rounded (Fig.9a-b, f-g). Metabolic movement was observed.Amphidiniumtheodoriwas encountered in Hainan (Xisha Islands), South China Sea (station 21; Supplementary Fig.S1; Table 1).
3.1.10AmphidiniumtomasiiKarafas
Cells ofA.tomasiistrain TIO31 were oval to elliptical and slightly flattened dorsoventrally(Fig.10). Cells were 22.1-33.2-μm long (27.5±4.2 μm,n=30) and 16.7-25.6-μm wide (21.2±3.8 μm,n=30),with the length/width ratio varying from 1.1 to 1.6(1.3±0.2,n=30). The epicone was curved and leftdeflecting and 13±1.7-μm long (n=6, Fig.10a-b). The nucleus was round, located in the posterior end of the cell (Fig.10b & c). A single and reticulate chloroplast was present (Fig.10d-e). No pusule could be observed by LM. Cell were actively swimming in the water column, and asexual divisions took place by binary fission in the motile cell (Fig.10e-h), No immobile or encysted cells were observed. The cingulum was displaced and the proximal and distal ends nearly contacted the ventral ridge and sulcus to form a “v”shape (Fig.10i). The sulcus originated around the ventral midpoint of the cell and widened gradually toward posteriorly (Fig.10i). The ventral ridge was short and straight (8.5±1.6-μm long,n=6), and connected the two flagella insertion points (Fig.10i).The anterior shoulders of the hypocone were asymmetrical with a higher left side, and the posterior of the hypocone were rounded (Fig.10i). The left side of the hypocone varied from straight to convex, while the right side of the hypocone was always convex.Longitudinal cleave was not observed on the dorsal hypocone (Fig.10j-k). The longitudinal flagellum was inserted in the middle third of the cell at the beginning of the sulcus, which was shallow and did not reach the antapex of the cell (Fig.10i). Hexagonal amphiesmal vesicles were observed on the cell surface, but body scales were not observed (Fig.10i).Amphidiniumtomasiiwas encountered in Hainan,South China Sea (stations 14 and 16; Supplementary Fig.S1; Table 1).
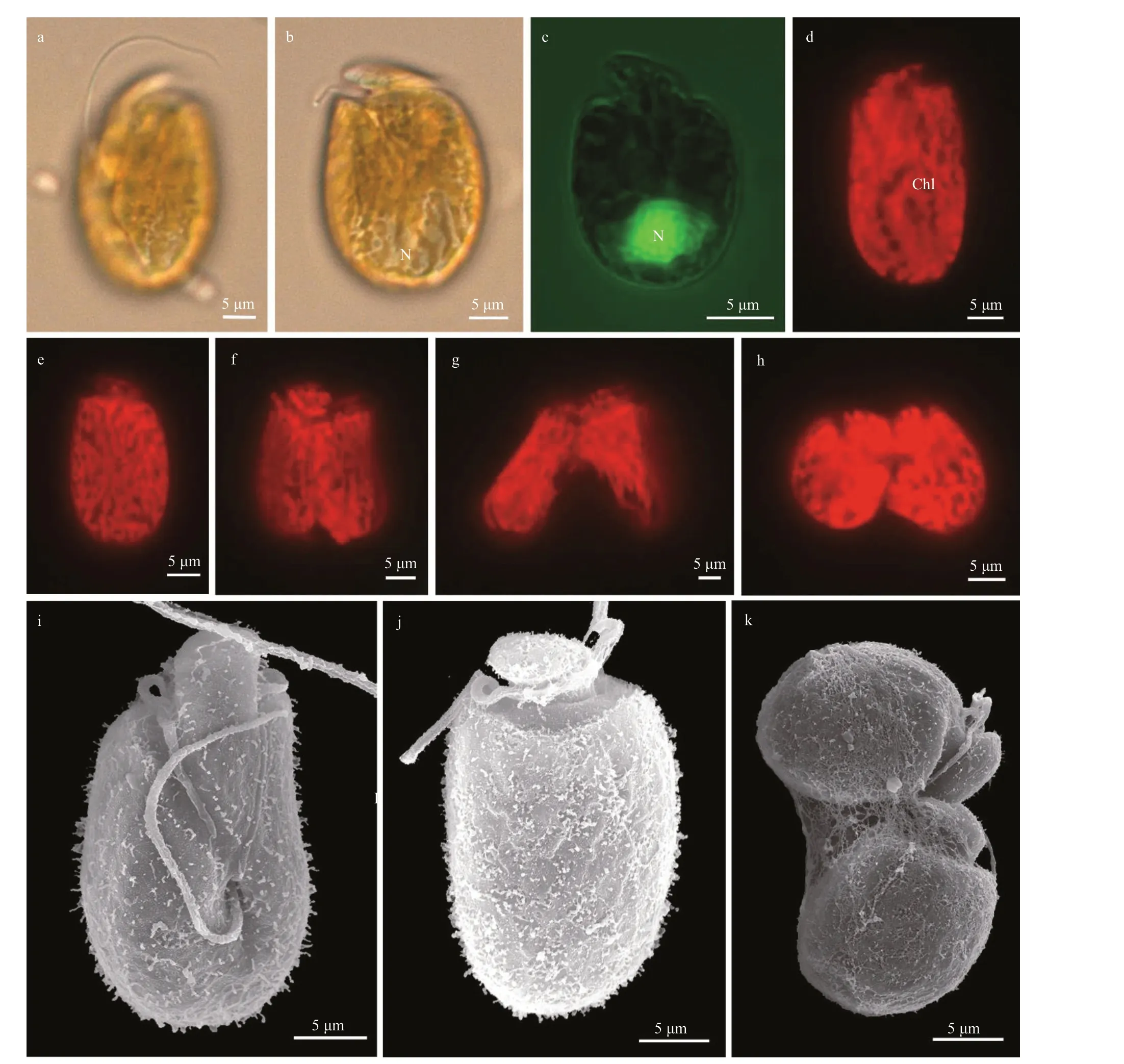
Fig.10 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium tomasii
3.1.11Amphidiniumsp.
Cells ofAmphidiniumsp. strain 5XS22 were oval to elliptical and dorsoventrally flattened (Fig.11). The epicone was minute and triangular, left-deflecting(Fig.11). Cells were 20.3-30.9-μm long (26.3±5.0 μm,n=30) and 15.9-24.8-μm wide (21.3±2.5 μm,n=30),with the length/width ratio varying from 1.1 to 1.5(1.2±0.3,n=30). Many lipid globules were observed in the periphery of the cells, but no pyrenoid or pusule were observed by LM (Fig.11a-b). Chloroplast appeared to radiate from the center of the cell and extend to the periphery (Fig.11c). The ventral ridge(8.97±1.31-μm long,n=4) was long and straight and connected the two flagella insertion points (Fig.11d-e).The cingulum was displaced and the proximal and distal ends were distant from the sulcus (Fig.11d-e).The cingulum ends nearly contacted the ventral ridge and formed a “v” shape (Fig.11e). The longitudinal flagellum was inserted in the lower third of the cell and at the beginning of the sulcus (Fig.11d-e). The sulcus was wide and reached to the antapex of the cell(Fig.11d-e). The anterior shoulders of the hypocone were nearly symmetrical (Fig.11d). The posterior of the hypocone were rounded (Fig.11). Pentagonal or hexagonal plate-like structure was observed on the cell surface (Fig.11d-e).Amphidiniumsp. strain 5XS22 was encountered in Hainan (Xisha Islands), South China Sea (station 21; Supplementary Fig.S1; Table 1).
3.2 Molecular phylogeny
From a comparison of LSU rRNA gene sequences(Fig.12), it was found that 22 strains of ChineseA.carteraediffered from each other in 24-56 positions(95.4%-98.1% similarity). Ten strains of ChineseA.fijiensisshared identical sequences, and they differed from the type strain Amfi0508-1 ofA.fijiensisin 34 positions (97.4% similarity). Three strains of ChineseA.gibbosumshared identical sequences with the USVI strain SI-36-50 ofA.gibbosum. Three strains of ChineseA.massartiidiffered from each other in 6-23 positions (97.1%-99.2% similarity). The ChineseA.massartiistrain TIO12 differed from the USA strain Amma0607-1 and Japanese strain HG115 in 31 and 35 positions (97.6% and 96.9% similarity),respectively, and differed fromA. cf.massartiistrain WZD23 isolated from Guangxi, South China Sea, in 50 positions (96.1% similarity). The ChineseA. cf.massartiistrains WZD23 and 3WZD7 differed from one another in 7 positions (99.2% similarity). Six strains of ChineseA.pseudomassartiishared identical sequences, and they differed from the type strain AKLV01 ofA.pseudomassartiiin 18 positions(98.6% similarity). Nine strains of ChineseA.operculatumdiffered from each other in 0-17 positions (97.9-100% similarity). Three strains ofA.operculatum(TIO175b, TIO175d, and TIO175e)isolated from the South China Sea shared identical sequences, and they differed from the strain TIO40 from the South China Sea, Australian strain K-0663,and New Zealand strain CAWD42 in 2, 41, and 19 positions (99.2%, 96.8%, and 97.9% similarity),respectively. Three strains of ChineseA.steiniidiffered from each other in 53-66 positions (93.1%-94.4% similarity). ChineseA.steiniistrain TIO311 differed from Australian strain CS-741 and Brazilian strain SM12 in 2 and 19 positions (99.2% and 97.9%similarity), respectively. Nine strains of ChineseA.thermaeumshared identical sequences, and they differed from the type strain UoABM-Atherm1 ofA.thermaeumin a single position (99.8% similarity).The ChineseA.tomasiistrain TIO31 differed from the type strain Amth1412-1 ofA.tomasiiin three positions(99.4% similarity). Two strains of ChineseA.trullashared identical sequences, and they differed from the type strain CAWD68 ofA.trullain 11 positions(99.1% similarity). The ChineseA.theodoristrain 3XS55 differed from the type strain Amth0702-1 ofA.theodoriin ten positions (98.7% similarity).
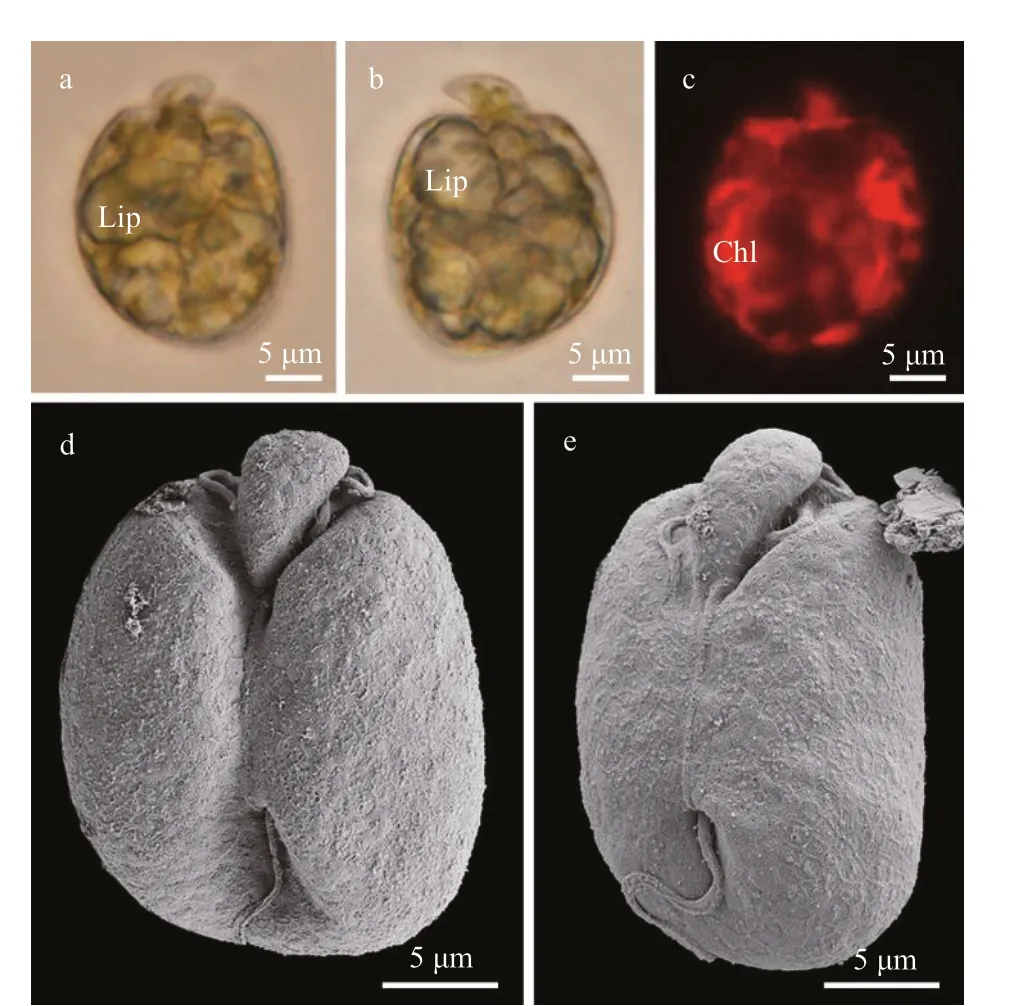
Fig.11 Light microscopy (LM) and scanning electron microscopy (SEM) of Amphidinium sp.
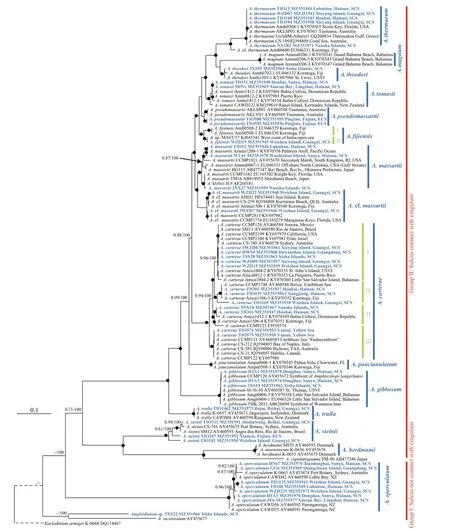
Fig.12 Molecular phylogeny of Amphidinium inferred from partial large subunit rRNA sequences based on Maximum likelihood (ML)
The maximum likelihood (ML) and Bayesian inference (BI) analyses based on partial LSU rRNA sequences yielded similar phylogenetic trees. The ML tree is shown in Fig.12. TheA.incoloratumCampbell andAmphidiniumsp. strain 5XS22 were the earliest branch in theAmphidinumsensu stricto but with low support.Amphidiniumoperculatumwas the second earliest branch followed byA.steinii,A.cupulatisquamaM. Tamura & T. Horiguchi,A.herdmaniiKofoid & Swezy, andA.mootonorumMurray & Patterson clade. Two groups (I and Ⅱ) were resolved by representing the sulcus connect with the cingulum or not (Fig.12). AllA.carteraestrains were grouped together in a fully supported clade that was the sister to a clade containingA.massartii,A.fijiensis,A.pseudomassartii,A.tomasii,A.theodori,A.magnumKarafas & Tomas, andA.thermaeum.Amphidiniumcarteraefrom various parts of the world that comprised four well-supported subclades,referred as subclades I, Ⅱ, Ⅲ, and Ⅳ. ThreeA.carteraesubclades (I, Ⅱ, and Ⅳ) were found in the South China Sea and subclade Ⅲ was found in the Yellow Sea. Strains ofA.massartiiandA. cf.massartiiwere sister clades with each other, and both were encountered in the South China Sea. Strains ofA.pseudomassartiiwere grouped together in a fully supported clade, which consisted of two clades from the East China Sea and Australia, and were referred as subclades I and Ⅱ, respectively. TheA.fijiensisfrom the South China Sea were grouped together and formed a well-supported clade (subclade Ⅱ) with west coast of India-open sea strains, which was a sister clade to the type strain ofA.fijiensis(subclade I).Amphidiniumthermaeumfrom various parts of the world were grouped together in a well-supported clade, which was positioned at the end of the branch of the phylogenetic tree.
Sequence similarity and genetic distances based on ITS sequences amongAmphidiniumspecies are listed in Table 2. The genetic distances betweenAmphidiniumspecies ranged from 0.215 to 0.519, with an average of 0.352. The largest interspecific distances were found betweenA.steiniiand the other species ofAmphidinium(0.472-0.519). The ML and BI analysis based on ITS sequences generated similar trees, and the ML tree is shown in Supplementary Fig.S5.Amphidinumspecies were resolved in accordance with traditional morphometrics based taxa units, which is consistent with the LSU rRNA sequences-based phylogeny.Amphidiniumsteiniidiverged early, followed by the rest of theAmphidiniumsensu stricto formed a well resolved clade, with strong support (1.00, 100).
3.3 Pigment profile
Eleven pigments, including peridinin, pheophorbidea, diadinoxanthin, antheraxanthin, diatoxanthin,pheophorbide, andβ-carotene, and four different chlorophylls were detected in all tested strains(Table 3). Peridinin, pheophorbide, chlorophylla,antheraxanthin, and chlorophyllc2were the dominant pigments in all five species. The content ratio of pheophorbide ranged from 19.24% (A.carteraesubclade Ⅱ) to 35.46% (A.carteraesubclade Ⅳ)(Table 3).
4 DISCUSSION
4.1 Morphology and phylogeny
There is a history of complication and confusion over the identification and placement ofAmphidiniumspecies due to their high morphological and genetic variability (Steidinger and Tangen, 1997; Daugbjerg et al., 2000; Saldarriaga et al., 2001). Jørgensen et al.(2004) and Murray et al. (2004) made notable strides in redefining the morphological characters of the type species of the genus and emended the genus definition.Presently, theAmphidiniumsensu stricto includes both heterotrophic and autotrophic forms, possessing a characteristically minute epicone that is deflected towards the left (Jørgensen et al., 2004; Murray et al.,2004). About 20 species have been confirmed to fit the revised definition of the genus (Jørgensen et al.,2004; Murray et al., 2004; Dolapsakis and Economou-Amilli, 2009; Tamura et al., 2009; Karafas et al.,2017). Two major clades in the autotrophicAmphidiniumhave been reported based on partial LSU rRNA sequences, i.e., the Herdmanii and Operculatum clades (Jørgensen et al., 2004; Karafas et al., 2017), but the morphological features delimiting these clades are not clear. Our study identified two groups within theAmphidinium(Groups I and Ⅱ) and highlighted the taxonomic significance of the position of sulcus inAmphidinium(Fig.12). Group I was characterized by the separation between sulcus and cingulum, and only includedA.operculatum,A.incoloratum, andAmphidiniumsp. strain 5XS22 so far. Group Ⅱ was characterized by the connection between sulcus and cingulum, and included other 14 species.
4.1.1Amphidiniumcarterae
Amphidiniumcarteraeis a well-known worldwide species that was first described asA.klebsiiKofoid and Swezy (Carter, 1937), but was later revised as a new species, based on its smaller size and unlikeA.klebsii, it has a single chloroplast (Hulburt, 1957).Phylogenetic analyses based on partial LSU rRNA sequences have revealed a high genetic diversitywithin theA.carteraestrains from around the world,and have shown many subclades (Murray et al., 2004,2012; Lee et al., 2013). Karafas et al. (2017) proposed a division into four subclades based on the similarities and differences of the secondary structure of the ITS2 region among the different subclades. Our phylogeny based on ITS and partial LSU rRNA sequences seems to support this proposal (Fig.12 and Supplementary Fig.S5). Amphiesmal vesicles were reported on the surface ofA.carteraesubclade I (culture CCMP124,genotype 2 in Murray et al. (2004); Fig.1e-g in the present study), and were also found inA.carteraesubclade Ⅱ here (Fig.1h). However, threadlike body scales were only found on the surface of Chinese strains ofA.carteraesubclades Ⅲ and Ⅳ, and are reported here for the first time (Fig.1i-j; arrows). The morphology and phylogeny results seem to suggest thatA.carteraemight contain several different species (Karafas et al., 2017; this study).
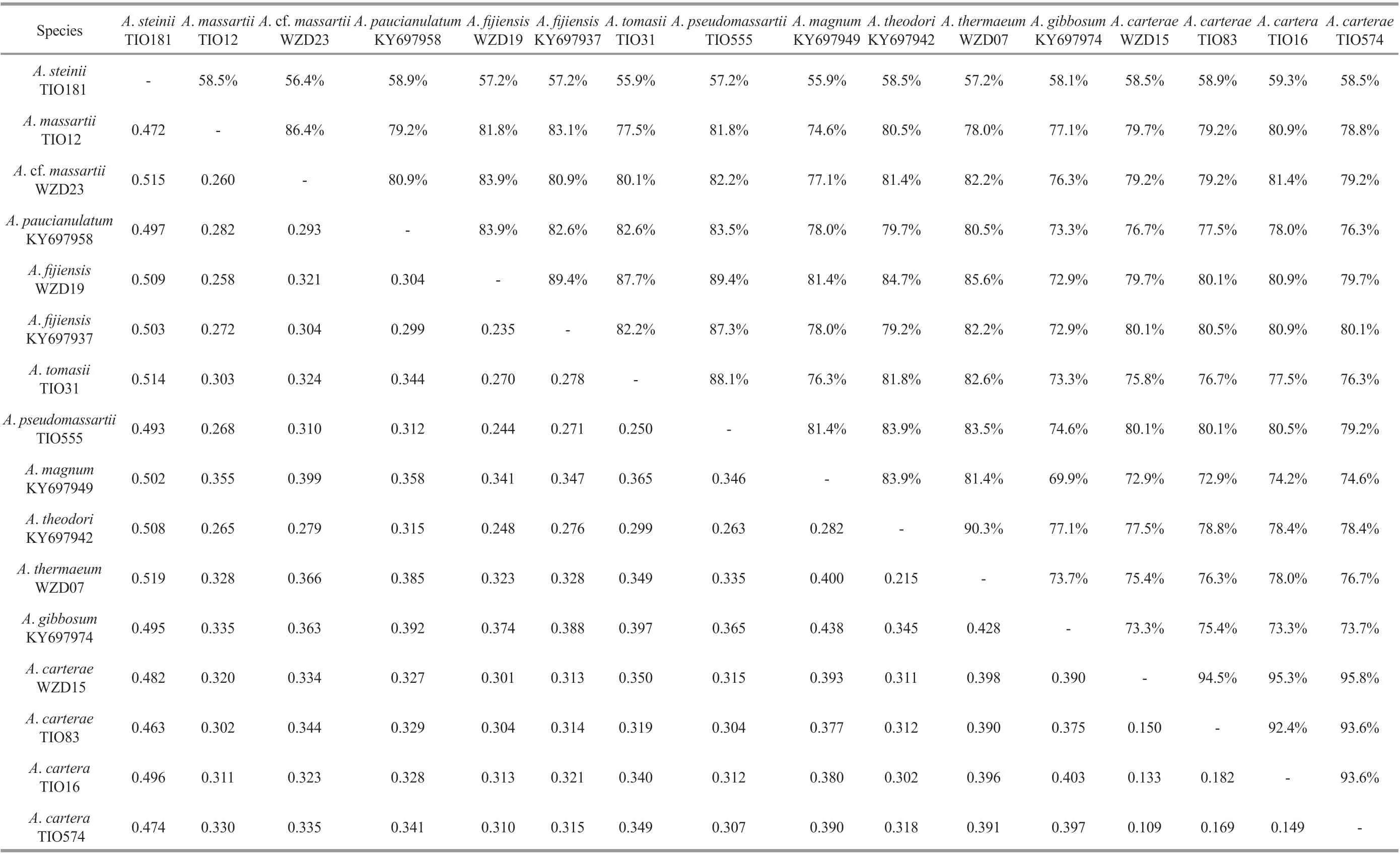
Table 2 Internal transcribed spacer (ITS) region sequences compared between Amphidinium species
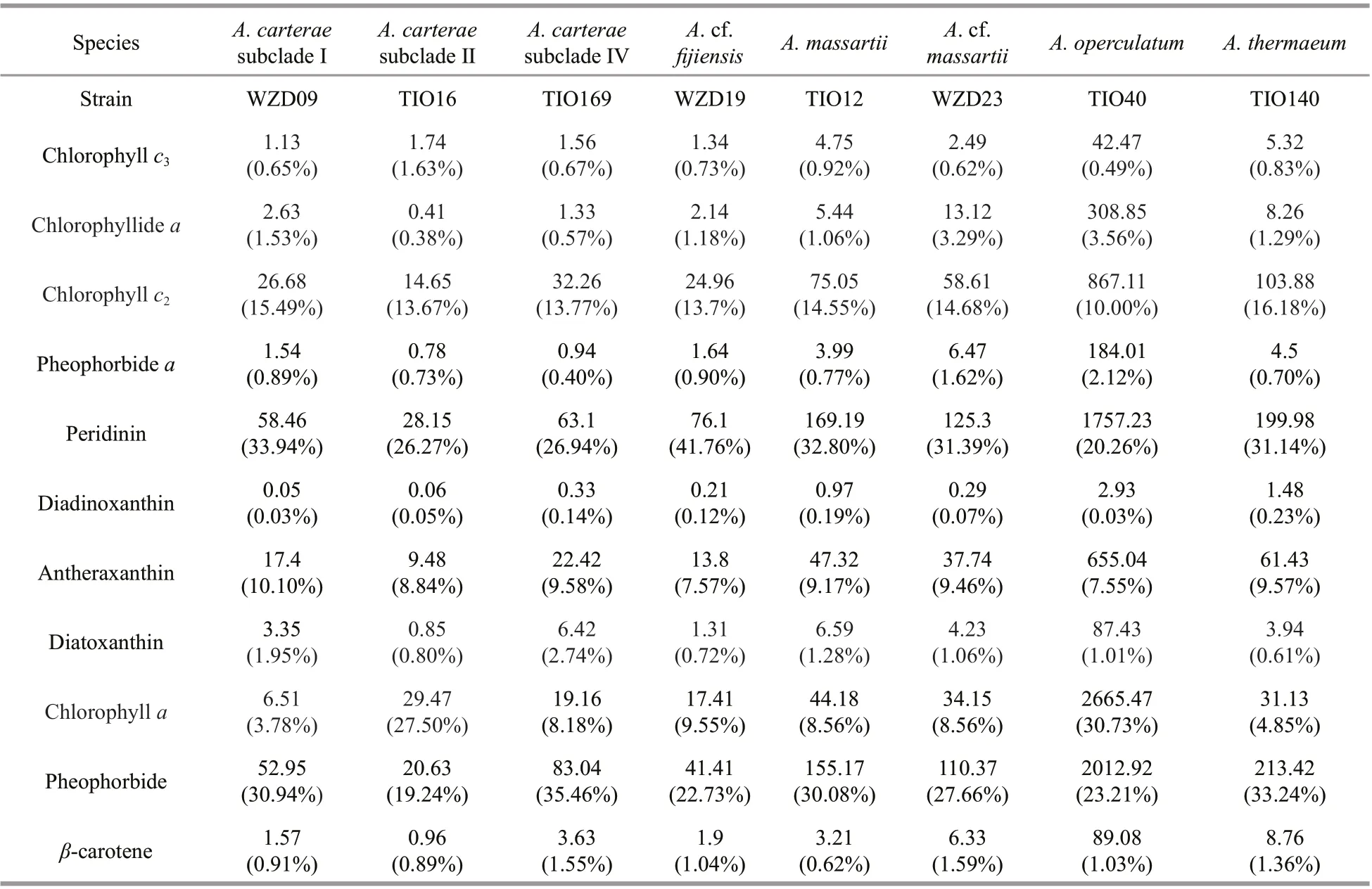
Table 3 Pigment profiles of selected Amphidinium strains, including pigment cell quotas (pg/cell) and percentage in brackets
Strains ofA.carteraesubclade Ⅲ were encountered in the Yellow Sea, but not from the East and South China Seas (Table 1). Strains ofA.carteraesubcladeⅢ have also been reported from Italy, Canada, and Tasmania in Australia and were also reported as an“endosymbiont” in the jellyfishCassiopeiaxamachanafrom the Caribbean Sea. It may suggest that the free-livingA.carteraesubclade Ⅲ strains are distributed only in the higher latitude of the temperate zone. In the case ofA.carteraesubclades I, Ⅱ, and Ⅳno biogeographical distribution was found that was related to the molecular result (Fig.12).
4.1.2Amphidiniumfijiensis
Amphidiniumfijiensiswas first described from a fish tank containing “live rock” imported from Korotoga Fiji (Karafas et al., 2017). ChineseA.fijiensisis consistent with the original description in terms of the small size, the insertion of the longitudinal flagellum in the middle third, the central pyrenoid, the posteriorly located nucleus, and reproduction within temporary hyaline cysts (Karafas et al., 2017).Hexagonal amphiesmal vesicles were seen on the surface of cells of strain WZD19 (Fig.2j), but whether they were also present in the type strain was not clear.The phylogeny based on ITS and partial LSU rRNA sequences revealed two well supported subclades between the type strains and South China Sea strains.The genetic distances between the type strain Amfi0508 and strain WZD19 from Guangxi, South China Sea, was 0.235, which was below the average value for differentAmphidiniumspecies (0.352).Until a stable character distinct from the type strain is clarified, the identity of Chinese specimens is retained asA.fijiensis.
4.1.3Amphidiniumgibbosum
Amphidiniumgibbosumwas originally identified under the name,A.operculatumvar.gibbosum(Maranda and Shimizu, 1996), and later changed toA.gibbosumdue to their hump-backed cell shape and different molecular sequences fromA.operculatum(Murray et al., 2004). The ChineseA.gibbosumstrain 3XS19 fits the original description (Murray et al.,2004), in addition to round pores recorded on the cell surface ofA.gibbosumhere for the first time. The phylogenetic position ofA.gibbosumwas ambiguous(Karafas et al., 2017), and the addingAmphidiniumsequences clarified its phylogenetic status. The LSU rRNA based phylogeny represented here revealed thatA.gibbosumis located as an independent taxon betweenA.paucianulatumandA.trulla(Fig.12). In the ITS phylogeny (Supplementary Fig.S5),A.gibbosumis shown to be separated fromA.massartiiandA. cf.massartii. Both the morphology and phylogeny results represented here confirm thatA.gibbosumcomposes its own unique species as an independent taxon (Murray et al., 2004; this study).
4.1.4Amphidiniummassartii,A. cf.massartii, andA.pseudomassartii
Amphidiniummassartiiwas originally described from France (Biecheler, 1952). LaterA.massartiiorA. cf.massartiiwere reported in the Mediterranean,and the coastal waters offAustralia, USA, Japan,Korea, and Canada (Murray and Patterson, 2002;Murray et al., 2004; Lee et al., 2013; Karafas et al.,2017). However, three distinctly different molecular clades have been reported among them (Murray et al.,2004, 2012; Lee et al., 2013; this study). The first and second were sister clades, and were traditionally identified asA.massartii, characterized by possessing round doughnut-like body scales on the cell surface(Murray et al., 2012; Lee et al., 2013; this study).Round doughnut-like scales were observed on the surface of the flagella of the secondA.massartiiclade(Lee et al., 2013, Fig.4j in the present study), although they were not specifically reported in the originally described French strain. Here, we found that round doughnut-like scales were present on the surface of the flagella of the firstA.massartiiclade for the first time (Fig.4g), and there were no morphological differences between the two subclades. The ITS sequences-based distance between Chinese strains TIO12 (the firstA.massartiiclade) and WZD23 (the secondA.massartiiclade) was 0.260, which was below the average (0.352) of the differentAmphidiniumspecies, but higher than the smallest one (0.215). In summary, a notable genetic diversity was confirmed,but morphological differences were not observed between the first and secondA.massartiiclade of the Chinese strains, as also reported in previous studies(Murray et al., 2012; Karafas et al., 2017). More morphological and ultrastructure details are needed before their systematic status can be determined, and for the time being the first clade can be referred to asA.massartiiand the second clade asA. cf.massartii(Karafas et al., 2017). The third clade included two Australian strains that were genetically distinct from the previous two clades (Murray et al., 2004, 2012;Lee et al., 2013). Karafas et al. (2017) assigned the third clade to a novel species,A.pseudomassartii,based on the lack of the round doughnut-like scales and wider epicones thanA.massartii. The discovery ofA.pseudomassartiifrom the East China Sea is the first record outside its type locality.
4.1.5AmphidiniumoperculatumandAmphidiniumsp.
Amphidiniumoperculatumis the type species of the genusAmphidinium, but its morphology was very different to the otherAmphidiniumsensu stricto species. The longitudinal flagellum ofA.opeculatumis inserted in the lower third of the cell at the beginning of the sulcus, and did not connect with the cingulum.A similar location of flagellum insertion was only found inA.incoloratum, the only heterotrophicAmphidiniumsensu stricto species. Here we reported a third one,Amphidiniumsp. 5XS22 with the sulcus not connected to the cingulum.Amphidiniumsp.5XS22 was phylogenetically close toA.incoloratum(Fig.12), but is an autotrophic species which is distinguishable fromA.incoloratum. Morphological details of strain 5XS22 are needed before confirming its taxonomic status. The separation between sulcus and cingulum distinguishesA.opeculatum,A.incoloratum, andAmphidiniumsp. 5XS22 from the otherAmphidiniumsensu stricto, and they comprised theAmphidiniumGroup I (Fig.12). The divergent nature ofA.operculatumwas also supported by having the greatest number of specific nucleotide changes in the partial LSU rRNA (Jørgensen et al.,2004; Murray et al., 2004). The long branch length in both the maximum likelihood and Bayesian inference analysis suggested a large divergence of this species from the others (Murray et al., 2004; Karafas et al.,2017). In addition, the ITS sequence ofA.operculatumwas not available, although we tried the primers ITSA/ITSB (Adachi et al., 1996), ITS1 and ITS4(White et al., 1990; Gottschling and Plötner, 2004), as well as newly designed primers at the 3′ end of SSU and the 5′ end of LSU, which also indicated the divergence of this species from the otherAmphidinium.Considering the divergent morphology and molecular sequences ofA.operculatum, the other species currently included inAmphidiniummay warrant the establishment of new genera.
4.1.6Amphidiniumsteinii
Amphidiniumsteiniiwas first identified asA.operculatum; however, unlike the original description ofA.operculatum(Stein, 1883), the insertion of the transverse flagellum is in an anterior position and division cysts are produced. We confirm that the most important characters distinguishingA.steiniifromA.operculatumare asexual division by means of a division cyst, as well as the insertion of the transverse flagellum in the anterior third of the cell(Murray et al., 2004). In addition, more differences were revealed, i.e., 1) the nucleus ofA.steiniiis round to oval, but inA.operculatumit is elongated (Figs.6c& 7c); 2) the chloroplast of bothA.steiniiandA.operculatumradiates from the center, but the former has more bulky and sparse lobes than the latter(Figs.6d & 7d). The morphology ofA.steiniiis also similar to that ofA.trullain terms of cell size, epicone shape, the anterior position of insertion of the transverse flagellum, and the possession of a central pyrenoid from which the chloroplast lobes radiate.The asexual divisions ofA.trullaoccur in the motile cell (Murray et al., 2004), but inA.steiniithey occur in division cysts (Fig.7).
The LSU rRNA sequences ofA.steiniihas been previously reported for strains in Australia and Brazil(Murray et al., 2004). Here, we found a large genetic differentiation of LSU rRNA sequences inA.steinii(Fig.12).Amphidiniumsteiniiwas also recorded in Wismar, Germany (Stein, 1883), and New Jersey and Maryland, USA (Thompson, 1951; Barlow and Triemer, 1988), but molecular sequences are not yet available. Additional strains ofA.steiniifrom different locations are needed for a thorough examination of the genetic variation within this species.
4.1.7Amphidiniumthermaeum
The ChineseA.thermaeumstrain WZD07 fits the original description regarding the plastic cell shape(metabolic movement), a pusule surrounded by small radiating vesicles, and asexual division within hyaline cysts (Dolapsakis and Economou-Amilli, 2009). Two pusules were reported in the original description ofA.thermaeum(Dolapsakis and Economou-Amilli,2009), but only one pusule was observed in Chinese strain WZD07. No pusules were observed in Dominican Republic strains (Karafas et al., 2017),suggesting that the number of pusules is also plastic.Very rarely, distorted cells may appear with furrows(striations or longitudinal cleave) on the hypocone in the original description (Dolapsakis and Economou-Amilli, 2009), but they were not observed in Chinese strains. Such furrows were also recorded in dividing cells ofA.tomasii(Karafas et al., 2017). This indicates that such furrows inAmphidiniumspecies are probably not common and may be restricted to certain life cycle stages. Metabolic movement has been reported inA.theodori,A.fijiensis,A.steinii, andA.thermaeum(Murray et al., 2004; Dolapsakis and Economou-Amilli, 2009; Karafas et al., 2017).Metabolic movement was observed in only a few cells in ChineseA.thermaeum, suggesting that this characteristic may only occur during certain life stages, such as the sexual stage in the life cycle(Murray et al., 2004; Karafas et al., 2017).Dinoflagellates display organellar evidence of secondary endosymbiotic events (Gould, 2012), as well as providing the basal position forAmphidiniumin the dinoflagellate evolutionary lineage (Zhang et al., 2007). The metabolic movement ability ofAmphidiniumpossibly indicates a constant relation between the ancestors of dinoflagellates and other cell plastic protist lineages.
4.1.8Amphidiniumtheodori
The ChineseA.theodoristrain 3XS55 fits the original description regarding the anterior 1/3 longitudinal flagellum insertion, a central pyrenoid,the poster located nucleus, plastic shape (metabolic movement), and asexual division within hyaline cysts(Karafas et al., 2017). One noticeable difference is related to the cell size, as cells of ChineseA.theodoristrain 3XS55 were about twice larger than the type species (length: 28.0 μm vs. 16.6 μm, width: 24.9 μm vs. 12.6 μm) (Karafas et al., 2017). In addition, ringlike scales were not observed on the surface of the ChineseA.theodoristrain 3XS55, possibly due to improper fixation.Amphidiniumtheodoriwas previously reported in Korotoga Fiji (the South Pacific), and U.S. Virgin Islands (the North Atlantic)(Karafas et al., 2017), here we extend its distribution to Xisha Islands (the North Pacific). All of these three spots are located between the tropic of cancer,implying that the distribution ofA.theodorimay be limited to the tropical zone.
4.1.9Amphidiniumtomasii
The Chinese strain ofA.tomasiiwas characterized by an oval to elliptical shape, curved and rounded epicone, the insertion of the longitudinal flagellum in the middle third of the cell, and binary fission in the motile cell, which fits the original description (Karafas et al., 2017). A longitudinal cleave was observed on the dorsal hypocone of the division cell but not in the vegetable cell in the original description (Karafas et al., 2017). In Chinese strains no longitudinal cleave was observed in either the division cells or vegetable cells. A similar structure has been reported in the vegetable cells ofA.thermaeumandA.klebsii(Klebs,1884; Dolapsakis and Economou-Amilli, 2009). The furrows (longitudinal cleave) may only appear at a specific point in the life cycle and their taxonomic significance needs to be validated (Karafas et al.,2017; this study).Amphidiniumtomasiiwas previously only reported in the northwest Atlantic(Dominican Republic, Puerto Rico, and Florida) and New Zealand (Karafas et al., 2017), but here we extend its distribution to the South China Sea.
4.2 Pigment profile
The typical chloroplasts of dinoflagellates possess peridinin, chlorophyllsc2and chlorophyllaas major pigments (Zapata et al., 2012). This type of chloroplast is considered to have originated from red alga via a secondary endosymbiosis (red alga get their chloroplast from cyanobacterium at the primary endosymbiosis) (Schnepf and ElbräChter, 1999;Zhang et al., 1999; Gould, 2012). About half of dinoflagellates have lost their chloroplasts and have become colorless during their evolution (Cavalier-Smith, 1992; Saldarriaga et al., 2004; Moestrup and Daugbjerg, 2007). In addition, a small number of dinoflagellates have replaced the peridinin-containing chloroplast with fucoxanthin-containing chloroplast,which originated from haptophyte alga, via the tertiary endosymbiosis (Morden and Sherwood, 2002;Yoon et al., 2002; Hackett et al., 2004; Curtis et al.,2012; Gould, 2012). According to Zapata et al. (2012),six pigment composition groups are present in photosynthetic dinoflagellate species. In general,most of the dinoflagellates can be classed as peridininand fucoxanthin-containing, while the others are alloxanthin-containing or violaxanthin-containing.All of theAmphidiniumstrains are peridinincontaining and were classed as “chloroplast Type 1”,which contain chlorophyllsaandc2and peridinin as the major photosynthetic pigments (Table 3).
Sand-dwelling benthic dinoflagellates produce a far greater diversity of pigments than planktonic forms (Yamada et al., 2015). The finding of pheophorbide and pheophorbideapigments in all testedAmphidiniumstrains is unexpected.Pheophorbides are well-known photosensitizers that are used in photodynamic therapy (Dougherty et al.,1998; Dolmans et al., 2003). Pheophorbides are a cancer treatment modality that selectively destroys malignant lesions through a photochemical reaction activated by light of the appropriate wavelength(Dougherty et al., 1998; Dolmans et al., 2003). In particular, pheophorbidea, the dephytylation and demetallation product of chlorophylla, is formed in both algae and higher plants (Takamiya et al., 2000).This catabolic transformation is mediated by chlorophyllase and Mg-dechelatase enzymes (Matile and Schellenberg, 1996; Rodoni et al., 1997).Pheophorbideabased photodynamic therapy has been shown to completely inhibit the growth of a viral-induced hepatoma cell line Hep 3B (Chan et al.,2006). The cell-free extract of someAmphidiniumstrains has been reported to exhibit antifungal and antimicrobial properties (Echigoya et al., 2005; Kong et al., 2013; Nuzzo et al., 2014), whereas others have shown strong cytotoxic activity against a human tumor cell line (Bauer et al., 1995; Takahashi et al.,2007; Kumagai et al., 2017). Many polyketide bioactive compounds, such as caribenolide and amphidinolides, have been isolated from the cell-free extract (Bauer et al., 1995; Echigoya et al., 2005), but the pheophorbide / pheophorbidearatio has not been well studied. SomeAmphidiniumstrains, especially species identified asA.carteraeandA.operculatum,have higher growth rates and are easily grown in culture (Murray et al., 2004). The high concentration of pheophorbide found inAmphidiniumimplies that it may be a good candidate as a natural source of photosensitizers.
5 CONCLUSION
Field surveys were undertaken to investigate the diversity ofAmphidiniumin shallow waters of the China Sea from 2012 to 2020. Eleven species were identified:A.carterae,A.gibbosum,A.operculatum,A.massartii,A. cf.massartii,A.fijiensis,A.pseudomassartii,A.steinii,A.thermaeum,A.tomasii, and an unidentified species. The last seven species have not been previously reported in Chinese waters. In addition, two strains were attributed toA.trullabased on LSU rRNA sequences. LSU rRNA sequences-based phylogeny revealed two groups(Groups I and Ⅱ) withinAmphidinium, which were consistent with their sulcus in touch with cingulum or not. High pheophorbide / pheophorbidearatio was detected inAmphidiniumimplies that it may be a good candidate as a natural source of photosensitizers,which is a well-known anticancer drug.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Typhoon-induced wind waves in the northern East China Sea during two typhoon events: the impact of wind field and wave-current interaction*
- Effect of subsea dispersant application on deepwater oil spill in the South China Sea*
- Geochemical characteristics of cold-seep carbonates in Shenhu area, South China Sea*
- Examination of seasonal variation of the equatorial undercurrent termination in the Eastern Pacific diagnosed by ECCO2*
- Deviation of the Lagrangian particle tracing method in the evaluation of the Southern Hemisphere annual subduction rate*
- Immunostimulatory effect of quaternary degree and acetyl group of quaternized chitosan on macrophages RAW 264.7*
