Cytological and transcriptional analysis reveal phosphatidylinositol signaling pathway plays key role in mitotic division of Pyropia yezoensis*
Yunke ZHU , Xinran WANG , Bin SUN , Xianghai TANG ,**, Yunxiang MAO
1 Key Laboratory of Marine Genetics and Breeding (Ministry of Education), College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
2 MOE Key Laboratory of Utilization and Conservation for Tropical Marine Bioresources, College of Fisheries and Life Science,Hainan Tropical Ocean University, Sanya 572022, China
3 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
Abstract The phosphatidylinositol (PI) signaling system, a central regulator of eukaryotic metabolism,is widely found in eukaryotes for regulating a variety of cell activities. Most of the genes in the PI signaling system were found conserved in Pyropia yezoensis. In this experiment, wortmannin was used as an inhibitor to inhibit the activity of phosphatidylinositol-3 kinase ( PI3K), an important regulator of the PI signaling system. After wortmannin treatment, the mitotic division of P. yezoensis was significantly inhibited in a dose-dependent manner, and the mitotic division percentage was reduced by 68.1% and 91.9% in the 5- and 10-μmol/L groups, respectively. When thalli were treated with wortmannin, the levels of reactive oxygen species (ROS) were significantly decreased. Furthermore, the expression level of PI3K was inhibited and the expression levels of downstream genes regulated by PI3K was significantly changed. In the PI3KAGC signaling pathway, the expression levels of Serine/threonine protein kinase ( AGC) and cyclindependent kinases A ( CDKA) were downregulated, while WEE1 kinase gene ( WEE1) was upregulated.Three nicotinamide adenine dinucleotide phosphate (NADPH) oxidase genes were downregulated after wortmannin treatment. These results indicate that the PI signaling system plays an important role in the regulation of cell activity in P. yezoensis. It was speculated that the growth and development of P. yezoensis might be regulated by P. yezoensis PI3K, which promoted the expression of the AGC gene and further regulates the expression of downstream WEE1 and CDKA genes to advance mitotic division, and also promoted the expression level of NADPH oxidase that regulates ROS homeostasis.
Keyword: Pyropia yezoensis; phosphatidylinositol signaling system; reactive oxygen species; mitotic division
1 INTRODUCTION
Similar to other eukaryotes, the plant phosphatidylinositol (PI) signaling system is a complex network that participates in the regulation of key physiological processes essential to plant function and development. At present, inositol phospholipids,inositol phosphates, and related enzymes of the PI signaling system have been identified in plant cell nuclei (Dieck et al., 2012). Inositol 1,4,5-trisphosphate(InsP3), regulated by inositol polyphosphate 5-phosphatase, can induce the release of calcium ions and is crucial for maintaining pollen dormancy,regulating the early germination of pollen, and closure of stomata (Wang et al., 2012). In addition, evidence showed that inositol polyphosphate 5-phosphatase participated in the control of cell division direction,cell wall pectin deposition, and actin organization,essential for the formation of internode meristems(Sato-Izawa et al., 2012). The PI signaling system also plays a role in the regulation of DNA synthesis.In soybean cells, the inhibitory effect of the chemical inhibitor U-73122 on phosphoinositide-specific phospholipase C (PI-PLC) activity reduces InsP3content, resulting in a reduction in the DNA synthesis rate by more than 30% (Shigaki and Bhattacharyya,2002). InArabidopsis, phosphatidylinositol-4-phosphate 5-kinase (PIP5K) is highly expressed in specialized meristems and is involved in the control of cell proliferation, similar to that in animals and yeast (Elge et al., 2001).
Previous studies have shown that phosphatidylinositol-3 kinase (PI3K) plays an important role in cell cycle regulation, development,and signal transduction by participating in endocytosis,reactive oxygen species (ROS) production, and transcriptional activity (Marqués et al., 2009; Lee et al., 2010; Campa et al., 2015). InChlamydomonasreinhardtii, the inhibition or knockdown ofPI3Ksignificantly decreased the cell growth, changed the cell morphology, and increased the fat and starch contents (Ramanan et al., 2018). In wheat,PI3Kinhibitor treatment could inhibit the root growth of germinated seeds, reduce the mitotic index, and affect the structure and function of the meristemic nucleolus(Ni and Zhang, 2014). In animals, the role ofPI3Kin regulating the timing of cell division through the cell cycle progression rate has been widely confirmed(Alvarez et al., 2003; Dangi et al., 2003; Campa et al.,2015).PI3K-dependent signaling regulates mammalian cell proliferation by promoting the G1/S and G2/M phases of the cell cycle. The inhibition ofPI3Kdecreased the cyclinB1 expression, cell division cycle 2 (CDC2) activity, and the mitotic index (Dangi et al., 2003). Studies have shown that thePI3K-AGCpathway positively regulated the cell cycle at the G2/M boundary, which might be accomplished by regulating the expression of WEE1 kinase gene(WEE1) and the modification ofCDC2(Katayama et al., 2005; Wu et al., 2018; Xu et al., 2018). Furthermore,PI3Kparticipated in the production of ROS by producing phosphatidylinositol 3-phosphate (PtdIns(3)P) (Joo et al., 2005). As the second messenger of plant hormone signals, ROS regulates a variety of biological processes, such as seed germination, seedling growth,root gravitation, apical growth, and programmed cell death (Garg and Manchanda, 2009; Hakeem et al.,2014). Changes in ROS distribution could alter the stability of PLETHORA2 (PLT2) protein, thereby regulating the root stem cell niche (Yamada et al.,2020). Nicotinamide adenine dinucleotide phosphate(NADPH) oxidase is the primary source of ROS production, and could catalyzes the production of O2-and regulates a wide range of biological functions in different organisms (Kaur and Pati, 2016). NADPH oxidase deficiency inArabidopsisthalianaroot hair defective 2 (rhd2) mutant impaired cell expansion,and the resulting mutant has short root hair and dysplastic roots (Foreman et al., 2003). In plants,PI3Khas been demonstrated to change the quantity of reactive oxygen species by regulating the expression and activity of NADPH oxidase (Lee et al., 2008; Liu et al., 2012; Livanos et al., 2012).
Pyropiayezoensisis a representative species of Bangiales (Rhodophyta), which grows naturally in the intertidal zone. Some species ofPyropiahave been industrially cultivated on a large scale because of their high economic value, particularly along the East Asian coast (Blouin et al., 2011). The analysis of the growth,development, and environmental adaptation ofP.yezoensiswill help in the development of methods to increase its yield, allowing for greater economic benefits. InP.yezoensis,PI3Kparticipates in the asymmetric migration of F-actin, which is necessary for the establishment of cell polarity (Li et al., 2008).The role of ROS in biological and abiotic responses inP.yezoensishas been demonstrated (Tang et al., 2019).
PI3Kmay play an important role in regulating cell division and ROS production inP.yezoensis, but this aspect has not yet been extensively investigated. In this study, the thalli ofP.yezoensiswere treated with wortmannin, which has been widely used to studyPI3Kfunction in seed germination, root growth, and vesicle transport. Subsequently, the cell status, mitotic division, and ROS levels of the thalli were evaluated.The expression changes of thePI3K, Serine/threonine protein kinase (AGC),WEE1,CDKA(CDC2homologous gene), and 4 NADPH oxidase genes after wortmannin treatment, were also detected. The importance of the PI signaling pathway in regulating mitotic division and ROS homeostasis inP.yezoensisis discussed in detail.
2 MATERIAL AND METHOD
2.1 Materials and reagent
A lab-cultured pure line RiZhai (RZ) ofP.yezoensiswas used for experiment. Gametophytic thalli(approximately 5 cm) were aerobically cultivated in 500-mL flasks containing sterile seawater with PES(Provasoli’s Enriched Seawater Medium/Provasoli’s ES Medium) medium at 10 °C, photoperiod 12-h L∶12-h D, and a light intensity of 40 μmol photons/(m2·s). The culture medium was renewed every 3 d during the cultivation process.
Wortmannin is a fungal metabolite that is used as a highly selectivePI3Kinhibitor. It has no inhibitory effect on Ptdlns-4-kinase, protein kinase C, or phosphoinositide-specific phospholipase C (PI-PLC)(Powis et al., 1994). At present, wortmannin is widely used in the study ofPI3Kfunction in seed germination,root growth, and vesicle transport.
5-ethynyl-20-deoxyuridine (EdU) is a nucleotide analogue of thymidine that is incorporated into DNA during the S-phase of DNA synthesis (Fujinami et al.,2017). In the present study, the iClick™ EdU Alexa Fluor™ 488 Imaging Kit was used to characterize the percentage of cell division.
Similarly, ROS were detected using an ROS assay kit (S0033S; Beyotime). As a fluorescent probe,2,7-dichlorodi-hydrofluorescein diacetate (DCFHDA) has no fluorescence itself and can freely cross the cell membrane because ofits extremely strong lipophilicity. After entering the cell, it is hydrolyzed by esterase and forms polar dichlorofluorescin(DCFH), which is oxidized by ROS to dichlorofluorescein (DCF), which cannot penetrate the cell membrane, and has strong green fluorescence.DCF formation was directly proportional to the level of intracellular ROS. Thus, the fluorescence intensity of DCF, detected by fluorescence microscopy,represents the level of intracellular ROS (Rastogi et al., 2010; Aranda et al., 2013).
2.2 Identification and analysis of PI signaling system genes and NADPH oxidase genes in P. yezoensis
The genome ofP.yezoensiswas used as the reference genome (DDBJ/ENA/GenBank:GCA_009829735.1). Gene annotation of the genes mentioned in this article was generated according to genomic annotation results of the pure line RZ ofP.yezoensis(Wang et al., 2020). Software Snapgene was used to predict the relative molecular weights(Mw) and isoelectric points (pI) of the PI3K protein.The secondary structure of the PI3K protein was predicted using the NPS online service (http://npsapbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html). The homologous protein sequences of thePI3Kgene in other species were searched and downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/). Software ClustalX was used to perform multiple sequence alignments using the full-length amino acid sequence of thePI3Kgene. The maximum likelihood (ML) tree was constructed using the Poisson model with the MEGA-X tool, and 1 000 bootstrap replications were used to assess the reliability of the branches. The conserved domains of 11 NADPH oxidase proteins were searched using the NCBI Conserved Domains Database (CDD) Batch search (https://www.ncbi.nlm.nih.gov/), and TBtools were used for visual analysis (Chen et al., 2020).PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to predict and analyze the action elements in the promoter region 2 000 bp before the transcription start site of 11 NADPH oxidase genes.
2.3 Cell status and viability detection
Thalli were placed in a 24-well plate. Sterile seawater (2 mL) supplemented with PES was added to each well, and the concentrations of wortmannin were adjusted to 0, 5, and 10 μmol/L, and cultured for 3 d. After 3 d, cell morphology was observed using bright field microscopy, and cell autofluorescence was observed at an excitation light of 450-490 nm,528-553 nm, and 330-380 nm using a fluorescence microscope. The photosynthetic parameters of thalli were detected using the chlorophyll fluorescence imaging system FluorCAM MF800 and were analyzed by one-way analysis of variance (ANOVA) using SPSS software. After staining with 0.04% trypan blue dye solution (dissolved in sterile seawater) for 3 min,cell viability was detected using a microscope.
2.4 Mitotic division measure
Thalli were cultured in a 24-well plate, and the wortmannin concentrations were adjusted to 0, 5, and 10 μmol/L. Then, 20-μmol/L EdU, a thymine analogue, was added and the cells were cultured for 4 d. Immobilization, penetration, and staining were performed according to the iClick™ EdU Alexa Fluor™ 488 Imaging Kit instructions. Mitotic division was observed under 488-nm excitation light using a fluorescence microscope. Three random fields in the middle part of the thalli were selected for counting,and each concentration contained three biological replicates. Mitotic division percentage was calculated as follows:
Mitotic division percentage=(EdU-labelled cells/total number of cells)×100%.
Software Imaris was used for cell counting, and software Photoshop CC was used for image processing. Mitotic division percentage was analyzed by ANOVA using software SPSS.
2.5 Reactive oxygen species assay
ROS in the thalli were detected using an ROS assay kit (S0033S; Beyotime). The procedure for ROS detection in situ was as follows: thalli were cultured in 24-well plates with 0-, 5-, and 10-μmol/L wortmannin for three days. Other samples were treated with 5- and 10-μmol/L wortmannin for three days, then recovered under normal culture conditions for another three days. DCFH-DA (25 μmol/L) was added to the culture and incubated at 10 °C for 1 h.Then, the cells were washed three times with sterile seawater to fully remove the DCFH-DA that did not enter the cells. ROS staining fluorescence was observed under 488-nm excitation light using a fluorescence microscope. Three fields in the middle of the thalli were randomly selected for each sample,and three biological replicates were prepared for each concentration.
2.6 Expression of PI3K- AGC pathway-related genes and NADPH oxidase genes
Thalli were collected after being treated with 0-, 5-,and 10-μmol/L wortmannin for 3 d in 500-mL flasks,and total RNA was extracted using a Plant RNA Kit(Omega, USA) after pre-cooling and grinding with liquid nitrogen. After the genomic DNA was removed,1 μg of total RNA was used for reverse transcription using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) kit (TaKaRa), according to the manufacturer’s protocol. Primers were designed according to the coding sequence (CDS) sequences ofPI3K-AGCpathway-related genes (PI3K,AGC,WEE1kinase, andCDKA) and NADPH oxidase genes, using Oligo7 software (Table 1). After the PCR amplification of cDNA, the amplified fragment was transduced into the recombinant plasmid. The recombinant plasmids were sent to the Tsingke Company (Qingdao, China) for sequencing, and the sequencing results were compared using Snapgene software. The amplification efficiency and specificity of the primers were evaluated using a standard curve and a melting curve.

Table 1 List of primers used for quantitative PCR
ChamQTM SYBR Color qPCR Master Mix(Vazyme, China) was used for real-time quantitative PCR (qRT-PCR). The amplification system was used according to the instructions, and the PCR conditions were as follows: 95 °C for 1 min, 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 15 s. The amplification efficiency of qRT-PCR was detected by at least five serial ten-fold dilutions of plasmids containing the target cDNA. The reaction system was optimized to ensure that the melting curve had a single peak, and the primer amplification efficiency was between 95% and 105%. Then, 0-μmol/L wortmannin-treatment samples were used as the standard. Each treatment had three biological and three technical replicates. Under the correction of the internal reference gene (pyUBC), the relative expression of the target gene was calculated by the2-ΔΔCtmethod and was analyzed by ANOVA using software SPSS.
3 RESULT
3.1 Phosphatidylinositol signalling-related genes in P. yezoensis
After a genome-wide survey and RefSeq nonredundant proteins (NR) database annotation,homologs relative to almost all PI signaling genes were found in the genome ofP.yezoensis, indicating that the PI signaling pathway could be conserved in different eukaryotic kingdoms (Table 2; Fig.1).
The PI3K protein contained 868 amino acids with a deduced molecular weight (Mw) of 93.4 kDa and an isoelectric point (pI) of 7.21. Secondary structure prediction showed that this protein contained 39.75%alpha helix, 6.91% beta turn, and 34.79% random coils based on bioinformatics analysis. A phylogenetic tree based on thePI3Kgene was constructed using MEGA-X with the ML method based on thePI3Kgene amino acid sequence. The phylogenetic tree consisted of three large branches. The first branch contained two small clusters, the first of which was made up of fungi(such asSaccharomycescerevisiaeandFusariumoxysporum), and the second was made up of higher plants (such asArabidopsisthalianaandBrassicanapus) and green algae (e.g.,Coccomyxasubellipsoidea,Pycnococcusprovasolii). In the second branch,P.yezoensisand red algaeGracilariopsischordahad the highest homology and the closest evolutionary relationship. They gathered into a small cluster with the red algaePorphyridiumpurpureumandPyropiahaitanensis, and another small cluster composed of brown algaeAureococcusanophagefferens, golden algaeEmilianiahuxleyiCCMP1516, and green algaeKlebsormidiumnitens. The third branch of the phylogenetic tree was prokaryotic bacteria (e.g.,Parcubacteria group bacterium GW2011_GWA2_42_14 andRhodovulumsulfidophilum), which had the longest evolutionary distance. In addition, thePI3Kof red algae was found to be more evolutionarily independent and may have its own characteristics (Fig.2).
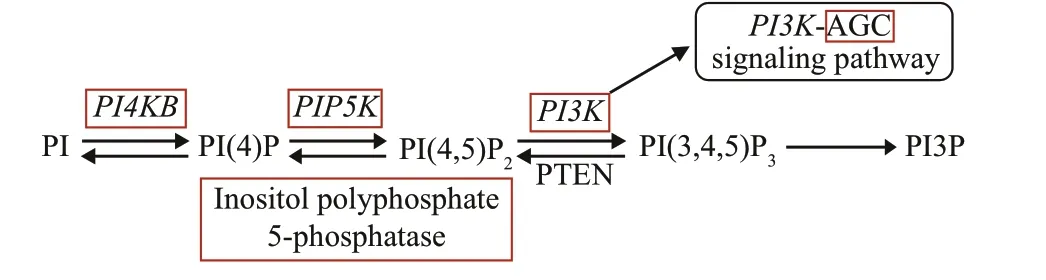
Fig.1 Pathway of phosphatidylinositol signaling system
3.2 NADPH oxidase genes and its cis-acting elements in P. yezoensis
In total, 11 NADPH oxidase genes were identified,of which 10 contained the NOX_Duox_like_FAD_NADP motif (Fig.3a), indicating that this motif plays an important role in the function of NADPH oxidase.In the action elements predicted in the promoter region of NADPH oxidase genes through the PlantCARE website, 1 927 cis-acting elements were obtained, of which 705 had clear functional annotations and could be divided into 18 different functional types. Among them, cis-acting elements related to stress response were the most common,accounting for 47.8%, and those related to hormone regulation accounted for 29.5% (Fig.3b). A total of 66 G-boxes and 9 W-boxes were identified. In addition,all 11 NADPH oxidase genes contained the 108 GCmotif, which is related to oxidative stress (Kaur and Pati, 2016).
3.3 Cell status and mitotic division measure
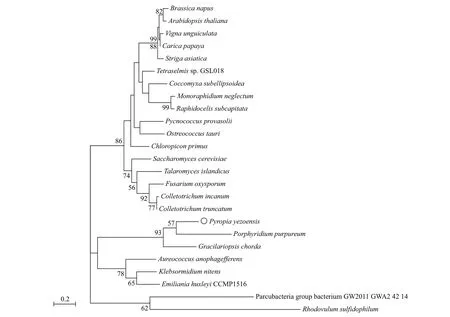
Fig.2 Maximum likelihood method tree based on PI3K amino acid
At concentrations of 5- and 10-μmol/L wortmannin,the cell morphology and auto fluorescence ofP.yezoensisthalli did not change noticeably (Fig.4a).Chlorophyll fluorescence imaging showed that there was no significant difference in theFv/Fmvalues among the three groups (P>0.05) (Fig.4b).Fv/Fmreflects the maximum photosynthetic potential of plants, which is known to decreases under stress(Maxwell and Johnson, 2000). In addition, no cell death was detected with 0.04% trypan blue (Fig.4a &c). The results indicated that wortmannin at the two concentrations did not change the morphology and photosynthetic physiology of the thalli nor caused the death of the thalli. Therefore, wortmannin at these concentrations could be used to study the function of PI3K in this experiment.
After 4 d of culture, significant differences were observed in the mitotic division rate ofP.yezoensisin different wortmannin treatment groups (P<0.05).Mitotic division percentage was calculated as follows:
Mitotic division percentage=(EdU-labelled cells/total number of cells)×100%.
The mitotic division percentage was 3.85%±0.64%in the 0-μmol/L group (Fig.5a & b), 1.23%±0.35% in the 5-μmol/L group (Fig.5c & d), and 0.31%±0.13%in the 10-μmol/L group (Fig.5e & f), suggesting that wortmannin inhibited mitotic division.
3.4 Reactive oxygen species detection
As shown in Fig.6, compared with the control group (Fig.6b), the ROS content decreased in the 5-and 10-μmol/L wortmannin-treated groups (Fig.6c &d), and increased after three days of recovery culture in normal seawater (Fig.6e & f). These results indicate thatPI3Keffectively regulated ROS production.
3.5 Expression levels detection of PI3K- AGC pathway-related genes and NADPH oxidase genes
After 3 d of wortmannin treatment, qRT-PCR was performed and the results showed that wortmannin effectively inhibited the expression of PI-related genes.As shown in Fig.7, after treatment with wortmannin,the expression levels of thePI3Kgene ( py05971. t1)andAGCgene ( py04695. t1) decreased significantly in a dosage-dependent manner, thereby upregulating the expression ofWEE1( py03205. t1), enhancing the inhibitory effect ofWEE1on cyclin-dependent kinase A activity (CDKA, py09517. t1). The expression level of theCDKAgene decreased (Fig.7). Four NADPH oxidase genes with high expression under normal culture conditions were selected for verification of gene expression. Among them, py00905. t1 did not change significantly, whereas the expression of py02900. t1, py02269. t1, and py07183. t1 decreased with an increase in the inhibitor concentration (Fig.7).
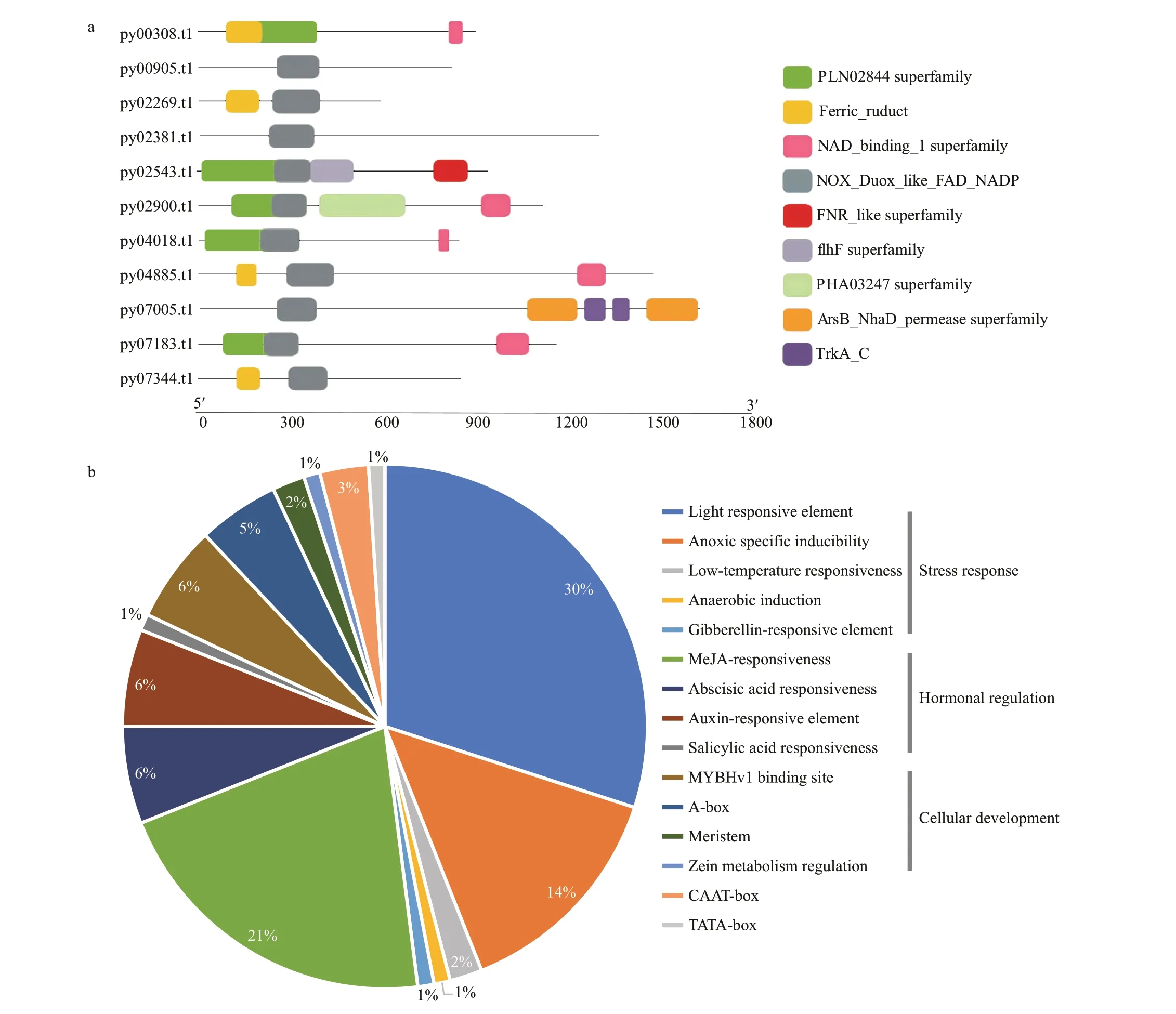
Fig.3 Bioanalysis of NADPH oxidase genes
4 DISCUSSION
As one of the central regulators of eukaryotic metabolism, the PI signaling system is widely present in eukaryotes (Heilmann, 2009). In animals and yeast,nuclear phosphoinositide regulates many processes,including vesicle transport, nuclear size, nuclear membrane reorganization, chromatin structure, DNA replication, DNA repair, transcription factor localization, transcription, RNA splicing, mRNA transport, and cell cycle progression (Dieck et al.,2012; Heilmann, 2016; Snider et al., 2017). In mammals, the PI signaling system plays a role in the orientation of the mitotic spindle and is involved in anaphase cell elongation (Cauvin and Echard, 2015).PI system enzymes have been identified in plant nuclei and are involved in DNA replication, chromatin remodeling, stress response, and hormone signaling(Dieck et al., 2012). Almost all homologous genes related to the PI signaling system were discovered inP.yezoensisthrough this experiment, which indicated that the PI signaling pathway could be conserved in different eukaryotic kingdoms.
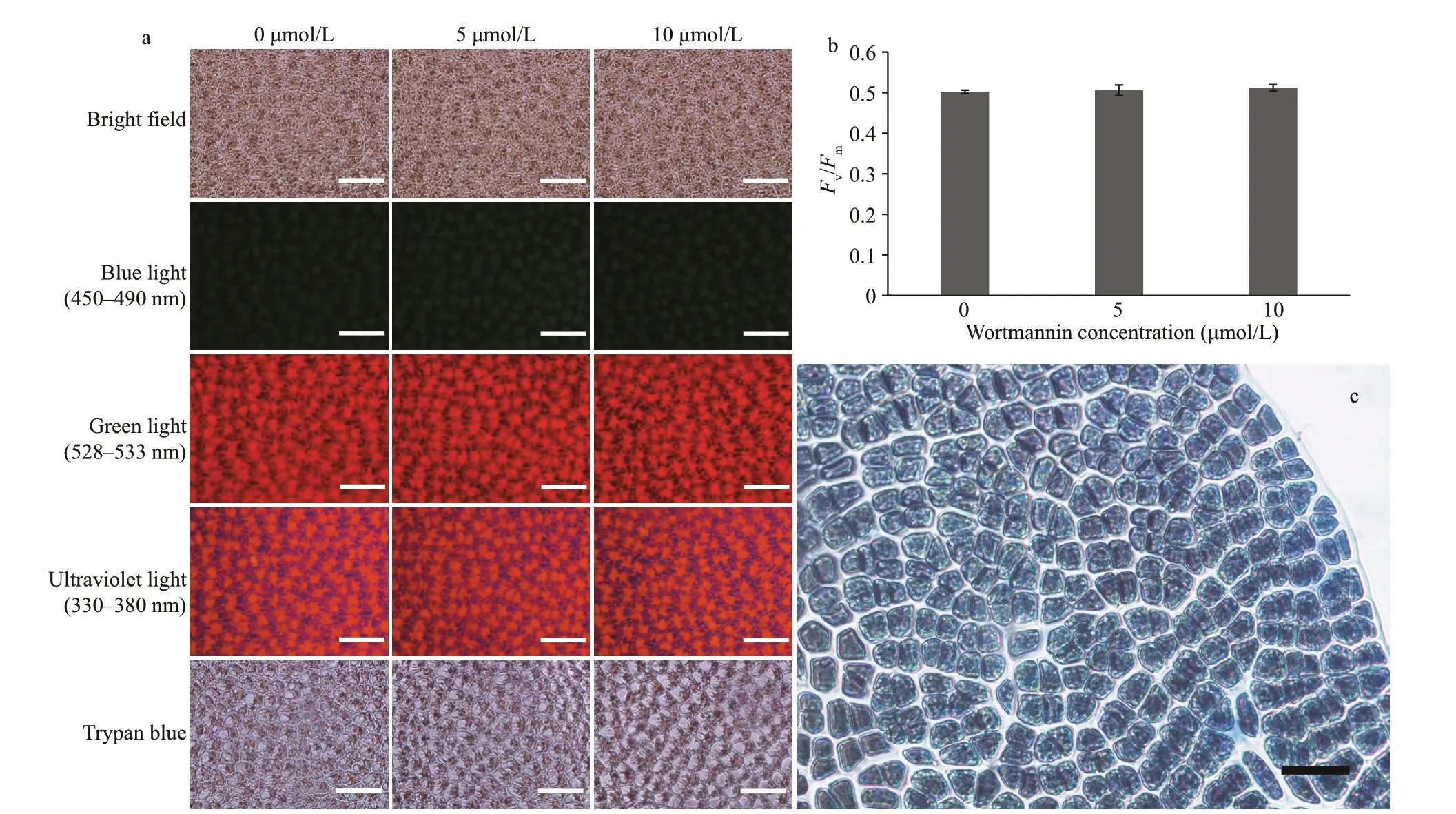
Fig.4 Cell state and viability under the treatment of different concentrations of wortmannin for three days
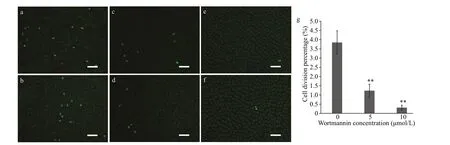
Fig.5 Mitotic division percentage in P. yezoensis treated with different concentrations (5 and 10 μmol/L) of wortmannin after EdU labelling for four days
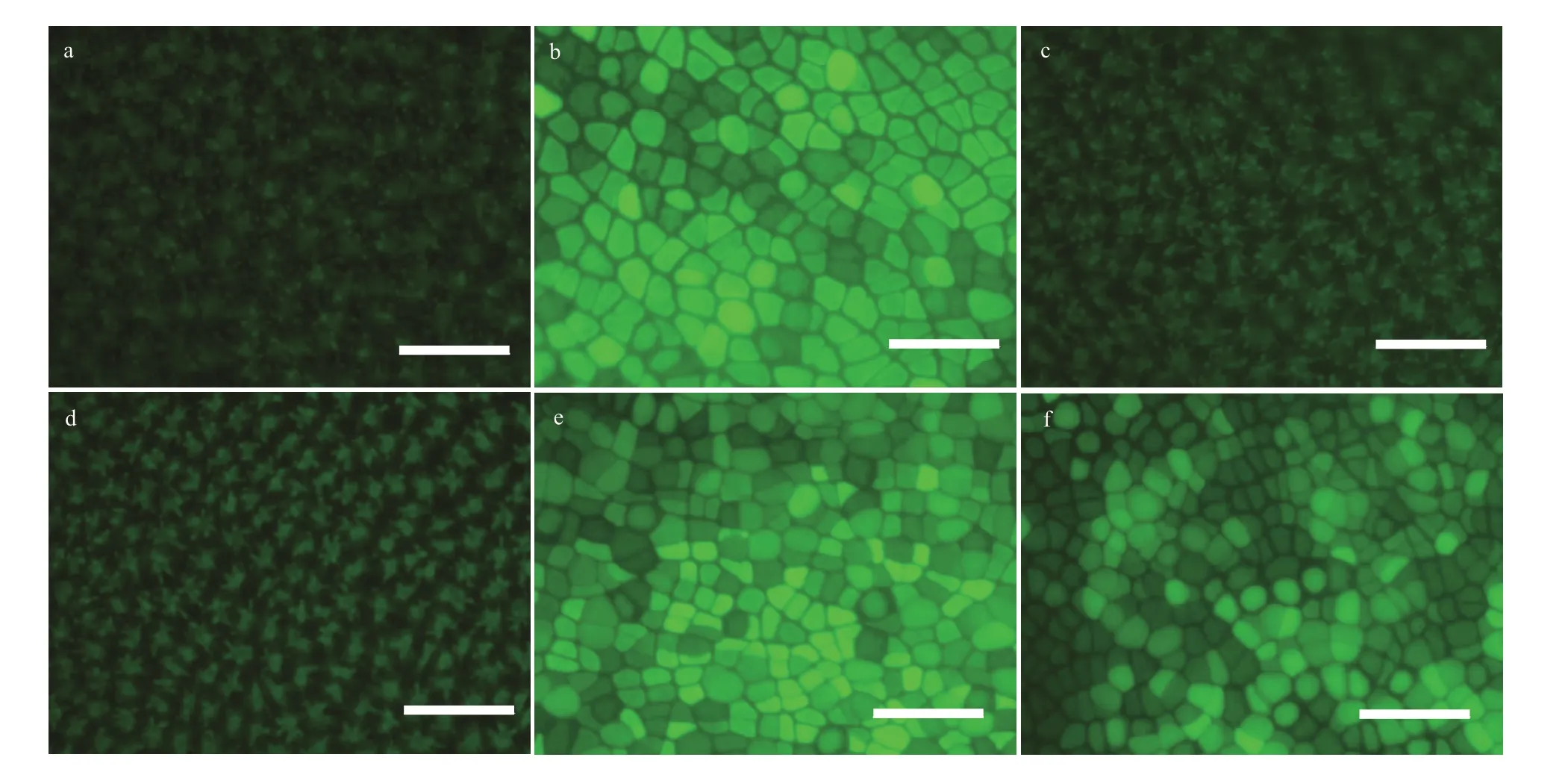
Fig.6 Content of ROS in P. yezoensis thalli that was wortmannin-treated for three days and recovered for another three days
PI3Kis involved in cell growth, morphogenesis,energy metabolism, vesicle transport, ROS regulation,and environmental adaptation in plants and algae (Liu et al., 2012; Cao et al., 2017). Therefore,PI3Kis considered essential for the growth and development of eukaryotes; however, the importance ofPI3Khas not yet been elucidated inP.yezoensis. To provide direct evidence for the role ofPI3Kin mitotic division during the growth and development ofP.yezoensis,wortmannin was used to treat the thalli. As a result,the mitotic division ofP.yezoensiswas found to be significantly inhibited in a dose-dependent manner: a dose of 5-μmol/L wortmannin reduced the mitotic division percentage by 68.1%, while 10-μmol/L wortmannin reduced the mitotic division percentage by 91.9%. This result is in agreement with previous findings, where the treatment of sea urchin eggs with wortmannin was found to inhibit the activation of maturation promoting factor (MPF) and interfere with centrosome replication, thereby preventing mitotic spindle formation and resulting in cell cycle arrest(De Nadai et al., 1998). Another study found that wortmannin plays an important role in regulating nucleolus structure and function during wheat seed germination and inhibits wheat root growth and cell division (Ni and Zhang, 2014). To determine the precise mechanism underlying the decline in mitotic division, the expression ofPI3K-AGCpathwayrelated genes was investigated, which have been previously shown to positively regulate the cell cycle(Wu et al., 2018). In this experiment, the inhibition ofPI3Kled to a decrease in downstreamAGCgene expression, weakened the inhibitory effect ofAGConWEE1, and upregulated the expression ofWEE1.Subsequently, the inhibitory effect ofWEE1onCDKAactivity was enhanced. The expression ofCDC2homologous geneCDKAdecreased. This could account for the decrease in the mitotic division percentage. The existing results only reflect the effect of inhibitors on gene transcription level, and the reason for other changes in gene expression under the action of wortmannin needs to be investigated further.Even so, the effect ofPI3Kon mitotic division inP.yezoensishas been preliminarily established.
In this study, when the thalli ofP.yezoensiswere treated with wortmannin, a specific inhibitor ofPI3K,the ROS content was suppressed. ROS control and regulate a variety of biological processes, including growth, cell cycle, and development (Garg and Manchanda, 2009). Therefore, it is believed thatPI3Kstimulates the production of ROS and participates in the mechanism of ROS balance, thus acting on the growth and development ofP.yezoensis. The release of ROS and its production sites depend on signal mechanisms, which involve the activity of ciselements present in the upstream region of the target genes (such as NADPH oxidase genes) (Kaur and Pati, 2016). InP.yezoensis, the analysis of the upstream elements of NADPH oxidase genes revealed a total of 66 G-boxes and 9 W-boxes. Common components related to ROS include GCN4, TACCA/T-motif, and G-box, while the W-box is one of the main elements of ROS (Kaur and Pati, 2016). The G-box element (CACGTG) is widely distributed among plant genes and is known to participate in hormone signalling and play a role in light- and abscisic acid-mediated responses. Light and abscisic acid are related to many physiological and developmental processes, including seed germination and seedling development (Seo et al., 2006). The W-box interacts with members of the WRKY transcription factor family, which is known to play a role in biotic and abiotic stress, leaf senescence, and seed development (Rushton et al., 2010). Therefore,NADPH oxidase gene expression could be regulated by the G-box and W-box elements in the growth and development ofP.yezoensis. Taken together, these results suggest thatPI3Kplays a role in ROS production by influencing the expression of NADPH oxidase genes inP.yezoensis. In plants, the hypothesis thatPI3Kactivates NADPH oxidase by regulating the transport of RAC1 protein from the cytoplasm to the plasma membrane has been proposed previously (Liu et al., 2012). However, the mechanism by whichPI3Kregulates the expression and activity of NADPH oxidase inP.yezoensisrequires further investigation.
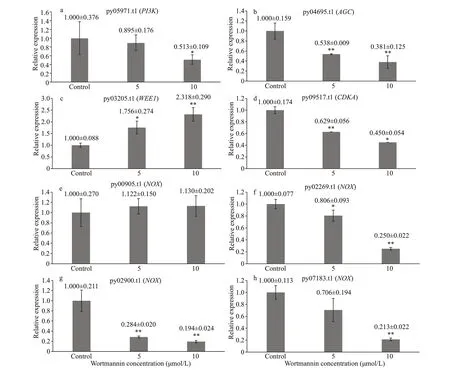
Fig.7 Expression changing of PI-related genes after wortmannin treatment for three days
5 CONCLUSION
PI3Kwas conserved inP.yezoensisand functioned in similar to those in other animals and plants,showing dual effects in regulating cell division and determining ROS levels. In addition, the PI signaling system played an important role in growth and development ofP.yezoensis. ThePI3K-AGCpathway was a key factor in promoting cell division.PI3Kweakened the expression of the downstreamAGCgene, and further upregulated the expression ofWEE1.CDKAactivity was inhibited and the expression ofCDKAwas decreased, both of which led to cell cycle arrest.PI3Kalso regulated the ROS homeostasis potentially by promoting the gene expression of NADPH oxidase (NOX), which codes for key enzymes in ROS production.
However, this study was solely based on the detection of key genes at the expression level, more complex regulatory network between genes and gene products, including their regulation mechanisms, will be clarified in future studies.
6 DATA AVAILABILITY STATEMENT
The data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Erratum to: Impact of typhoon Lekima (2019) on material transport in Laizhou Bay using Lagrangian coherent structures*
- Erratum to: Chinia gen. nov.—the second diatom genus simonsenioid raphe from mangroves in Fujian, China*
- Characterization of Amphidinium (Amphidiniales,Dinophyceae) species from the China Sea based on morphological, molecular, and pigment data*
- The role of Smad6 in immunity of the pearl oyster Pinctada fucata martensii*
- Carnosine concentration and expression profiles of carnosine related genes in Mytilus after beta-alanine injection*
- Microsatellite marker development and population genetic analysis revealed high connectivity between populations of a periwinkle Littoraria sinensis (Philippi, 1847)*
