Community structure and assembly of denitrifying bacteria in epiphytic biofilms in a freshwater lake ecosystem*
Guoqing LI , Dingbo YAN , Pinhua XIA , Haipeng CAO , Tao LIN , Yin YI ,3
1 Guizhou Province Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment,Guizhou Normal University, Guiyang 550001, China
2 College of Life Sciences, Guizhou Normal University, Guiyang 550001, China
3 State Key Laboratory of Southwest Karst Mountain Biodiversity Conservation of Forestry and Grassland Administration,Guizhou Normal University, Guiyang 550001, China
Abstract Denitrifying bacteria are a crucial component of aquatic ecosystem in nitrogen cycle. However,the denitrifying bacterial community dynamics and structure in epiphytic biofilms remain unexplored.The abundance of denitrification gene ( nir) and structure of nirS-denitrifying bacterial community in the epiphytic biofilms collected in July and November of 2018 from a typical plateau lake (Caohai Wetland,Guizhou, China) were studied by Real-time Quantitative Polymerase Chain Reaction (qPCR) and highthroughput sequencing. Results show that the gene abundance of nirK was higher than that of nirS ( P <0.05),and it was significantly different during the growth period (July) than the decline period (November). The denitrifying bacterial species was similar in the two months and shared 76.18% of OTUs. Proteobacteria(56.55%±22.15%) was the dominant phylum in all the samples. Epiphytic biofilms between growth period and decline period displayed significantly different microbial community structures due to differences in species abundance. Water temperature was the crucial factor that affected the denitrifying microbial community structure in our study. Environmental factors explain only partially the dynamic characteristics of denitrifying microbial communities, implying that the stochastic processes affected the construction of denitrifying microbial communities. As the null model analysis results show, dispersal limitation (stochastic)and undominated processes significantly influenced the assembly of denitrifying microbial communities.This study broadened our understanding of the denitrifying bacterial community structure and its function on epiphytic biofilms in freshwater ecosystems with new information provided.
Keyword: denitrifying bacteria; epiphytic biofilms; community assembly; null model
1 INTRODUCTION
Nitrogen is a crucial component of living organisms and a vital nutrient for eutrophication of water bodies(Howarth, 2008). Eutrophication caused by excessive nitrogen and phosphorus from agricultural activities and urbanization has become a severe environmental issue (De-Bashan and Bashan, 2010). Submerged plants have gained a lot of attention recently as they could effectively remove nitrogen and phosphorus from eutrophic water bodies (Søndergaard et al.,2007; Han et al., 2018). Interestingly, stems and leaves surfaces of submerged plant are populated with distinct epiphytic bacteria (Flemming and Wuertz,2019). A highly diverse nitrogen metabolism mechanism related to nitrogen fixation, nitrification,denitrification, and so on observed in those bacterial communities in stems and leaves surfaces (Levi et al.,2015; Yan et al., 2019). Thus, the self-purification function of the water body can be attributed to the submerged plant-epiphytic bacteria composite system. As per the previous report, submerged macrophytes are a crucial niche for nitrogen cycling bacteria (Coci et al., 2010). Previous studies have underestimated the role of epiphytic microorganisms in the water purification function of submerged plants in artificial or natural systems. However, the underlying mechanism for community composition and structure of epiphytic denitrifying bacteria in aquatic biofilms remains mostly unexplored.
Epiphytic biofilms on the surface of the stems and leaves of submerged plants contain microbes (algae,protists, bacteria, and fungi) and biomolecules(organic, inorganic, and extracellular polymers aggregate), which appears as an adhesion layer of varying thickness (Palmer and White, 1997; Lu et al.,2016). They form a microbial symbiosis with diversified functions and collaborative divisions. The epiphytic biofilm presents an obvious gradient of dissolved oxygen for generating a typical aerobic and hypoxic environment and shaping suitable living conditions for aerobic and anaerobic microorganisms(Sand-Jensen et al., 1985; Ji et al., 2015). Thus,epiphytic biofilm has shown the potential to remove simultaneous nitrogen by aerobic nitrification and anaerobic denitrification. Ribot et al. (2012) validated the crucial role of biofilm in the nitrogen transformation process. A few research studies had demonstrated that a large number of microbes were involved in the nitrogen cycle (Kuypers et al., 2018).Those microbes with related functional genes colonized the epiphytic biofilms onPotamogetoncrispusandWolfiaaustralia(Xie et al., 2015; Yan et al., 2018). Yan et al. (2019) validated outstanding denitrification capabilities of epiphytic bacterial communities using functional prediction analysis.Furthermore, Mu et al. (2020) highlighted the substantial role of epiphytic biofilms in nitrogen removal using isotope labeling.
A myriad of studies have shown that the environmental factors (pH, temperature, light, redox potential, water flow, nutrient availability, and dissolved oxygen concentration) influence the dynamics of epiphytic bacterial communities (Kuehn et al., 2014; Hao et al., 2017). Arnon et al. (2007)reported hydrodynamic force mediated regulation of dissolved oxygen and nitrogen mass transport in the biofilms, which in turn affected the denitrogenation efficiency of the microbes. Recent studies stated that external environmental conditions mediated the regulation of epiphytic bacterial community structure(Bengtsson et al., 2010; He et al., 2014). Epiphytic biofilms showed host-specific microbial communities,which could be due to the complex physical or biochemical characteristics of distinct plant leaves or growth stages (Lachnit et al., 2011). However, as compared to the bacterial community, the community structure and seasonal variation of denitrifying bacteria in epiphytic biofilms remain poorly understood.
Microbial ecology research studies have been centered on the assembly rules of microbial communities and compositional dynamics prediction(Emerson and Gillespie, 2008; Zhou and Ning, 2017).Ecological processes (deterministic and stochastic)affect the assembly of local microbial communities simultaneously (Zhou and Ning, 2017). In aquatic ecosystem, hydrodynamics drives both deterministic and stochastic assembly processes by generating changes in selective pressures and by physically translocating microbes across geographic barriers(Graham et al., 2017). Recent studies stated the dominant role of stochastic processes in microeukaryotic/bacterial community assembly in aquatic ecosystem (Chen et al., 2019; He et al., 2020).Typical physicochemical characteristics of the epiphytic biofilms are liable for its complicated assembly mechanisms. For instance, extracellular polymeric substances serve as a physical barrier creating a “closed microenvironment” to reduce bacteria dispersal ratio (a stochastic process)(Seymour et al., 2017). Concurrently, the complex interactions between bacteria and algae may increase deterministic biotic selection (Foster et al., 2011).However, interaction of stochastic and deterministic processes remains ambiguous. The underlying mechanism for the assembly of the microbial community in copious habitats has been explored(Nemergut et al., 2011). However, none of the studies has explored the ecological mechanisms for the assembly of denitrifying bacterial community in epiphytic biofilms.
Denitrification is a crucial pathway to eliminate nitrogen from eutrophic ecosystems (Ji et al., 2015).Nitrite reductase (nir) catalyzes NO2ˉ reduction to NO,a limiting step in the denitrification process (Henry et al., 2004). Thenirgene types,nirSandnirK, are widely distributed in multiple ecosystems. These two genes serve as biomarkers for the identification of denitrifying bacteria (Fan et al., 2016).
Fig.1 Map of the sample sites (S1-S9) in the Caohai Lake,Guizhou Province, SW China
The objectives of study were to (i) explore the distribution characteristics of denitrifying bacterial community abundance and structure of the biofilms formed on the submerged macrophytes; (ⅱ) to unveil the effect of environmental factors on dynamics of the denitrifying bacterial community; and (ⅲ) to determine the relative significance of stochastic and deterministic processes in shapingnirS-denitrifying bacterial community assembly.nirSgene was employed as biomarker to investigate the denitrifying bacterial community structure and seasonal changes in epiphytic biofilms using Illumina MiSeq highthroughput sequencing. The null model analysis was performed to assess the roles in stochastic and deterministic processes in the assembly of denitrifying microbial communities, which commonly used to explore the construction mechanisms of a microbial community (Tripathi et al., 2018).
2 MATERIAL AND METHOD
2.1 Sample collection
Caohai Wetland (26°49′N-26°53′N, 104°12′E-104°18′E) located in karst area of Guizhou Province,southwest (SW) China is a national nature reserve since 1990. It is a typical wetland in the Yungui Plateau, 25 km2in area, 2-m average depth, 2 170 m in altitude, 10.6-℃ average temperature, and 1 000-mm average precipitation (Yan et al., 2019). It is the primary a wintering place for black-necked craneGrusnigricollisthat is endemic to China. Aquatic plants in this wetland areCeratophyllumdemersum,Potamogetonaceae,Otteliaacuminatavar.acuminate,Myriophyllumverticillatum, and so on. We gathered water and biofilms from nine sites in lakes of Caohai wetland in July and November of 2018 (Fig.1). For details in site description and physicochemical factors, please see Xia et al. (2020).
2.2 Real-time PCR analysis and sequencing
The Real-time Quantitative Polymerase Chain Reaction (qPCR) was employed to determine the abundance ofnirSgene using the cd3aF (5′-GTSAACGTSAAGGARACSGG-3′) and R3cdR (5′-GASTTCGGRTGSGTCTTGA-3′) primers (Palmer et al.,2012). F1aCu (5′-ATCATGGTSCTGCCGCG-3′) and R3Gu (5′-GCCTCGATCAGRTTGTGGTT-3′) were used as thenirKgene primers, as described previously(Fan et al., 2016). PCR products were checked on 1%agarose gel electrophoresis, and the melting curve was constructed to confirm that fluorescent signals were generated only by the PCR products and not from primer-dimers or other artifacts (Yu et al., 2018).The 10-fold serial dilutions of anirS/nirK-insert containing plasmid were carried out to construct standard curves for qPCR. The gene abundances were calculated from these standard curves (nirSR2=0.999,nirKR2=0.998), and the values were converted to copies per gram of biofilm’s fresh weight (BFW),assuming 100% DNA extraction efficiency.
The denitrifying bacteria were identified using primers: cd3aF and R3cdR. The same method was applied in scoring denitrifying bacterial communities from environmental samples (Jung et al., 2011; Yu et al., 2018). Multiple amplification products did not qualify the sequencing quality of thenirKgene, thus the community structure data of thenirKgene were eliminated. All samples were tagged with the forward primer used in the PCR in barcode sequence, which was synthesized using an ABI GeneAmp®9700 PCR thermocycler (ABI, CA, USA). The 20-μL PCR reaction mixture contained 4 μL of 5X FastPfu Buffer,0.8 μL of each primer (5 μmol/L), 10 ng of template DNA, 0.4 μL of FastPfu polymerase, BSA 0.2 μL,2 μL of 2.5 mmol/L dNTPs, and ddH2O up to 20 μL.Amplicons were purified and pooled in equimolar concentration. The paired-end were sequenced on Illumina MiSeq platform (Illumina, San Diego, USA),as per the standard protocols outlined by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).Raw sequences were de-multiplexed and quality filtered using QIIME 2. The reads that could not be assembled were discarded. 97% similarity cut-offwas used to cluster Operational taxonomic units (OTU)using UPARSE version 7.1 with the novel “greedy”algorithm that simultaneously executes chimera and OTU clustering. The taxonomy of thenirSgene sequence was analyzed by employing the RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the FGR functional gene database with a 70%confidence threshold (Fish et al., 2013). Subsequently,the “vegan” package in R was used to calculate the alpha diversity indexes as per the OTU level. Also,the denitrifying community beta diversity (at OTU level) was as per the Bray-Curtis distances between samples assessed using the “vegan” package in R software (Jiao et al., 2020).
2.3 Data analysis
We divided the growth stages of epiphytic biofilm in July and November into the growth period and decline period, respectively, as detailed in our previous study (Xia et al., 2020). The physicochemical data except for pH were log(x+1) transformed, and the microbial community data were “Hellinger”transformed before analysis (Borcard et al., 2011) for the homoscedasticity and normality. A Wilcoxon test(non-parametric method) was used to compare the seasonal variation of abundance, alpha, and beta diversity ofnirSandnirKgene. The Kruskal-Wallis test was employed to determine the significance ofnirSandnirKgene abundance, alpha, and beta diversity, and differences among the three submerged macrophytes. The “ggpubr” package in R was utilized for conducting the non-parametric test. The“VennDiagram” package in R compared the differences in the composition of denitrifying bacterial species between growth period and decline period also among three different submerged macrophytes.Non-metric multidimensional scaling analysis(NMDS) and the analysis of similarity (ANOSIM)based on Bray-Curtis distance were employed to visualize the denitrifying bacterial community difference through the “vegan” package in R.
Multiple regressions on distance matrices (MRM),a permutation-based method, unraveled the relationship between variations of bacterial communities in epiphytic biofilms and physicochemical factors of water by 1 000 permutations. Before MRM analysis, using the“ecodist” package, the “bioenv” function in the“vegan” package selected a subset of water’s environmental parameters that were best correlated to the community distance (Trumbo et al., 2013).Furthermore, redundancy analysis (RDA) determined the relative importance of physicochemical effect of water, and “ordiR2step” function determined the forward model choice based solely on adjustedR2andP-value by 1 000 permutations in the “vegan”package.
Relative influences of ecological processes were estimated using an operational framework described by Stegen et al. (2015). The between-community nearest taxon index (βNTI) and Bray-Curtis-based Raup-Crick (RCbray) were calculated through null model-based phylogenetic and taxonomic beta diversity metrics. The null model expectation used 999 randomizations. The classified ecological processes influencing community assembly were categorized as homogeneous selection (βNTI < -2),variable selection (βNTI > 2), homogenizing dispersal(-2 < βNTI < 2 and RCbray< -0.95), dispersal limitation(-2 < βNTI < 2 and RCBray> 0.95), and undominated(-2 < βNTI < 2 and -0.95 < RCBray< 0.95, a condition for which no single process dominates community assembly).
3 RESULT
3.1 Abundance of nirS and nirK gene
ThenirSandnirKgene abundance was summarized in Fig.2. The number ofnirSgene copy ranged from 5.54×106to 3.89×107copies per gram of BFW, and 2.92×106to 1.13×107copies per gram of BFW during the growth period and decline period of the plant,respectively (P<0.05). The gene abundance ofnirKranged from 6.71×106to 9.76×108copies per gram of BFW and 3.44×106to 1.93×107copies per gram of BFW during the growth period and decline period of the plant, respectively, which was higher thannirSgene abundance (P<0.05). Substantial differences(P<0.05) in the abundance of epiphytic denitrification genes in different host plants were observed, as depicted in Fig.2. The numbers ofnirSandnirKgene copies in the epiphytic biofilm on theP.lucenswere the highest during the growth period(2.79×107±1.96×107and 7.53×106±8.41×106copies per gram of BFW, respectively) and decline period(7.76×108±5.78×108and 2.91×107±6.67×107copies per gram of BFW, respectively). ThenirSandnirKgene copy number in the epiphytic biofilms of thePotamogetonlucensandM.verticillatumdecreased from growth period to decline period, while epiphytic biofilms on theC.demersumwere increased.
3.2 Alpha diversity and taxonomic analysis of the denitrifying bacterial community during growth period and decline period
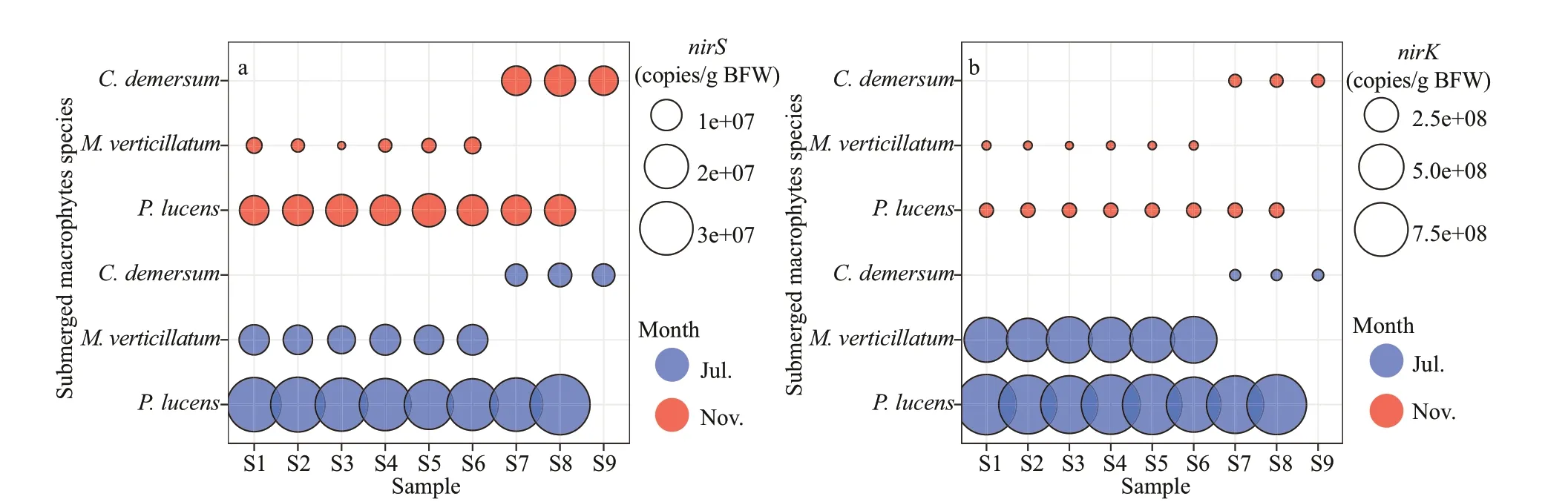
Fig.2 Bubble diagram showing the abundance (copies/g BFW) of the denitrification genes in epiphytic biofilm
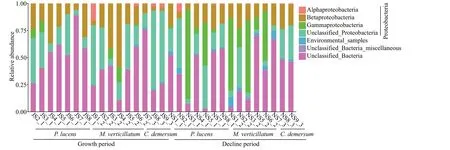
Fig.3 The relative abundance of different denitrifying bacteria in biofilms formed on Potamogeton lucens, Myriophyllum verticillatum, and Ceratophyllum demersum
Rare bacterial species were not considered in this analysis. Deficient reads were removed except for those who had ≥ 5 sequence reads in at least three samples or that 10% of the total samples contained these reads. 382 OTUs of denitrifying bacteria containing thenirSgene were identified from 641 656 quality sequences at 97% similarity level for the 32 samples (samples JS1_1, NS6_1, and NS4_2 were eliminated due to poor sequencing quality). Supplementary Table S1 summarizes the Good’s coverage and alpha diversity of the denitrifying bacterial community in the epiphytic biofilm at the OTU level of 97% similarity. The Good’s coverage ranged from 0.997 to 0.999 in the individual samples, and the integrated index of all the 32 samples was 0.998, indicating that the majority of the denitrifying bacterial taxa was identified from metacommunity. The abundancebased coverage estimator (ACE), Shannon-Weiner index, phylogenetic diversity (PD), and Pielou’s evenness did not change significantly between the growth period and decline period of macrophyte.ACE and Shannon index were slightly higher in winter than in summer. In addition, in each period,no sizeable differences were observed in the alpha diversity of the denitrifying bacterial community from the three host plants.
Most of thenirS-denitrifying bacteria belonged to phylum Proteobacteria (56.55%±22.15%), and others were unclassified bacteria (41.71%±22.17%) (Fig.3),amounting up to 91.39%-99.98% of the total reads from different samples. Proteobacteria growth showed no significant difference between growth period (July)and decline (November) period (54.50% vs. 58.54%),while the Gammaproteobacteria growth was faster during growth period than the decline period(P<0.005). The relative abundance of Proteobacteria in epiphytic biofilm onM.verticillatumwas the high during both the growth period and decline period(Fig.3).
3.3 Beta diversity of denitrifying bacterial community during growth period (July) and decline (November) period
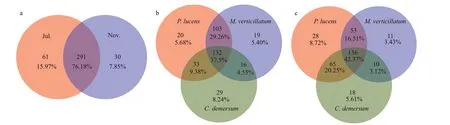
Fig.4 The Venn diagram of the denitrifying bacterial community (OTU level) showing species in common
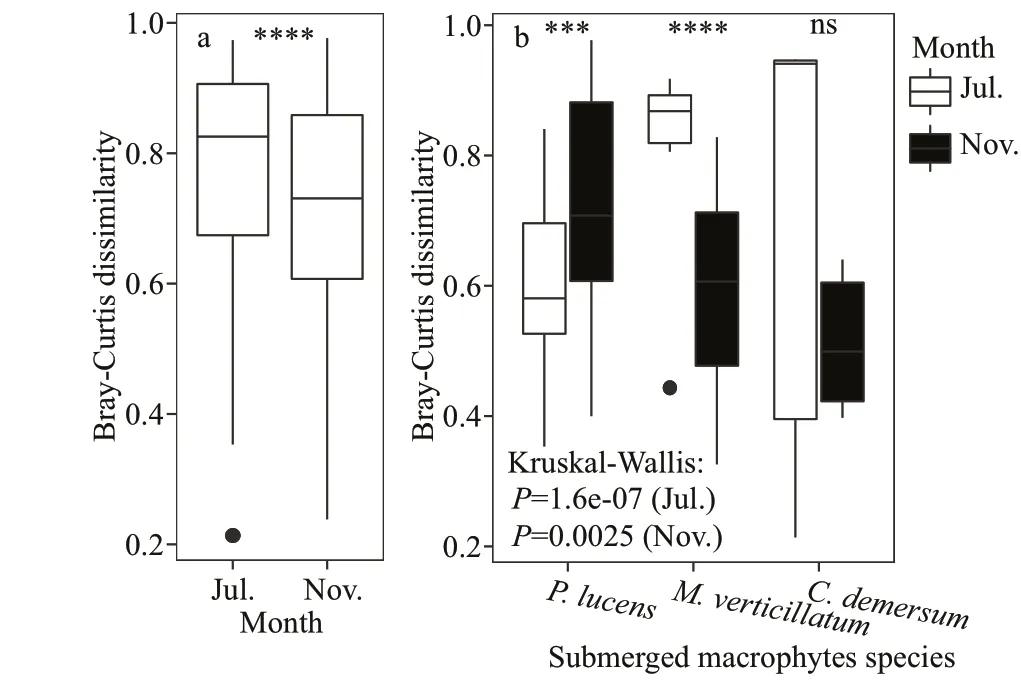
Fig.5 The box-diversity plots of biofilm denitrification functional communities in different plants in different months
Variances in composition of thenirS-denitrifying bacterial communities between growth period and decline period and among host plants were investigated using the Venn diagram analysis at OTU level (Fig.4).We found that 76.18% of the total 291 OTUs were shared between the two periods in the case ofnirSdenitrifying bacteria. Meanwhile, epiphytic biofilms formed during the plant growth period was twice of the decline period. Three submerged macrophytes had 132 common OTUs (37.5%) of the epiphytic denitrifying bacterial community during the growth period and 136 OTUs (42.37%) during the decline period. The denitrifying bacteria in epiphytic biofilms ofP.lucensandM.verticillatumhad the highest OTU sharing rate of 72.76% during the growth period and 64.84% during the decline period.P.lucensshowed the highest unique OTUs during growth period and decline period.
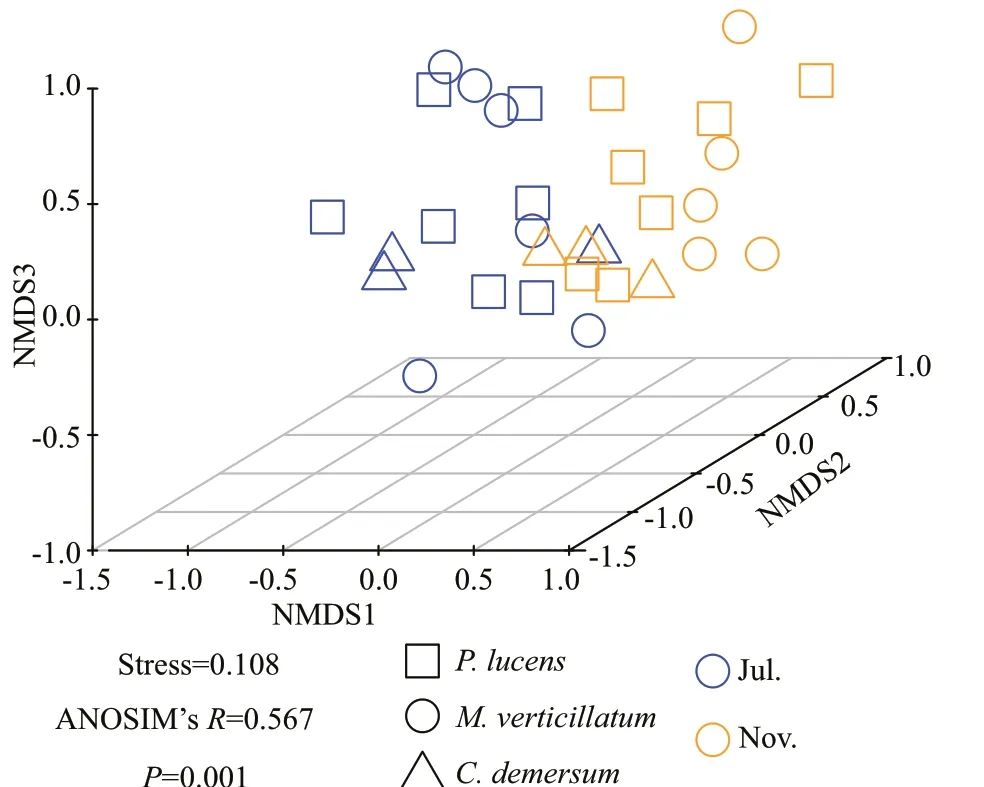
Fig.6 The NMDS similarities between all samples
The beta diversity of denitrifying bacterial communities was summarized in Fig.5. Beta diversity(P<0.000 1) of denitrifying bacteria was significantly higher in epiphytic biofilms during the growth period than in the decline period. Conversely, beta diversity was significantly higher in epiphytic biofilms during the decline period than the growth period inP.lucens(P<0.001), whereasM.verticillatumshowed the opposite trend. To unveil the differences innirSdenitrifying bacterial community structure between growth period and decline period, an NMDS analysis was performed based on the Bray-Curtis distance as per the OTU distribution (Fig.6). The denitrifying bacterial community structure during the growth period was remarkably different from the decline period (Stress=0.108). ANOSIM analysis further proved the variance in the denitrifying bacterial community structure between growth period and decline period (R=0.567,P=0.001). As shown in this analysis, denitrifying bacterial community structure was different in three submerged macrophytes during growth period and decline period (R=0.259,P=0.029 for growth period, andR=0.218,P=0.049 for decline period) (Fig.6).
3.4 Effect of environmental conditions on the structure of denitrifying bacterial communities
The Mantel test presented significant correlation between physiochemical factors, such as water temperature (WT), disolved oxygen (DO), pH, total phosphorus (TP), NH4+, and thenirS-denitrifying bacterial communities of the submerged macrophytes during the growth period (Mantel’sR=0.435,P=0.007; Supplementary Table S2). However, during the decline period, WT, pH, total nitrogen (TN), and chlorophylla(Chla) were significantly correlated to thenirS-denitrifying bacterial communities (Mantel’sR=0.299,P=0.015). Thus, denitrifying bacterial communities showed significant seasonal variation in correlation with WT, DO, and NO3ˉ (Mantel’sR=0.477,P=0.001). Multiple regression analysis indicated that using physicochemical factors of water column could not discern the diversity in denitrifying bacterial community from epiphytic biofilms (MRM’sR2was 0.14, 0.103, and 0.238 for growth period, decline period, and both, respectively). Alternatively, the RDA results demonstrated that variation in a few denitrifying bacterial communities could be explained through water parameters (R2was 0.182, 0.281, and 0.36 for the growth period, decline period, and both,respectively; Supplementary Table S3).
3.5 Phylogeny-based null model analysis
As indicated by the relative contribution of ecological process, physicochemical factors of water column played a minor role in the assembly of denitrifying bacterial community in epiphytic biofilm (Fig.7), while dispersal limitation process played a major role in shaping the denitrifying community structure in both growth period (49.22%) and decline period (41.78%).In addition, seasonal-change-related effect was crucial on the denitrifying bacterial community in epiphytic biofilms (64.52%). Secondly, the undominated process played an essential role in the assembly of denitrifying bacterial community during the growth period, decline period, and seasonal changes. Furthermore, the subdivision of ecological processes indicated that the stochastic process had a higher contribution than the deterministic process in growth period and decline period, including the seasonal changes. Notably, the undominated process played a minor role in community assembly in all seasons.
4 DISCUSSION
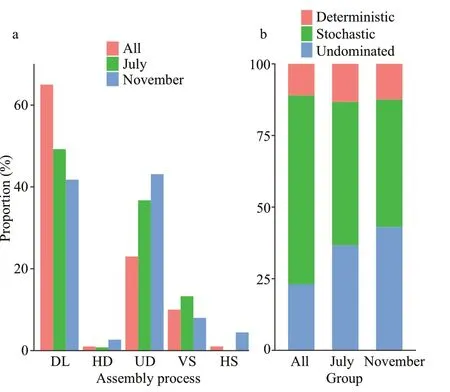
Fig.7 Proportions of denitrifying bacteria assembled by diffusion-limited (DL), homogeneous diffusion(HD), uncontrolled (UD), factor selection (VS), and homogeneous selection (HS) according to the null model analysis (a); the proportion of denitrification communities assembled from deterministic,stochastic, and undominated processes (b)
In the current study, we observed a highernirSandnirKgene abundance during the growth period (July)than the decline period (November), which was in line with the previous studies (Lindemann et al., 2016;Huang et al., 2018). Multiple reports accentuated the fact that environmental factors affect the abundance and activity of genes involved in the biogeochemical nitrogen cycle (Sunagawa et al., 2015). As stated in our previous study on the abundance of epiphytic bacteria, temperature affects substantially the microbial abundance (Xia et al., 2020), and so the gene abundance. In this study, the epiphytic denitrification genes were isolated from the microbiome present on leaf surfaces of three submerged macrophyte species. We observed that the abundance ofnirKgene was higher than that of thenirSgene in summer and winter. Previous studies have shown that the abundance ofnirKgene was higher in multiple environmental samples, such as sediment(Saarenheimo et al., 2015), epiphytic biofilms (Vila-Costa et al., 2014), soil (Dandie et al., 2011), and so on than that ofnirS. It may be due to the oxygen-rich microenvironment of biofilms, which might have hampered the growth of oxygen-sensitivenirS-type bacteria and promoted the growth of aerobicnirK-type bacteria (Knapp et al., 2009). We also observed that the abundance of thenirKgene was higher in the growth period than the decline period. In addition,higher variation ofnirKgene abundance between different seasons than that ofnirSgene can be seen in Fig.2. It was suggested that thenirK-type denitrifying bacteria were more sensitive to environmental variations than that of thenirS-type denitrifying bacteria (Dandie et al., 2011; Yuan et al., 2012).
A myriad of studies demonstrated denitrification activity in the submerged epiphytic biofilms of macrophytes in freshwater ecosystem (Fan et al.,2016; Yan et al., 2019). Interestingly, the denitrification gene abundances in biofilms was higher than in other denitrification spots, such as sediments and water columns. The most probable number (MPN) method was widely employed to calculate the density of denitrifying bacteria in submerged macrophytes(Körner, 1999; Eriksson, 2001). Wuhle River (Berlin,Germany) that serves as a dumping site for the effl uent of Falkenberg sewage treatment plant, showed the denitrifying bacterial density of 9.4×104to 7×104MPN/mL in water and 3.3×106to 2.5×106MPN/cm3in sediment. The average number of denitrifying bacteria was higher (8.7×107±1×107MPN per gram of plant dry weight) in the epiphytic biofilms of 6 submerged macrophytes includingCeratophyllumdemersum,PotamogetonnatansL.,PotamogetoncrispusL.,SagittariasagittifoliaL.,SparganiumemersumrehmannL., andPotamogetonpectinatusL.(Körner, 1999). In the Donghu (East) Lake, a eutrophic lake in Wuhan, Hubei Province, the abundance ofnirSgene ranged from 1.47×104to 1.67×105copies per gram of dry sediment, and that ofnirKgene ranged from 2.02×107to 9.71×107copies per gram of dry sediment (Hou et al., 2013). However, the abundance ofnirSandnirKgene obtained in our analysis was significantly higher than that of the Donghu Lake. Besides, Fan et al. (2016) reported a higher abundance ofnirSandnirKgenes in epiphytic bacterial communities of plantsPotamogetonmalaianusandC.demersumthan the water samples from Taihu Lake. Mu et al. (2020) identified the critical contribution of epiphytic biofilms ofMyriophyllumspicatumin nitrogen removal by isotope labeling. Thus, biofilms on leaves of submerged macrophytes in aquatic ecosystem have outstanding denitrification potential. These are a novel approach for controlling nitrogen levels in aquatic ecosystem in the foreseeable future.
In this study, most of the denitrification microbes in the biofilms belonged to phylum Proteobacteria(56.55%±22.15%) while the rest of the bacteria were unclassified (41.71%±22.17%) (Fig.3). Previous phylogenetic analysis showed that the aerobic denitrifying bacteria mainly belonged to Alpha-,Beta-, and Gamma-Proteobacteria (Ji et al., 2015).Occurrence of diverse aerobic denitrifying bacteria and alternating anaerobic-oxygenic environments in biofilms on plants (Sand-Jensen et al., 1985; Ji et al.,2015) created a conducive environment for denitrification. In addition, epiphytic biofilms can uptake a sea of nitrogen nutrients in the surrounding water (Levi et al., 2015), and the nitrogen could be further utilized for the development of epiphytic biofilms and microbial growth involved in the nitrogen cycle (Levi et al., 2015; Yan et al., 2018).Moreover, secretion from the plant leaf surface provide a carbon substrate for microbes in the nitrogen cycle (Florez et al., 2017), and epiphytic algae respiration increases the pH of the epiphytic biofilms.The microenvironmental factors, such as physical barrier (diffusion limitation) of extracellular polymer in the biofilms, enhanced the denitrification function(Yan et al., 2019). The specific microbial community composition and microenvironment of epiphytic biofilms promote the nitrogen removal from the water.
Denitrifying microbial community in epiphytic biofilms showed the highest species commonality during the growth period and decline period. However,differences in the structure of the denitrifying microbial community between growth period and decline period were apparent (R=0.5,P=0.001). Concurrently,according to the outcomes of the functional gene quantification (Fig.2), the denitrifying bacterial density in epiphytic biofilms during the growth period was significantly higher than that of the decline period.Thus, we believe that difference in the denitrifying microbial community structure of epiphytic biofilm was due primarily to depleted microbial abundance.First, higher biomass in July, i.e., the plant growth period, was resulted from water temperature, which stimulated the growth and reproduction of microbes(Bengtsson et al., 2010). Secondly, strong photosynthesis of plants during growth period and sufficient oxygen and carbohydrates accelerated the growth and reproduction of aerobic microbes (Yan et al., 2019). However, in November (the plant decline period), as the ambient temperature dropped, the growth and metabolism of microbes were also depleted. Also, the decreased photosynthetic activity of the host plant hampered the growth of the primary dominant species and introduced or promoted the growth of organic-matter-decomposing bacteria(Doughari et al., 2011; Mancuso et al., 2016; Xia et al.,2020). These bacteria also went through the selection pressure of the complex organic molecules released by the plant during the decline period (Seymour et al.,2017; Zhang et al., 2018). At the same time, biofilms gradually matured from July to November, drawing more nutrients from water. Yan et al. (2018)demonstrated that a higher nutrient concentration led to the inhibition of denitrifying microorganisms and the reduction of the biomass in the biofilms. Finally,the abundance and activity of the denitrifying bacteria in the epiphytic biofilm was depleted.
Temperature was the key factor in limiting the seasonal dynamic characteristics of microbial communities as per previous studies (Casartelli and Ferragut, 2015). In this study, MRM, Mantel test, and RDA analysis indicated that temperature was the primary factor on seasonal dynamics of denitrifying microbes in epiphytic biofilms. Our study of the effect of aquatic environmental factors on the dynamics of biofilm’s denitrifying microbial communities indicated that multiple factors affected the assembly process of denitrifying microbial communities. It implies the involvement of biotic and abiotic factors on the epiphytic denitrifying bacterial community. These factors, such as epiphytic biofilm, algal secretion, and respiration, increased the pH in the ambience of epiphytic biofilms, promoted the buildup of epiphytic microbial community and the heterotrophic microbial activity, and affected the ecological function of the microbial community (Kuehn et al., 2014; Song et al.,2015). Also, the low effect of aquatic environmental factors implies that the stochastic process may be the vital driving force to the construction of denitrifying microbial communities. Results of the null model analysis (Fig.7) indicated that the dynamic characteristics of the denitrifying microbial community were affected by stochastic dispersal limitation(64.52%) and undominated (23.20%) processes. The underlying driving mechanism for microbial community assembly in aquatic ecosystems had been controversial. Previous research reports revealed that the deterministic process forms bacterioplankton communities (Sunagawa et al., 2015; Wang et al.,2019), while stochastic process regulates microbial community structure (Evans et al., 2017; Zhou and Ning, 2017).
In this study, the biofilms created a nearly closed microenvironment, and the physical barrier made of extracellular polymer limited the microbial dispersion(Liu et al., 2016; Wu, 2016). Many microbes, such as Alphaproteobacteria and Betaproteobacteria, do not have motor flagella and thus show low motor ability(Roger et al., 2017). This suggests that the construction of denitrifying bacteria community was significantly influenced by the morphological (type, size, and shape) and habitat (openness, geographical distance)of the bacterial community. It was in line with the size-dispersal hypothesis (a stochastic process) (Liu et al., 2020) and distance-decay patterns (a stochastic process) (Astorga et al., 2012). Overall, the community assembly ofnirS-type bacteria was influenced by both stochastic and deterministic processes, but the stochastic processes such as dispersal limitation emerged as the dominant mechanism as per our analysis.
5 CONCLUSION
Abundant denitrification genes was found in the biofilm formed on the surface of leaves of submerged plants with seasonal changes. Meanwhile, abundance ofnirKgene was higher than that ofnirSgene in biofilm. In addition,nirSgene showed more environmental stability thannirKgene did.Proteobacteria was the dominant phylum in thenirStype denitrifying bacterial community in the epiphytic biofilms and showed distinct seasonal differences. ThenirS-type denitrifying community dynamics could not be well explained by environmental factors assessed in this study. Combining the null model analysis results, we suggested that stochastic and deterministic processes affected the community assembly ofnirStype bacteria. And the stochastic process, such as dispersal limitation, emerged as the principal mechanism in this study. However, the process of temporal changes in the abundance, composition,structure and assembly process of denitrifying bacteria from the growth period (July) to decline period(November) of the epiphytic biofilms remains unresolved. Therefore, research on the denitrification function and community dynamic mechanism of the epiphytic biofilms demands further investigations.
6 DATA AVAILABILITY STATEMENT
The data that support the finding of this study are available from the corresponding author.
7 ACKNOWLEDGMENT
The authors thank the editor and reviewers in helping to improve the manuscript. We also thank Xu SONG, Yu YANG, Xiangchen TANG, Tianyou WANG, Xin DU, and Mengmeng FAN from Guizhou Province Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment for their generous help in sampling and laboratory works.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Typhoon-induced wind waves in the northern East China Sea during two typhoon events: the impact of wind field and wave-current interaction*
- Effect of subsea dispersant application on deepwater oil spill in the South China Sea*
- Geochemical characteristics of cold-seep carbonates in Shenhu area, South China Sea*
- Examination of seasonal variation of the equatorial undercurrent termination in the Eastern Pacific diagnosed by ECCO2*
- Deviation of the Lagrangian particle tracing method in the evaluation of the Southern Hemisphere annual subduction rate*
- Immunostimulatory effect of quaternary degree and acetyl group of quaternized chitosan on macrophages RAW 264.7*
