Microbial community coexisting with harmful alga Karenia mikimotoi and microbial control of algal bloom in laboratory*
Li SUN , Peike GAO ,**, Yu LI , Chao WANG , Ning DING , Junfeng CHEN ,
Yuhao SONG 1, Chunchen LIU 1, Lun SONG 2, Renjun WANG 1,**
1 College of Life Sciences, Qufu Normal University, Qufu 273165, China
2 Key Laboratory of Marine Biological Resources and Ecology, Liaoning Ocean and Fisheries Science Research Institute, Dalian 116023, China
Abstract Algicidal bacteria have been frequently isolated from algal blooming areas. However,knowledge regarding the microbial communities coexisting with microalgae and their potential application in preventing harmful algal blooms (HABs) is limited. In this study, we investigated the composition of the microbial community coexisting with harmful alga Karenia mikimotoi and its responses to algal control via nutrient stimulation or by adding algicidal strain in microcosms. The microorganisms inhabiting the K. mikimotoi culture consisted of 24 identified phyla, including dominant Proteobacteria (relative abundance 76.24%±7.28%) and Bacteroidetes (22.67%±8.32%). Rhodobacteraceae, Phaeodactylibacter,and Maritimibacter predominated during the algal cultivation. Both the added nutrient and fermentation broth of algicidal strain Pseudoalteromonas QF1 caused a massive death of K. mikimotoi and substantial changes in the coexisting microbial community, in which Rhodobacteraceae and Phaeodactylibacter significantly decreased, while Halomonas and Alteromonas increased. Core operational taxonomic units(OTUs) analysis indicated that 13 OTUs belonging to Rhodobacteraceae, M aritimibacter, Marivita,Nisaea, Phaeodactylibacter, Citreicella, Halomonas, Alteromonas, Marinobacter, Muricauda, and Pseudoalteromonas dominated the changes of the microbial communities observed in the K. mikimotoi culture with or without treatments. Collectively, this study indicated that microbial community inhabiting K. mikimotoi culture includes potential algicidal bacteria, and improves our knowledge about microbial community succession during biocontrol of K. mikimotoi via nutrient stimulation or by adding isolated algicidal strains.
Keyword: Karenia mikimotoi; microbial community; nutrient stimulation; algicidal bacteria;Pseudoalteromonas
1 INTRODUCTION
Kareniamikimotoiis a dinoflagellate species known to cause harmful algal blooms (HABs) (Aoki et al., 2017). Blooms induced byK.mikimotoihave been reported frequently in past decades worldwide,such as the coast of China (Sakamoto et al., 2021),Imari Bay (Aoki et al., 2017), the east Johor Straits of Singapore (Kok and Leong, 2019), the French Atlantic Shelf (Sourisseau et al., 2016), the waters offwestern Ireland (O’Boyle et al., 2016), the north-west European continental shelf (Gillibrand et al., 2016),the Kachemak Bay Alaska (Vandersea et al., 2020),and the Arabian Sea (Kumar et al., 2018). The largescale HABs have adverse impacts on aquatic ecosystem, can cause mass mortality of benthic and pelagic organisms by the competition of nutrient salt,the dramatic reduction of underwater light, and the secreted toxic substances. A number of researches reported that the type of HABs can cause mortality of marine organisms, such as oysters (Mizuno et al.,2015), zooplankton,PenaeusvannameiandScophthalmusmaximus(Li et al., 2017), and rotifers(Li et al., 2018). Thus, the control ofK.mikimotoihas recently attracted more attention.
Physical and chemical methods have been developed and applied to control HABs (Nagai et al.,2016; Park et al., 2017). Algaecides, such as copperbased products and natural clays, had been used to control HABs. However, copper-based algaecides may cause environmental issues, such as toxicity to aquatic organisms (Closson and Paul, 2014), and are banned internationally. Due to the low f locculation efficiency and high field consumption, it is difficult to achieve large-scale application of natural clay.Recently, eco-friendly controlling methods, such as using ultrasonic technology, modified clays,allelochemicals secreted by macroalgae, algaecides produced by bacteria, and nutrient competition between organisms, are receiving increasing attention.Among them, algicidal bacteria have strong applied potential in control of HABs because of the accessibility and excellent biocompatibility.
To date, a large number of algicidal bacteria have been isolated from seawater (Zheng et al., 2018),reservoirs (Shimizu et al., 2017), lake sediments (Su et al., 2016), mangroves (Yu et al., 2018), and soils (Cai et al., 2019). The reported algicidal microorganisms and algicidal mechanisms were collated in Supplementary Table S1 (Zheng et al., 2019). These microorganisms were mainly affiliated with Bacteroidetes, α-Proteobacteria, β-Proteobacteria,γ-Proteobacteria, Actinomycetes, and Firmicutes. The algicidal bacteria inhibit algae by influencing algal cell integrity, enzymatic activities, gene expression,photosynthesis, respiration, and reproduction. For example,MyxococcusparasitizedPhormidiumand resulted in algal cell lysis (Burnham et al., 1984), and most algicidal bacteria inhibit algae via producing algicidal substances (Zhang et al., 2020a, b).
Bacteroidetes (Flavobaterium,Cytophaga, andCellulophaga), α-Proteobacteria (Paracoccus,Rhodobacteraceae), β-Proteobacteria (Thalassospira),γ-Proteobacteria (Alteromonas,Idiomarina,Vibrio,Pseudoalteromonas,Halomonas, andMarinobacter),Actinomycetes (Kocuria), and Firmicutes (Bacillus)have proven to be able to inhibit or killK.mikimotoi(Imai et al., 2006; Lu et al., 2016; Zheng et al., 2018),showing potential application in controllingK.mikimotoi. In our previous work, an isolatedPseudoalteromonasshowed algicidal activity againstK.mikimotoi, and caused the accumulation of reactive oxide species (ROS) and the apoptosis of algal cells(Zheng, 2019). Previous studies also reported thatPseudoalteromonascould inhibit algae from Dinophyceae (Gymnodiniumcatenatum,Cochlodiniumpolykrikoides,Akashiwosanguinea,andAlexandriumtamarense), Raphidophyceae(ChattonellamarinaandHeterosigmaakashiwo), and diatom (Skeletonemacostatum) (Lovejoy and Bowman, 1998; Lee et al., 2000; Oh et al., 2011; Sun et al., 2016; Lyu et al., 2019). Due to its algicidal activity, the isolatedPseudoalteromonasQF1 was added into the culture ofK.mikimotoito investigate its influences on the co-existing microbial community.
Recently, the microbiomes coexisting with algae have received extensive attention for better understanding the process and mechanism of algal bloom formation and extinction. However, the algicidal effects of the coexisting microbial communities on microalgae have rarely been studied.In this study, the microbial community coexisting withK.mikimotoi, their responses to nutrient addition and added algicidal strainPseudoalteromonasQF1 were investigated in microcosms. The hypothesis of nutrients stimulation is: microalgae are photoautotrophic; compared with microalgae,microorganisms have shorter growth and metabolism cycles, and give priority to nutrients; the added nutrients is limited, and not enough to cause massive growth of microalgae.
2 MATERIAL AND METHOD
2.1 Material
2.1.1Kareniamikimotoiand cultivation
Kareniamikimotoiwas donated by Ocean University of China and was preserved in our laboratory. The culture was incubated and preserved in a sterile f/2 medium prepared with seawater(Guillard and Ryther, 1962) in 500-mL glass conical flasks capped with aseptic breathable parafilm. The used seawater was first filtered through a 0.45-μm filter membrane to eliminate microorganisms. The f/2 medium was autoclaved for 20 min at 121 °C before use. Unless otherwise noted, all the algal cultivation was performed at 25± 1 °C under a 12-h∶12-h lightdark cycle with a light intensity of 3 000 lx in a light incubator. The cultures were manually shaken three times per day to prevent the algae from growing against the wall of the flask.
Axenic algae culture was obtained via repetitive filtration using a 5.0-μm mixed cellulose ester membrane filter, which allows the microorganisms existing in theK.mikimotoiculture to pass through the membrane, while the algal cells are trapped (Baker and Kemp, 2014). The number of cultivable bacteria remained on the axenic culture were measured based on colony forming units (CFUs) counts: plating aliquots of samples on 2216E agar plates that were incubated at 25± 1 °C for 24 h. The 2216E agar medium consisted of 1-g yeast extract, 5-g peptone,15-g agar, and 1-L seawater, with pH of 7.2, and was autoclaved for 20 min at 121 °C before use. After the aseptic processing, the bacteria in the algal culture decreased from 1.1×107to 2.1×102cells/mL.
The algal cells were fixed with Lugol’s solution and counted under a light microscope using hemocytometer. The Lugol’s solution consisted of 4-g iodine (I2) and 6-g potassium iodide (KI) in 100 mL of distilled water.
2.1.2PseudoalteromonasQF1
PseudoalteromonasQF1 can effectively inhibit the growth ofK.mikimotoithrough indirect way (Zheng et al., 2018). Strain QF1 was previously isolated from estuarine of the Yellow Sea, China. The strain was cultured in 200-mL sterile 2216E medium in 500-mL conical flasks at 25± 1 °C.
2.2 Method
2.2.1 Algicidal experiment
Because the lack of organic carbon source in the f/2 medium, it cannot meet the growth of microorganisms. Therefore, 2216E medium was used to stimulate the growth of microorganisms inhabitingK.mikimotoiculture. Algicidal experiments consisting of control group (C), nutrient-stimulated group (E),and QF1 treated group (J) were conducted. The control group had no 2216E medium and QF1 fermentation broth and was used to investigate the succession of the coexisting microbial community. In the nutrient-stimulated group, 4.5 mL (3%, 2216E medium volume/algal culture volume) and 9 mL (6%)of sterile 2216E medium were respectively added to 150-mLK.mikimotoiculture with an initial algal cell concentration of 8.0×104cells/mL to investigate the influence of the 2216E medium onK.mikimotoiand the coexisting microbial community. In addition,4.5-mL and 9-mL sterile 2216E was respectively added to 150-mL axenicK.mikimotoiculture to form the control group of nutrients (A-E) to test the influence of the 2216E medium on the growth ofK.mikimotoi. In the QF1 treated group, 4.5-mL (3%)and 9-mL (6%) QF1 cultures in the stationary phase(8.5×108cells/mL) were respectively added to 150-mLK.mikimotoiculture to investigate the influence of the added algicidal strain onK.mikimotoiand the coexisting microbial community. Each of the experiments above was conducted in triplicate. The algal cells in each group were counted every 24 h, and the algal inhibition ratio, representing the algicidal activity, was estimated according to the following equation:
A lgicidal rate (%)=(1-Nt/Nc)×100,whereNcrefers to the number of algal cells in the control group, andNtrefers to the number of algal cells in the treatment group.
For microbial DNA extraction, 50-mL microalgae suspension from each group was collected on the 3rd,6th, and 9thday, respectively, and were centrifuged at 8 000 r/min at 4 °C for 5 min to collect microbial cells. The pellets from centrifugation were frozen at-80 °C before DNA extraction. Additionally, the number of cultivable microorganisms inK.mikimotoicultures was measured based on counts of CFUs on 2216E plates.
2.2.2 Microbial community 16S rRNA gene sequencing and analysis
Microbial genomic DNA was extracted using glass bead grinding method combined with an AxyPreP bacterial genomic DNA kit. Universal prokaryotic primers 336f (5′-GTACTCCTACGGGAGGCAGCA-3′) and 806r (5′-GTGGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3-V4 region of microbial 16S rRNA gene. PCR products of the same sample in triplicate were mixed to minimize bias. The 16S rRNA amplicons were examined by DNA electrophoresis on 2% agarose gel, and were recovered using AxyPrep DNA gel Recovery Kit. Amplicons were paired-end sequenced on the MiSeq platform based on PE300 (2×300 bp) in Allwegene Technology Co. Ltd., Beijing, China. Fastq data were processed using QIIME, version v.1.8.0 (Caporaso et al., 2010).After filtering low quality reads and chimeras, 16 714 effective sequences were selected at random for each sample for community analysis to reduce sequencing deviation. Sequences were assigned to operational taxonomic units (OTUs) at a 97% sequence similarity level using the UPARSE pipeline (Edgar, 2013). Goods coverage was used to assess the sequencing depth for the samples. The representative sequence sets were aligned and given a taxonomic classification by ribosomal database project (RDP) against the SILVA Small Subunit rRNA database at an 80% confidence threshold (Cole et al., 2014). The representative sequences of OTUs with the highest abundance were selected for tree building using ‘Mafft’ and ‘Fasttree’.The tree was visualized using R software (https://www.r-project.org/) based on the abundance and evolutionary relationship at the genus level.‘Unweighted uniFrac’ only considers whether the microbial taxon appears in the community, but does not consider the abundance. However, the relative abundance of different microbial taxon can be critical for describing the community changes. Therefore,‘weighted uniFrac’ that considers on abundance information during calculations was used here to illustrate the responses of the microbial community existing inK.mikimotoiculture to the added 2216E medium and QF1 broth. Non-metric multidimensional scaling analysis (NMDS) based on Bray-Curtis of the OTUs and heatmap based on weighted unifrac distances were performed to visualize the changes in the microbial community in the control and treated groups.To determine the significant differences in microbial β-diversity, analysis of molecular variance (AMOVA)depending on the weighted unifrac distance matrixes was analyzed using the ‘anosim’ function of ‘ade4’package in R. Then, a Wilcoxon rank-sum test was performed to determine the microbial populations with statistically significant difference between the control and treated groups.
3 RESULT
3.1 Growth of K. mikimotoi in microcosms

Fig.1 The growth curves of K. mikimotoi in microcosms
The influences of the added 2216E medium and QF1 broth on the growth ofK.mikimotoiwere investigated in the algicidal experiments consisting of control group (C), nutrient-stimulated group (E), and QF1 treated group (J). For the control group, the algae rapidly reproduced, with algal cell density increasing from 8.4×104to 55.8×104cells/mL in 9 days. Adding both nutrients and QF1 fermentation broth caused mass mortality ofK.mikimotoi, and the algal inhibition ratio reached up to 90% after 3 days of cultivation.The same amount of sterile 2216E medium was also added to the same volume of axenicK.mikimotoiculture, and no inhibiting effects on the growth ofK.mikimotoiwere observed (Fig.1): the algal cell density increased from 8.4×104to 52.0×104cells/mL in 9 days for groups with 3% 2216E medium (A-E3),and 48.8×104cells/mL for groups with 6% 2216E medium (A-E6). The results indicated that adding nutrients and QF1 broth could efficiently inhibit the growth ofK.mikimotoiin non-sterile environment.Additionally, the microorganisms existing inK.mikimotoiculture would be stimulated by nutrients.
3.2 Sequencing information and the shared microbial OTUs
The sequencing coverage of the microbial communities in each sample ranged from 99.70% to 99.96%, which could reflect the whole of the microbial community in each sample. The responses of microorganisms existing inK.mikimotoiculture to the added 2216E medium and QF1 broth were further investigated. A total of 424 OTUs were detected in the control group (average: 85± 41 OTUs/sample),nutrient-stimulated group (average: 87 ± 43 OTUs/sample), and QF1 fermentation broth treated group(average: 82± 34 OTUs/sample). Additionally, 140 shared OTUs were detected in all groups. Among them, 13 OTUs were simultaneously detected in each sample, and were distributed in Rhodobacteraceae,Maritimibacter,Marivita,Nisaea,Phaeodactylibacter,Citreicella,Halomonasmeridiana,Alteromonas,Marinobacter,Muricauda, andPseudoalteromonas(Fig.2). The 13 OTUs accounted for 88.10%-97.04%,75.05%-96.03%, and 8.98%-88.18% of the microbial total relative abundances in the control, nutrientstimulated, and QF1 fermentation broth treated groups, respectively.
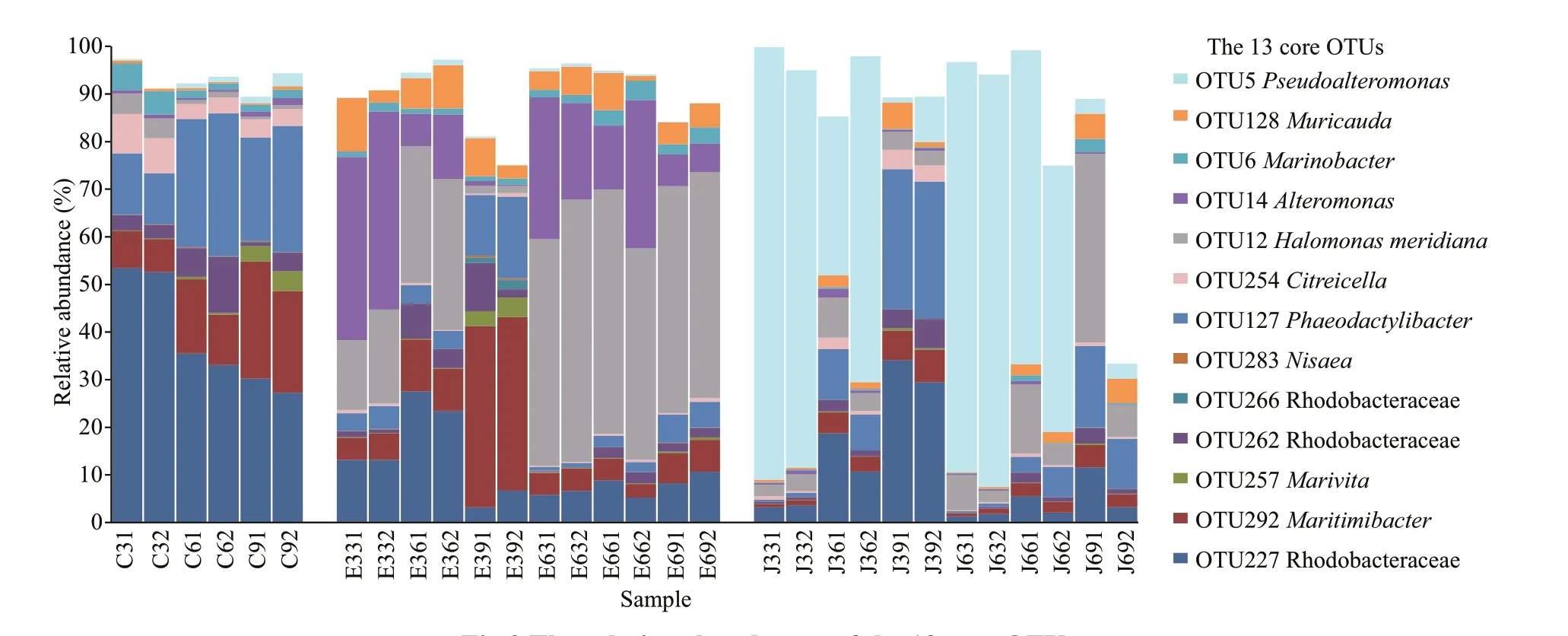
Fig.2 The relative abundances of the 13 core OTUs
3.3 Microbial community coexisting with K. mikimotoi
The number of cultivable microorganisms in the non-sterileK.mikimotoiculture was approximately 1.2×107-1.9×107cells/mL during algal cultivation. As shown in Fig.3, many microorganisms with diverse phylogenetic relationships coexist with well-grownK.mikimotoi. These microorganisms were affiliated with 1 unidentified phylum and 24 identified phyla.The main phyla were Proteobacteria and Bacteroidetes,accounting for 76.24%±7.28% and 22.67%±8.32% of the whole community, respectively (Fig.3). A total of 38 identified classes were detected, including the dominant Alphaproteobacteria, Sphingobacteriia,Gammaproteobacteria, and Deltaproteobacteria,which accounted for 66.29%±5.60%, 21.51%±7.99%,8.69%±3.21%, and 1.24%±1.16% of the microbial community, respectively (Fig.4a). An unidentified genus belonging to Rhodobacteraceae,Phaeodactylibacter,Maritimibacter,Citreicella,Marinobacter,Halomonas,Marivita,Oceanococcus,andPseudoalteromonaspredominated inK.mikimotoiculture, and accounted for 45.87%±10.97%,21.49%±7.99%, 14.45%±7.34%, 4.91%±2.29%,2.84%±1.93%, 1.96%±1.75%, 1.45%±1.80%,1.44%±2.46%, and 1.07%±0.97% of the microbial community, respectively (Fig.4b).
As shown in the NMDS graph (Fig.5a) and heatmap of the weighted unifrac dissimilarity matrix (Fig.5b),microbial communities from the 3rd, 6th, and 9thday of theK.mikimotoiculture were clustered together.However, a slight succession of the microbial community was observed during the cultivation ofK.mikimotoi(Figs.4 & 5): the unidentified Rhodobacteraceae genus decreased from 57.52%±0.35% (3rdday) to 33.19%±0.47% (9thday),Citreicelladecreased from 7.84%±0.60% (3rdday) to 3.67%±0.17% (9thday),Halomonasdecreased from 4.20%±0.12% (3rdday) to 0.67%±0.16% (9thday),whilePhaeodactylibacterincreased from 11.80%±1.46% (3rdday) to 24.13%±3.34% (9thday),andMaritimibacterincreased from 7.33%±0.53% (3rdday) to 22.99%±2.29% (9thday).
3.4 Responses of the microbial community to nutrient stimulation
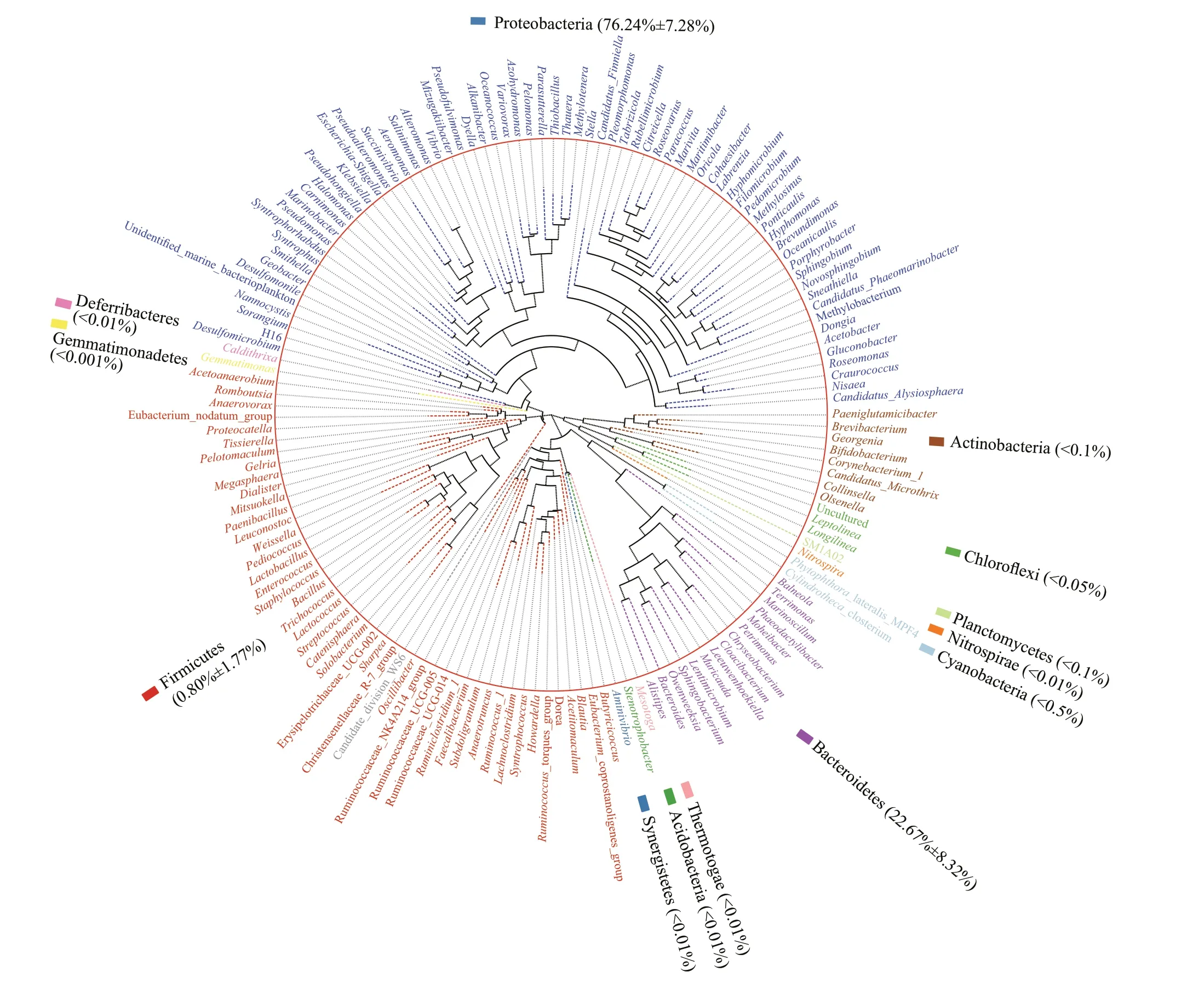
Fig.3 Phylogenetic tree and abundance at phylum level of the microorganisms coexisting with K. mikimotoi
Compared with the control group, the number of coexisting microorganisms increased to 2.5×108-3.9×108cells/mL in the nutrient-stimulated group, and the microbial community compositions showed an obvious change (Figs.4 & 6a), forming a distinct cluster in the NMDS graph (Fig.5a), with a significant weighted unifrac dissimilarity (Fig.5b; AMOVA,Fs=12.637 7,P<0.001). The Wilcoxon rank-sum test revealed the genera with significantly different abundances between the control and nutrientstimulated groups (Fig.6a,P<0.05). Compared with the control group, the genus that belongs to Rhodobacteraceae,Phaeodactylibacter, andMaritimibactersignificantly decreased, whileHalomonasandAlteromonasbecame dominant on the 3rdand 6thdays in the 3% nutrient-stimulated group. It is worth noting thatHalomonasandAlteromonasdecreased again on the 9thday of algal cultivation,whilePhaeodactylibacterandMaritimibacterobviously increased. Accordingly, the microbial community on the 9thday in the 3% nutrient-stimulated group showed a higher similarity with those in the control group (Fig.5b), and clustered together in the NMDS plot (Fig.5a). Differing with the 3% nutrientstimulated group,HalomonasandAlteromonasalways dominated during the algal cultivation process in the 6% nutrient-stimulated group, accounting for 44.38%-55.13% and 5.97%-31.13% of the microbial community, respectively. The community in the 6%nutrient-stimulated group had a higher dissimilarity with those in the control group (Fig.5).
3.5 Responses of the microbial community to added algicidal strain QF1
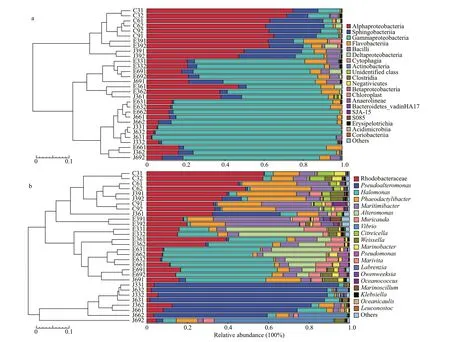
Fig.4 Composition and succession of the microbial communities at the class (a) and genus (b) level
QF1 fermentation broth treatment significantly changed the microbial community coexisting withK.mikimotoi(Figs.4 & 6b; AMOVA,Fs=10.204 9,P<0.001). The changes in the microbial community were also significantly different from those in the nutrient-stimulated group (Figs.4 & 6c; AMOVA,Fs=12.399 8,P<0.001). In the 3% QF1 treated group,the relative abundances ofPseudoalteromonaswere 83.52%-90.83% at the 3rdday, decreasing to 33.42%-68.45% at the 6thday, with further decreases to 1.10%-9.50% at the 9thday. Conversely, the genus that belongs to Rhodobacteraceae,Phaeodactylibacter,andMaritimibactersignificantly increased from 3.74%-5.52%, 0.42%-0.89%, and 0.62%-1.14% on the 3rdday to 37.10%-39.49%, 28.86%-29.45%, and 6.19%-6.80% on the 9thday, respectively. The microbial community at the 9thday in the 3% QF1 treated group showed a higher similarity with those in the control group (Fig.5b), and clustered together in the NMDS plot (Fig.5a). The 6% QF1 treated group had community compositions similar to those of the 3% QF1 treated group at the 3rdand 6thday, andPseudoalteromonassignificantly decreased on the 9thday. Differing with 3% QF1 treated group, the unidentified Rhodobacteraceae genus andPhaeodactylibacterdid not obviously increase on the 9thday. As a result, the microbial community in the 6% QF1 treated group at the 9thday showed higher dissimilarity with those in the control group.
4 DISCUSSION
This study detected a number of microbial populations inK.mikimotoiculture without nutrients and QF1 broth stimulation. The dominant populations were assigned to Rhodobacteraceae and Saprospiraceae, which were broadly observed in marine algal-associated microbial communities, such asUlvaaustralis(Burke et al., 2011)andGymnodinium-diatomm bloom cycles (Shao et al.,2020). Rhodobacteraceae is frequently detected in marine environments, consisting of diverse species which can utilize various organic and inorganic compounds and play important roles in sulfur and carbon biogeochemical cycling (Pujalte et al., 2014).This type of microorganism includesCitreicella,Marivita,Labrenzia,Maritimibacter, andRoseovarius.Species from Saprospiraceae are also frequently isolated from marine environments, which are generally aerobic and chemoheterotrophic, and can hydrolyze complex carbon sources (Mcilroy and Nielsen, 2014). In this study,Phaeodactylibacter, a genus of Saprospiraceae,was detected in theK.mikimotoiculture. Previous researches have isolated the species fromPhaeodactylibacterfrom algaPhaeodactylumtricornutumandPicochlorumsp. (Chen et al., 2014; Lei et al., 2015). These microorganisms likely benefit from the microalgae, which produce dissolved organic carbon compounds and oxygen through photosynthesis, and in turn may improve algal growth by providing nutrient,such as vitamins, iron, and ammonia (Amin et al., 2012).

Fig.5 NMDS analysis (a) based on Bray-Curtis distance and heatmap (b) of weighted unirfac distance of the microbial communities in control and treated groups
Except for the dominant populations,Halomonas,Alteromonas,Pseudoalteromonas, andMarinobacterwere also detected inK.mikimotoiculture without nutrients and QF1 broth stimulation. Previous studies have reported that these populations could inhibit the growth of some harmful microalgae. Extracts ofHalomonassp. HSB07 could inhibit the red-tide microalgaeGymnodinium(Pyrrophyta) (Liu et al.,2013).Alteromonassp. A14 caused a significant decrease inCochlodiniumpolykrikoides(Lee et al.,2008).PseudoalteromonasS1 can lyseAkashiwosanguineain both direct and indirect ways (Sun et al.,2016). In our previous work, algicidal strains fromHalomonas,Alteromonas,Pseudoalteromonas, andMarinobacterwere also isolated from co-cultures ofK.mikimotoiwith sea water and estuarine soil of the Yellow Sea, China, and showed a high inhibition ofK.mikimotoi(Zheng et al., 2018). Although these microbial populations increased slightly during the cultivation ofK.mikimotoi, they never predominated in the coexisting microbial community. In addition,the number of cultivable microorganisms in theK.mikimotoiculture was low during algal cultivation.This could possibly explain why the growth of algal cells was not obviously inhibited, even when coexisting with these microbial populations. This phenomenon indicated that the limited nutrient in the culture ofK.mikimotoiwere not sufficient to promote the growth of the coexisting microorganisms. The limit nutrient refers to the f/2 medium, which contains 75-mg/L NaNO3and 5-mg/L NaH2PO4that can satisfy the growth of microalgae. However, it cannot meet the massive growth of microorganisms because the lack of organic carbon source. As for achieving the purpose of algal inhibition in nutrient-stimulated groups, it was demonstrated later.
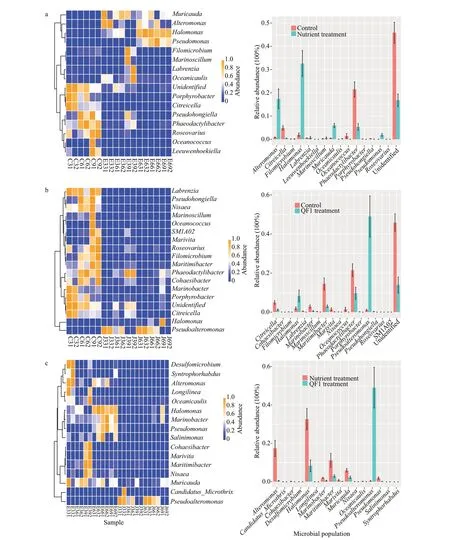
Fig.6 Heatmaps and Histograms with error bar of the microbial populations revealed by Wilcoxon rank-sum test
Nutrient addition increased microbial abundance and obviously changed the composition of the microbial community existing in theK.mikimotoiculture. After nutrient stimulation, the relative abundance of an unclassified genus belonging to Rhodobacteraceae andPhaeodactylibactersignificantly decreased, whileHalomonasandAlteromonasgradually became the dominant populations. In nutrient-stimulated groups, a high mortality ofK.mikimotoiwas observed.Furthermore, the 6% nutrient stimulation resulted in a higher mortality ofK.mikimotoithan the 3% nutrientstimulated groups. Algal inhibition was not observed when we added the same nutrient to the axenicK.mikimotoiculture, indicating that the growth of some microorganisms rather than the nutrient components inhibited algal growth in nutrientstimulated group. Therefore, it has been concluded that nutrient stimulated the growth and metabolism of the coexisting microorganisms in theK.mikimotoiculture,and the latter inhibited algal growth. Almost as remarkably, the microbial communities of 3% nutrients treated groups became similar with those of the control groups at the 9thday, and the relative abundance ofHalomonasandAlteromonasdecreased, but always dominated in the 6% nutrient-stimulated group during the cultivation process. These results indicated the robustness of the microbial community in theK.mikimotoiculture to low-dose nutrient disturbance.
The QF1 fermentation broth had a high algicidal activity against theK.mikimotoi. Although the added QF1 fermentation broth rapidly changed the composition of the microbial community existing inK.mikimotoiculture in the initial phase, the relative abundance ofPseudoalteromonassubstantially decreased in the microbial community on the 9thday.Furthermore,Pseudoalteromonaswas detected inK.mikimotoiculture, yet it did not become a dominant population in the nutrient stimulation process. It seems thatPseudoalteromonasdoes not have competitive edges in the microbial community existing inK.mikimotoiculture. In addition, the robustness of the microbial community in theK.mikimotoiculture to low-dose QF1 fermentation broth disturbance was also observed: the microbial communities of QF1 treated groups became similar with those of the control groups on the 9thday. Therefore, adding fermentation broth ofPseudoalteromonasQF1 has potential application on controllingK.mikimotoi.
It should be noted that 13 OTUs were simultaneously detected in each sample, and predominated inK.mikimotoiculture with or without treatment. These OTUs were assigned to Rhodobacteraceae,Maritimibacter,Marivita,Nisaea,Phaeodactylibacter,Citreicella,Halomonasmeridiana,Alteromonas,Marinobacter,Muricauda, andPseudoalteromonas.Furthermore, these OTUs include the dominant populations inK.mikimotoiculture, such as Rhodobacteraceae,Phaeodactylibacter,Maritimibacter,and the dominant populations in nutrient-treatedK.mikimotoiculture, such asHalomonasmeridianaandAlteromonas. This phenomenon indicated that the aforesaid 13 OTUs are the principal parts of the microbial communities in theK.mikimotoiculture, and the changes of microbial communities observed in theK.mikimotoiculture with or without treatments could be represented by the changes of the 13 OTUs.
5 CONCLUSION
Having investigated the succession of the microbial community existing inK.mikimotoiculture, and the influences of added nutrients and exogenous algicidal strain on the growth ofK.mikimotoi, diverse microbial populations were detected inK.mikimotoiculture and could be selectively stimulated by nutrients, thereby inhibiting the growth ofK.mikimotoi. Adding algicidal strainPseudoalteromonasalso effectively inhibited the growth ofK.mikimotoi. Moreover, the microbial community existing inK.mikimotoiculture showed robustness to low-dose nutrients and QF1 disturbance. This study provides insights for microbial control ofK.mikimotoivia nutrient stimulation of the coexisting microorganisms or by adding fermentation broth ofisolated algicidal strains. However, the amount of the added nutrients or fermentation broth ofisolated algicidal strain will be studied to avoid secondary pollution to the marine environment.
6 DATA AVAILABILITY STATEMENT
The raw reads from 16S rRNA gene Illumina MiSeq sequencing have been submitted to the NCBI Sequence Read Archive (SRA) with the accession number SUB6782573. The 16S rRNA gene sequence of QF1 have been deposited in the GenBank database with the accession number MG457253.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Typhoon-induced wind waves in the northern East China Sea during two typhoon events: the impact of wind field and wave-current interaction*
- Effect of subsea dispersant application on deepwater oil spill in the South China Sea*
- Geochemical characteristics of cold-seep carbonates in Shenhu area, South China Sea*
- Examination of seasonal variation of the equatorial undercurrent termination in the Eastern Pacific diagnosed by ECCO2*
- Deviation of the Lagrangian particle tracing method in the evaluation of the Southern Hemisphere annual subduction rate*
- Immunostimulatory effect of quaternary degree and acetyl group of quaternized chitosan on macrophages RAW 264.7*
