Effects of phytoplankton community and interaction between environmental variables on nitrogen uptake and transformations in an urban river*
Jing YANG, Haiguang PEI, Junping LÜ, Qi LIU, Fangru NAN, Xudong LIU, Shulian XIE,Jia FENG
School of Life Science, Shanxi University, Taiyuan 030006, China
Abstract Phytoplankton are not only the main bearer of the nitrogen cycle, but also a key link driving nitrogen cycle. However, most phytoplankton cannot directly use N 2, and they must uptake nitrogenous nutrients (ammonium, nitrate, and urea) to meet their photosynthesis needs. We examined the uptake characteristics of several nitrogenous substrates using stable isotope technique and identified the potential nitrogen transformations in the Fenhe River. Results revealed that spring phytoplankton community composed of mainly Fragilaria, Ulothrix, Microcystis, and Synedra. Urea can meet the spring partial nitrogen requirement of phytoplankton. The large uptake rate of urea depended on urease, chlorophyll a, and nitrate concentrations as shown in random forest models. Cyanobacteria explained more than 42.8% of the total abundance at all sites in summer. Upstream was dominated by Actinastrum, and Chlorella was relevant in the downstream section. The uptake rates of ammonium were higher than those of nitrate and urea. In addition, the random forest model demonstrated that ammonium, urease, and dissolved oxygen (DO) were the major contributors to the ammonium uptake rates. Ammonium was taken up preferentially in autumn and phytoplankton ( Cyclotella, Chlorella, and Pseudanabaena) appeared to be able to respond to changes in nitrogen forms by adjusting their community composition. Structural equation models demonstrated that temperature-induced changes in DO directly affected the transformations of different forms of nitrogen.At the same time, dissolved organic carbon can directly act on nutrients and then indirectly affect enzyme activity. There were great differences in the positive and negative effects of different paths in the process of nitrate reduction to nitrite and then reduction to ammonium in time and space. These findings provide a better understanding of the underlying mechanism of nitrogen uptake and the influences of interaction between environmental variables on nitrogen transformations in urban river ecosystems.
Keyword: phytoplankton; environmental variables; nitrogen uptake; transformation; urban river
1 INTRODUCTION
Human activities have more than doubled the global input of reactive nitrogen, and these excessive anthropogenic nitrogen emissions have become one of the most serious global environmental problems,which may potentially cause irreversible ecological problems (Tan et al., 2019). For example, a series of environmental problems such as algal blooms, water pollution, global warming, and acid rain are all related to man-made nitrogen overload, especially in the urbanized area. At present, more than half of the world’s population lives in cities (Grimm et al., 2008).The discharge ofindustrial and domestic wastewater has increased nitrogen pollution, which has greatly changed the regional and global nitrogen biogeochemical cycle (Gu et al., 2012; Zhang et al.,2015), seriously threatening the aquatic ecosystem and the health of urban residents. Therefore, studying the process of nitrogen transformation in urban rivers is of great significance for water quality protection and improvement.
High-load nitrogen is considered to be one of the main causes of black and odor in rivers (Hobbie et al.,2017; Song et al., 2017; Liang et al., 2018; Cai et al.,2019). The self-purification function of the river ecosystem can remove nitrogen pollutants through a biologically driven process (Jobin and Namour,2017). However, the assimilation of nitrogen is affected by both environmental factors and phytoplankton community (Steele and Heffernan,2014; Ibekwe et al., 2016; Kuypers et al., 2018).Studies have shown that pollutants from urban rivers affect the community structure and integrity of phytoplankton (Staley et al., 2013; Lin et al., 2014).The affected phytoplankton community in turn disrupted the self-purification process and aggravated the accumulation of water degradation. Therefore, it is critical to understand the processes that impact phytoplankton community composition and abundance changes under the background of both natural phytoplankton community succession and undesirable interference (Treonis et al., 2004; Fierer et al., 2007). Besides, nitrogen assimilation processes depend on various environmental factors such as temperature, the supply of oxygen, light availability,organic carbon concentration, etc. (Gücker and Pusch,2006), and seasonal changes also can dramatically alter biogeochemistry processes, denitrification(Koike and Sørensen, 1988), nutrient remineralization(York et al., 2007), algal growth, and nutrient uptake are more active at the higher temperature.
Phytoplankton assimilation is a key pathway in eutrophic lakes, previous studies only focused on the assimilation of nitrogen at the cellular and individual levels in oceans and lakes (Kang et al., 2017; Bhavya et al., 2020). However, the ecological effects of urban rivers still have not received widespread attention.Research on the nitrogen assimilation of phytoplankton in urban rivers is still scarce, and the impact of interactions between environmental factors on nitrogen transformations is largely unclear. Thus,there is still a lack of prediction models that nitrogen uptake and transformation driven by phytoplankton community composition and environmental factors in time and space. Most importantly, the nitrogen assimilation process cannot proceed normally without the system of enzyme that requires energy, nitrogen,and carbon for expression and synthesis. At present,although there has been a lot of information about the enzyme activity of a single phytoplankton species under specific conditions, research on enzyme dynamics of phytoplankton community in situ is still rare.
Our study area was a section of Fenhe River that running through the busy central urban areas of Taiyuan City, Shanxi Province (see Section 2.1). As a critical part of urban environment, it functions not only to absorb urban waste (human activities,industrial, and agricultural sewage), but also to have a positive impact on flood control and drainage. In addition, the river significantly reduced the urban heat island effect. Therefore, to evaluate the ecological impact of agricultural and domestic wastewater on nitrogen pollution in the urban river section, it is necessary to understand the pollution,assimilation, and transformation of nitrogen in contaminated areas, thereby promoting nitrogen removal through biogeochemical processes(Hayakawa et al., 2006; Reinhardt et al., 2006). The key research issue of this study was the nitrogen uptake characteristics of phytoplankton community and the potential nitrogen transformation pathways involved in enzyme activities and environmental parameters. We hypothesized that dissolved oxygen(DO), pH, and dissolved organic carbon (DOC) may affect the transformation pathway of different forms of nitrogen in the process of nitrogen assimilation by phytoplankton, thereby affecting the activity of nitrogen assimilation enzymes. The study of mutual transformations and uptake of various forms of nitrogen can provide a theoretical basis for the dynamics in the nitrogen cycle and nitrogen nutrition ecology of freshwater ecosystems, which is conducive to the sustainable development of urban rivers.
2 MATERIAL AND METHOD
2.1 Sites description
The Fenhe River is the largest river in Shanxi Province, northern China (35°N-39°N; 110°E-114°E),and the second largest tributary of the Huanghe(Yellow) River (Fig.1). The annual mean precipitation is 504.8 mm and the catchment area is 39 471 km2(Yang et al., 2018). According to the Shanxi Provincial Hydrology and Water Resources Survey Bureau, the water flow of the Fenhe River ranges from 2.12 to 9.44 m3/s, and the speed ranges from 0.35 to 0.58 m/s.Six sampling sites were selected from upstream to downstream from Mar. 2019 to Nov. 2019 (winter is the icing period). We collected the samples from surface water (0-0.5 m) with a 2.5-L Niskin water sampler, the water samples were immediately prefiltered with a 200-mesh screen to remove particulate matter, thereby eliminating the influence of zooplankton and impurities on nitrogen uptake(Truesdale et al., 2003; Moschonas et al., 2017; Arias-Andres et al., 2018; Kaboré et al., 2018), and kept them fresh at 4 °C in the dark and transported to the laboratory measurements within 1-2 h.
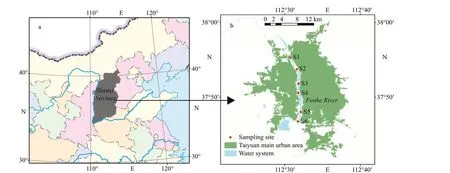
Fig.1 The location of the sampling sites in Fenhe River
2.2 Physiochemical measurement, phytoplankton identification, and enzyme activities determination
(Olympus BX-51). The phytoplankton species were identified according to the methods described in the literature (Hu and Wei, 2006; Wen and Xu, 2010). We mainly reflected the algal cell density by calculating the average cell number of each colony and filament(Yamamoto and Nakahara, 2009; Cataldo et al., 2012;Pan et al., 2014). The Shannon index and Margalefindex were calculated as per Yang et al. (2016). The water quality was evaluated by Saprobic index according to previous studies (Junqueira et al., 2010;Galán et al., 2011; Polla et al., 2016; Taboada et al.,2018).
Ammonia nitrogen (NH4+), nitrite nitrogen (NO2ˉ),nitrate nitrogen (NO3ˉ), and phosphate (PO43ˉ) were determined using nano-reagent spectrophotometry,naphthylethylenediamine spectrophotometry,ultraviolet spectrophotometry, and molybdate spectrophotometry, respectively (Kazi et al., 1987).Urea nitrogen was measured using the diacetgl monoxime method (Revilla et al., 2005);measurements of DOC were made using total organic carbon analyzer (Shimadzu Corporation, Kyoto,Japan). Water temperature (WT) and DO were measured by a Multi-parameter water quality meter(Hydrolab DS5, Hach Company, USA). Chemical oxygen demand (CODCr), permanganate index(CODMn), and chlorophylla(Chla) were measured according to Wang et al. (2008) and Wang et al. (2011b).
The activity of nitrate reductase (NR) was determined by the kit method (Beijing Solabao Bio Co., Ltd.). The determination of glutamine synthetase(GS) activity was optimized based on the method of Magalhaes and Huber (1991). The GS activity is represented by A540/(g·h). The measurement of urease activity was optimized based on the method of Solomon et al. (2007).
2.3 Nitrogen uptake experiments
Phytoplankton samples were immediately fixed with Logol’s solution, and concentrated after 48-h sedimentation, then the concentrated phytoplankton samples (30 mL) were counted under the microscope
The uptake rates of nitrate, ammonium, and urea by phytoplankton community were determined by stable tracer technique (Bhavya et al., 2018). The 250-mL water samples from each sampling site were added to 300-mL polycarbonate bottles, and followed by the addition of three different forms15N tracer(15NH4Cl,15NaNO3, and15Urea), then incubations lasted 2 h. Each tracer was added at ≤ 10% of the ambient concentrations of the nitrogen nutrients(Dham et al., 2005). Three replicates were set for each sample. The saturated ZnCl2solution was added to terminate the reaction after culture. The water sample was filtered by pre-combusted Whatman GF/F filters(450 °C for 3 h), and then 5 mL of deionized water was filtered to wash offthe tracer adsorbed on the membrane. Finally, the phytoplankton filter samples were determined the isotope ratio by an Isotope Ratio Mass Spectrometer (Thermo Fisher Scientific, Inc.,Bremen, Germany). The relative uptake rateV(/h),absolute uptake rateρ(μmol/(L·h)), and relative priority index (RPI) are calculated as follows:
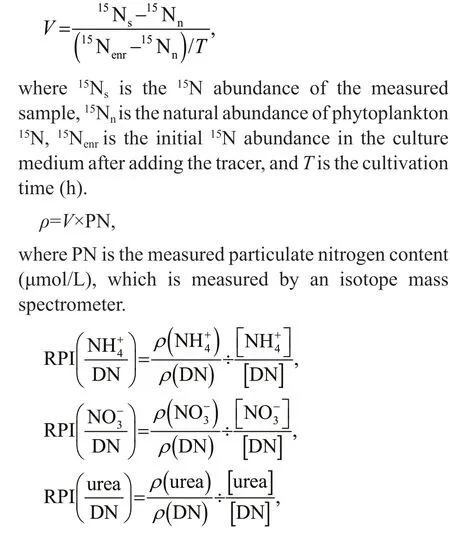
whereρ(NH4+),ρ(NO3ˉ), andρ(urea) are the absolute uptake rate of phytoplankton community to NH4+,NO3ˉ, and Urea, respectively;ρ(DN) is the sum of absolute uptake rates of NH, NO, and Urea by phytoplankton; [NH4+], [NO3ˉ], and [urea] is the concentration of nitrogen in the culture medium,respectively; [DN] is the sum of the concentrations of NH4+, NO3ˉ, and Urea. Turnover time (TT) is the ratio of nitrogen concentration to absolute uptake rate.
2.4 Statistical analysis
Phytoplankton relative abundance was analyzed using a histogram and the “ggplot2” package. The variations of nitrogen uptake and enzyme activity were implemented using software Origin 9.1. The significance (ANOVA variance) analysis of all parameters was conducted by SPSS 26.0. Redundancy analysis (RDA) was implemented with the “vegan”package to determine the relationship among phytoplankton community structure, enzyme, and environmental variables. Random forest (RF) models were applied in the “random forest” function to determine the important variables contributing to nitrogen uptake rates temporally and spatially.Structural equation model (SEM) was completed by the maximum likelihood method using the R package“semPlot” and “lavaan” to identify the possible nitrogen transformation pathways. In the SEM, the absolute fitness index chi-square valueP>0.05,indicating that the covariance matrix of the data itself and the covariance matrix of the model are matched,goodness of fit index (GFI) value greater than 0.9 indicates that the model is ideal, and root mean square error of approximation (RMSEA) value less than 0.05 indicates that the model adaptation is very good. If the parameters after fitting cannot meet the requirements,the model needs to be properly modified until it meets the requirements. All statistics were performed using the Origin 9.1 software and R programming language(version 3.5.1; R Project for Statistical Computing,Vienna, Austria).
3 RESULT
3.1 Temporal and spatial variations of water quality indicators and enzyme activities
In the seasonal cycle of physicochemical parameters at each sampling site, WT in the Fenhe River varied significantly with time (ranged from 15.3 °C in spring to 28.3 °C in summer) (Table 1).The maximum DO occurred in spring while the minimum DO in summer. The concentrations of DOC, CODMn, and CODCrin spring were low during the investigation period, which verified the relatively low organic matter enrichment. The ammonium,nitrate, and urea covered a wide range of concentrations, from 0.032 mg/L (autumn) to 1.082 mg/L (summer), 0.115 mg/L (summer) to 2.500 mg/L (spring), and 0.076 mg/L (spring) to 1.960 mg/L (autumn), respectively. Other nutrients(nitrite and phosphorus) also covered a wide range of concentrations, with 0.014 mg/L to 0.282 mg/L and 0.010 mg/L to 0.373 mg/L, respectively. According to the Saprobic index, the Fenhe River was mainly between α-mesosaprobic and β-mesosaprobic(Supplementary Table S1), and the pollution degree in summer was higher than that in other seasons.
The seasonal and spatial changes of nitrogen assimilation enzymes in Fenhe River were shown in Fig.2. NR, GS, and urease all had significantdifferences in season (P<0.01), but no significant differences in space (P>0.05) (Supplementary Table S2). The activity of NR in autumn was higher than that in summer and spring. GS activity was highest in summer and reached its peak at S4 sampling site. The urease activity was autumn > spring > summer.
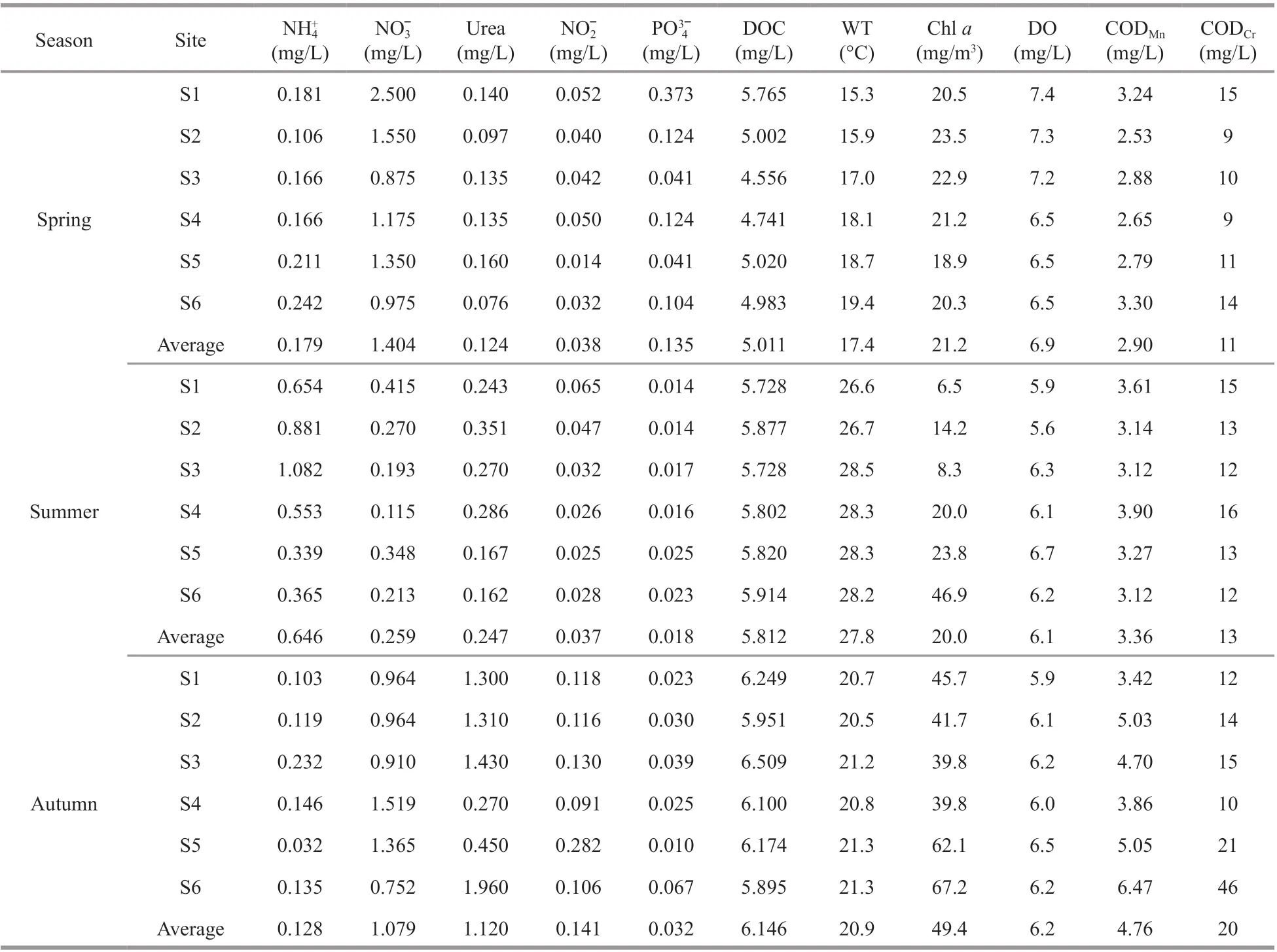
Table 1 Statistics of main physical and chemical parameters in the Fenhe River
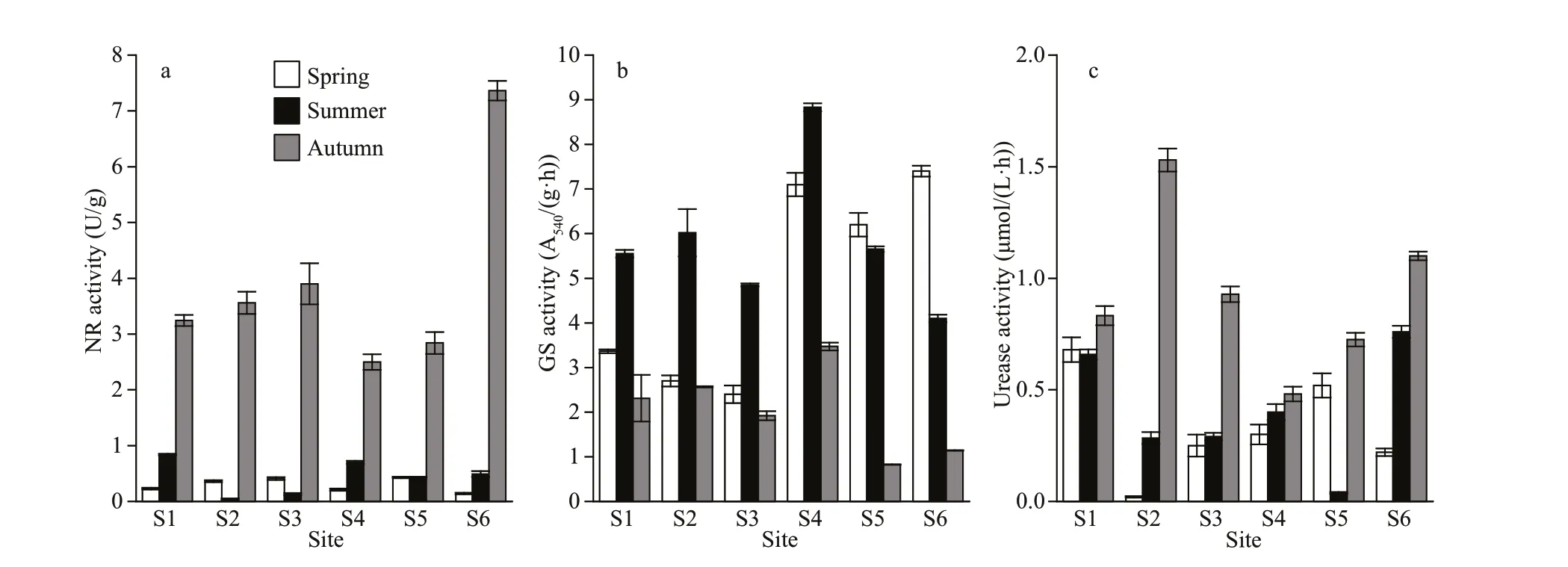
Fig.2 Temporal and spatial variations of enzyme activities
3.2 Temporal and spatial variations in phytoplankton community composition, algal relative abundance, and richness index
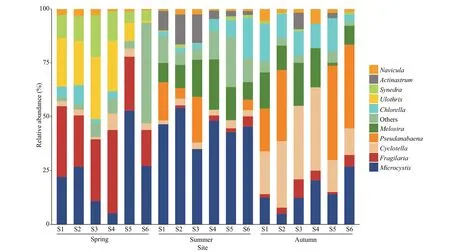
Fig.3 The relative abundance of dominant genera in temporal and spatial realms
The relative abundance of algal cell density of different phylum in temporal and spatial realms of our study area was shown in Supplementary Fig.S1. In general, the relative abundance of cyanobacteria in summer was higher than in other seasons. The relative abundance of Bacillariophyta was relatively higher in spring and autumn. The Shannon diversity index was between 1.50-2.09, and the maximum appeared at the S3 sampling point in spring. Margalef richness index ranged from 0.94 to 1.31. There were also certain differences in dominant genera between seasons(Fig.3). Spring communities of Fenhe River were dominated byFragilaria,Ulothrix,Microcystis, andSynedra, and the relative abundance of diatoms at the upstream was remarkably larger, whereas cyanobacteria were relatively abundant in the downstream. Cyanobacteria explained more than 42.8% of the total abundance at all sites in summer.Upstream was also dominated byActinastrum, andChlorellawas relevant in the downstream section.Cyanobacteria and green algae accumulated as temperature and light intensity increase in summer.Diatoms such asCyclotella,Melosira, andNaviculaand cyanobacteria (Pseudanabaena) had the highest contribution to the relative abundance of phytoplankton community in autumn.
3.3 Temporal and spatial variations of uptake rates, relative preference index, and turnover time
In spring, the15N-normalized uptake rates of nitrate by phytoplankton were higher than those of the other two nitrogen forms at the site S1 (Fig.4). The ranking in the order of average uptake rates (± SD) of different forms of nitrogen was urea > ammonium > nitrate at the site S2, and urea > nitrate > ammonium at the sites S3-S6. In contrast, our study showed uptake rates of nitrate and urea were comparable and often lower than those of ammonium in summer. This trend continues during autumn sampling periods as well. A strong preference for ammonium exceeded urea and nitrate in autumn. It is therefore unlikely that ammonium preference is just a matter of available substrate, but rather a sign that there is a basic difference in the metabolic strategies of phytoplankton in rivers. And this fundamental metabolic difference hypothesis supported that the nitrate uptake rates were relatively lower than other nitrogen substrates throughout sample periods.
RPI was used to determine which nitrogen source is the preferred form of phytoplankton uptake and utilization (McCarthy et al., 1977). RPI value of 1 denotes that the uptake of nutrients by phytoplankton is proportional to the amount provided by it, a value greater than 1 or less than 1 means the phytoplankton has a faster or slower uptake rate relative to the amount of nutrient provided. Urea showed the greatest RPI by phytoplankton in spring followed by ammonium and for nitrate it was less than 1 when it was available (Supplementary Fig.S2). This means nitrate would be used only if other forms of nitrogen were less available. In summer, RPI showed that the nitrate was predominant at the upstream, however, its value was less than 1, and ammonium was typically preferred at the downstream and its value was greater than 1. Ammonium was the dominant preferred substrate in autumn.
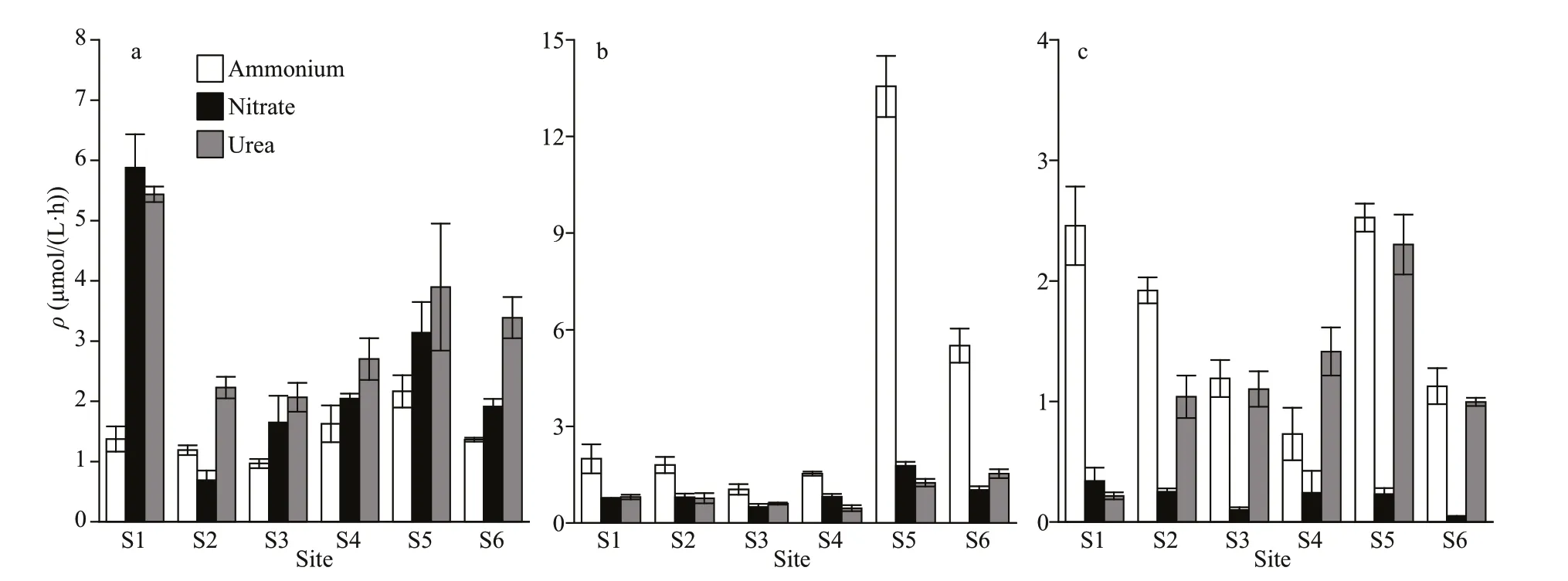
Fig.4 Absolute uptake rates of ammonium, nitrate, and urea in temporal and spatial realms
The relative use of different forms of nitrogen by phytoplankton affected the turnover time and the fate of nitrogen. The high nitrate concentrations and low uptake rates resulted in a long turnover time of nitrate in spring (Supplementary Fig.S3). Warming relatively increased the nitrate RPI, but decreased the turnover time of nitrate. The turnover time of nitrate was higher than those of urea and ammonium at all sites except S1 in autumn.
3.4 Important contribution variables to absolute uptake rates temporally and spatially
RF is one of the powerful and efficient statistical classifiers in ecology. And they were generally used to calculate the contribution of variables to the classification models. Therefore, we used RF models to determine the importance of variables that contribute to absolute uptake rates in time and space(Fig.5).
In spring, the contribution of urease, Chla, and nitrate to the uptake rates of urea were higher according to the important scores of 15 environmental variables. In summer, ammonium, urease, and DO were the major contributors to absolute uptake rates of ammonium. The most important variables for predicting the absolute ammonium uptake rates in autumn were ammonium, phosphate, and nitrite. In our results, the uptake rates of ammonium, nitrate,and urea at downstream were higher than at upstream.Therefore, we identified the important variables that affect the uptake rates of three different forms nitrogen at downstream (Fig.5d-f). Specifically, urease,ammonium, and nitrate were the variables contributing to the uptake rates of ammonium. NR, CODMn, and nitrate were the primary contributors to uptake rates of nitrate. The most important variables for predicting the absolute uptake rates of urea were WT, DOC, and Chla. The pollution was more serious at the downstream because of heavy residential and industrial activities, organic carbon pollutants, and organic nitrogen pollutants, which became important contributing variables to the uptake rate of nitrogen.
3.5 The possible mechanism affecting nitrogen transformations
Nitrogen transformations in lakes are a microbialled biochemical process, and the type, quantity, and activity of microbes depend not only on carbon and nitrogen content, but also on environmental factors such as temperature and nutrients. Therefore, changes in environmental conditions will inevitably affect the nitrification and other processes of nitrogen transformations in water. Our starting point is a“conceptual model”, that is SEM, which is a priori method that can be used to visualize the causal links between variables through fitting data (Hu et al.,2017). Therefore, we utilized this model to analyze direct and indirect pathways between several latent variables (i.e., physical environment, chemical variables, enzymes, and phytoplankton dynamics)and nitrogen transformations (Figs.6-7).
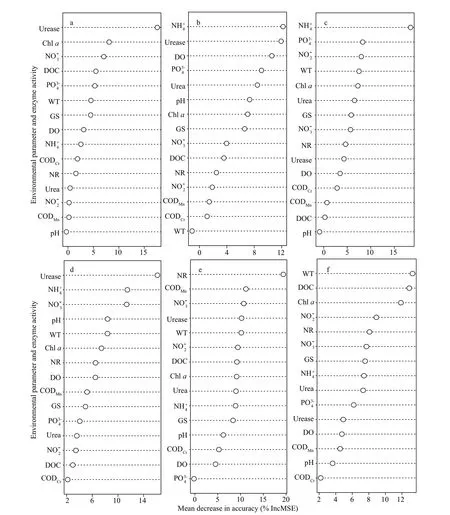
Fig.5 Predict important contribution of each variable to nitrogen uptake rate by random forest (RF) model
As represented in our structural equation models,WT always imposed a positive impact on pH (P<0.01),whereas negative impact on DO (P<0.01), this relationship persisted from spring to autumn. One relationship lied in the alkaline changes in pH driven by DO, which was due to the increased photosynthetic activity of planktonic algae to produce oxygen, or to the chemical nature of water (Fathi et al., 2001).Temperature-induced changes in DO had direct impacts on different forms of nitrogen and become the critical limiting factor in this process. Specifically,DO had directly negative effect on ammonium and urea in spring and autumn, while only had a negative effect on urea in summer. In spring and autumn, pH only had a positive effect on urea, and positive effect on both nitrite and urea in summer. The main transformation pathways of nitrate: nitrate is first converted nitrite and then to ammonium, these two steps were all negatively correlated in spring and summer, while the transformation processes in autumn were all positively correlated.
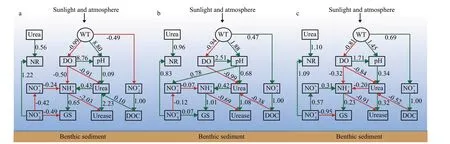
Fig.6 The possible mechanism of affecting nitrogen transformation based on structural equation model (SEM) in temporal realm
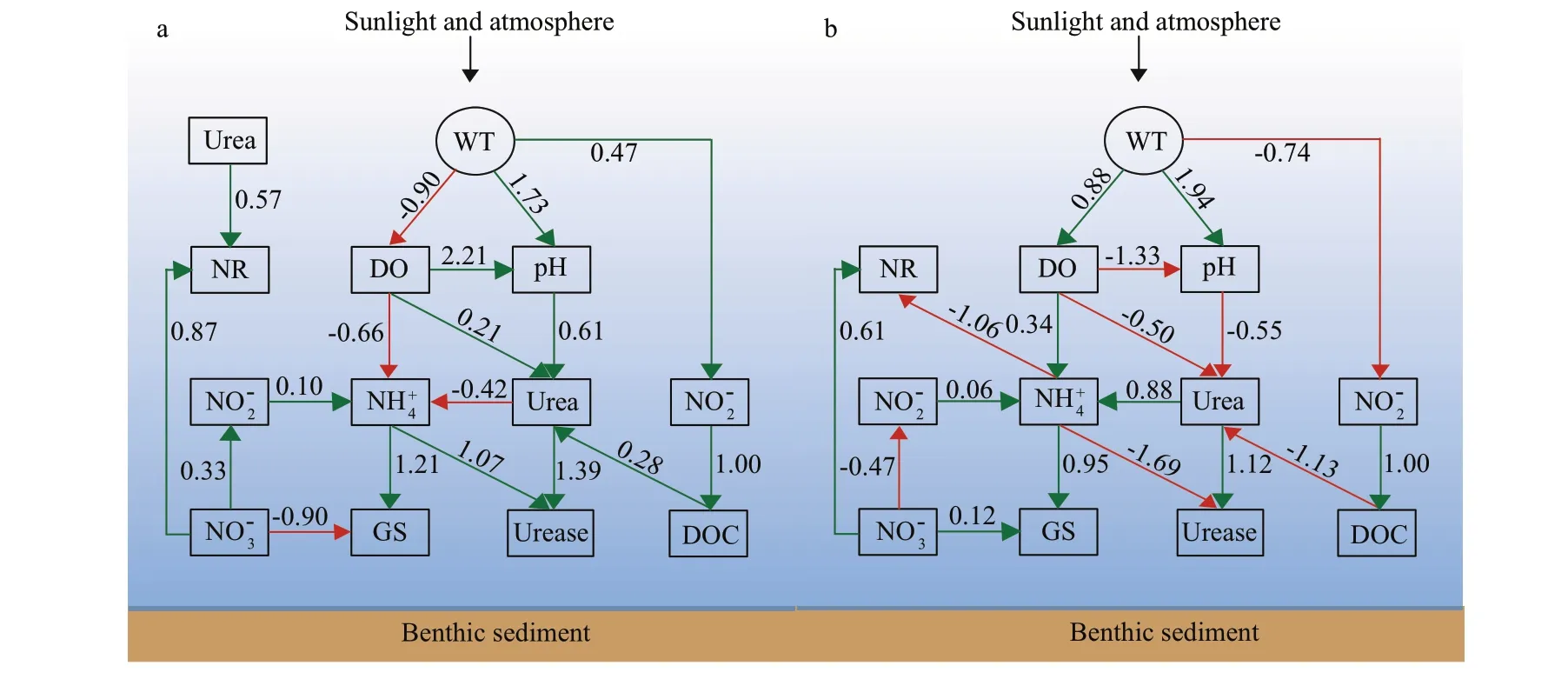
Fig.7 The possible mechanism of affecting nitrogen transformation based on structural equation model (SEM) in spatial realm
The effects of DO and pH on enzyme activities were directly mediated by ammonium, nitrate, and urea. DOC had a positive effect on urea in spring,whereas negative effects on urea in summer and autumn (P<0.05), and urea concentrations across Fenhe River followed a positive relationship with measurements of urease activity (i.e., urease activity will increase with increasing urea concentrations)(P<0.01). At the same time, complete the transformation of urea to ammonium, an important link in the nitrogen cycle in aquatic ecosystems, then ammonium had directly negative effect on urease and positive effect on GS.
In our study area, downstream occurs black and odorous water bodies, the reason is caused by insufficient DO and the discharge of pollutants in water. Wang et al. (2011a) pointed out that the phenomenon of black odor in rivers is actually a biochemical phenomenon, the organic matter in water body consumes more oxygen than reoxygenation during the decomposition process, resulting in an anoxic environment. Microorganisms decompose the organic matter to produce a large amount of odorous gas that escapes from the water surface into the atmosphere, causing the water body to be black and smelly. Urban rivers not only supply water, but also have become the main discharge place for urban industrial wastewater and domestic wastewater,which increasing the loads of organic carbon pollutants, organic nitrogen pollutants, and phosphorus-containing compounds in rivers. From the proportional relationship between DO and urea(P<0.05) at downstream, it can be seen that the production of urea was accompanied by a large consumption of DO (Fig.7). DOC can produce both positive effect (upstream) and negative effect(downstream) on urea, and then completed the conversion to ammonium. These results demonstrated that the content of nitrogen can be adjusted by DO and DOC concentrations.
4 DISCUSSION
DO is one of the important state factors that can be used for water quality evaluation, inadequate oxygen in water can lead to the migration of phytoplankton species, which threatens ecosystem health. The seasonal difference of DO was mainly attributed to the water temperature, as solubility of oxygen in water decreased with increasing temperature. The highest value of nitrate reflected the direct impact of the agricultural runoffin spring (York et al., 2007;Fathi and Al-Kahtani, 2009), while the lower value is because of nutrients uptake by primary producers(phytoplankton) in summer and autumn (Fig.4). In general, urea concentration in natural water would not exceed 1 mg/L (Finlay et al., 2010), however, the agriculture activities, local animal communication capabilities (such as bird aggregation, krill populations), and direct excretion and decomposition of undigested feed can increase the supply of urea(Huang et al., 2017). The relatively higher DOC concentrations were found in autumn and summer,suggesting a large net in-stream release of DOC at this time, which may be from terrestrial input. The period of high DOC concentration in summer demonstrated that the DOC concentration-temperature relationship occurred, which increased the changes in the high DOC concentration-emission relation. The temperature in autumn is still sufficient for organisms to produce higher DOC concentrations (Dawson et al., 2008). Some measurements performed in the United States and Asia (Avery et al., 2006, 2013)indicated that anthropogenic emissions in urban areas(waste incineration, traffic, industrial metallurgical processes, steel industry, coal combustion,construction works, etc.) can be the source of hydrophobic portion of DOC.
The enzyme-mediated processes are intimately related to nitrogen uptake in aquatic systems, and the variations in interrelated enzyme activities also can directly reveal the physiological rate of phytoplankton and the nitrogen assimilation (Liu et al., 2015). The NR activity in autumn was much higher than in other seasons (Supplementary Table S2), the assemblages were dominated by green algaeChlorellaand diatom such asCyclotellaandMelosira(Fig.3). And NR activity was easily repressed during ammonium growth in summer, while the enzyme was synthesized during urea growth in autumn. In spatially, the NR activity was largest at site S6. The GS activity was higher in summer except sites S5 and S6. This reflected a key finding that regarding enzyme activities: GS activity was related to high ammonium, low nitrate,and high autotrophic biomass (Fig.2 and Table 1).However, GS activity was higher where the total dissolved inorganic nitrogen concentration was low.Some studies demonstrated that nitrogen-deficient algal cells have higher GS activity than algal cells grown under high-level external nitrate conditions(Iriarte et al., 2005). The phytoplankton assemblages were characterized by cyanobacteria (MicrocystisandPseudanabaena) and the green algaeActinastrum,they were major contributions to the phytoplankton relative abundance during high GS activity. Urease activity attached a peak in autumn, which might be due to the accumulation of urea. Solomon et al. (2010)pointed out urea could induce the urease activity.
Lomas and Glibert (2000) demonstrated that diatom blooms are usually related to higher nitrate supply because they have physiological adaptability that allows them to utilize nitrate (Arora et al., 2020;Arora and Philippidis, 2021). Furthermore, the uptake of nitrate by diatoms has demonstrated a temperature dependence (uptake rates decrease with increasing temperature), however, it has been reported to occur only under aerobic conditions(Tantanasarit et al., 2013; Kamp et al., 2015). Our results revealed that urea meet the partial nitrogen requirement of phytoplankton in spring in the study area. Little or no measurement of urea uptake in previous studies, we found that it can provide a high nitrogen demand for phytoplankton community(intact, surface-water community) and make an important contribution to the overall assimilation process. As the most abundant nitrogen pool, the uptake rates of nitrate were always lower than that of urea. The lower nitrate rates may be because of the metabolic cost of reducing nitrate to ammonium.According to the RF models, ammonium concentration in summer and autumn were the reasons for high ammonium uptake rates. As shown in Fig.6, although the summer temperature did not directly affect ammonium, it can produce a positive effect on ammonium through DO, thereby enhancing the uptake rates. It is known that light has a stimulating effect on phytoplankton nitrite excretion during nitrogen assimilation (Al-Qutob et al., 2002).The concentration of nitrite was relatively higher in autumn and accumulated in the aquatic ecosystem,causing the phytoplankton to quickly absorb ammonia. Killberg-Thoreson et al. (2021)demonstrated that the ammonium uptake rate of York River in autumn accounted for the largest proportion of total uptake, which is consistent with the result in this study. The source of ammonium nitrogen appears to be the remineralization of organic matter and release from the sediments as the water body stratified and oxygen depleted (Lai et al., 2021).
In addition, on the whole, the uptake rates of urea and nitrate were mainly spring > autumn > summer(P<0.01) (Supplementary Table S2), while the uptake rate of ammonium had no significant difference between seasons. Phytoplankton usually absorb nitrogen and phosphorus in a certain proportion according to their physiological structure and substance synthesis needs. Therefore, the assimilation of nitrogen is also strongly associated with the availability of phosphorus, and the estechiometric ratios provide a good idea for studying the coupling process of nitrogen and phosphorus. We performed the correlation between the assimilation of nitrogen and the availability of phosphorus through SPSS.This study showed that the availability of phosphorus was significantly positively correlated with the uptake rates of nitrate and urea. It can be seen that sufficient phosphorus is very important to the assimilation of nitrogen, and insufficient phosphorus content will greatly affect the assimilation of nitrogen. Our study showed uptake rates of nitrate and urea were comparable and often lower than those of ammonium in summer. In contrast, studies from other regions have found that the uptake rate of nitrate was usually equal to or more than ammonium uptake, which is more important due to “new” rather than “regenerated”production (Dugdale and Goering, 1967; Baer et al.,2017).
For RPI, ammonium appeared to be the preferred nitrogenous nutrient by phytoplankton (Supplementary Fig.S2), which is also a common observation in other aquatic systems (Domingues et al., 2011). According to the known rates of urea hydrolysis to ammonium(Solomon et al., 2010), this enrichment of urea-N can quickly become a source of ammonium-N enrichment.And the preference for ammonium was thought to be partly due to the reduced energy demand for cells,ammonium is more easily transported through cell membranes under N-limiting conditions. Delayed uptake of nitrate relative to ammonium was observed in this study, the literature indicated that the diatomdominated community in autumn stopped absorbing nitrate when the concentration of ammonium is greater than 10 μmol/L, and subsequently the uptake of nitrate and diatom growth were restored when the consumption of ammonium is less than 4 μmol/L(Glibert et al., 2016).
In our study, nutrients (especially urea) were subject to the direct positive/negative effects of organic carbon, further regulating the fundamental microbial processes and interactions, and ultimately coupling the biogeochemical cycles of carbon and nitrogen (Taylor and Townsend, 2010; Lunau et al.,2013). As the main medium of biological processes,enzymes can closely involve in the catalytic reactions necessary for the decomposition of organic matter,nutrient cycling, energy transfer, and environmental quality. GS is a highly regulated enzyme that participates in various reactions related to N metabolism, its synthesis and activity are easily controlled by various environmental variables (Hong et al., 2011). McCarty (1995) pointed out the inhibitory effect of ammonium on urease production in summer was related to the assimilation effect of GS activity on ammonium. In addition, nitrate also had a strong positive effect on NR activity (P<0.01). There is a positive correlation between NR and nitrate assimilation in phytoplankton (Yu et al., 2018). The high DO content in spring reduced the production of ammonium, but enhanced the nitrate concentration and NR activity, which resulting in an increase of uptake rates. Li et al. (2012) indicated that there are 184 industrial and household pollution sources in Fenhe River. The main sources ofindustrial pollution including coal mining and preparation, iron making,coking, and chemical industry. There is also a portion of untreated municipal and industrial wastewater directly discharged into adjacent rivers or lakes through a large number of pipes and sewers. Results demonstrated that we can control the content of nitrogen by adjusting DO and DOC concentrations.
5 CONCLUSION
Our results demonstrated that the uptake rates of nitrate and urea were highest in spring, and the phytoplankton assemblages were mainlyFragilaria,Ulothrix, andSynedra. The uptake rate of ammonium in summer was significantly higher than other seasons,ammonium, urease, and DO were the major contributors. SEM revealed that temperature-induced changes in DO had direct impacts on different forms of nitrogen. DOC also had a positive (spring) and negative (summer and autumn) effect on urea, then indirectly acted on ammonium. Besides, urea concentrations followed a positive relationship with urease and NR. GS activity was affected by ammonium(positive effect) and nitrate (positive/negative effects).There were great differences in the positive and negative effects of different paths in the process of nitrate reduction to nitrite and then reduction to ammonium in time and space. Our results provide insights into the dynamics of phytoplankton and environmental variables to the nitrogen cycle in the region, thus providing a technical reference for pollution control and management of other urban rivers.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We are grateful to the Dr. Zhonghua ZHAO(Nanyang Technological University, Singapore) for the editorial assistance with the English.
 Journal of Oceanology and Limnology2022年3期
Journal of Oceanology and Limnology2022年3期
- Journal of Oceanology and Limnology的其它文章
- Typhoon-induced wind waves in the northern East China Sea during two typhoon events: the impact of wind field and wave-current interaction*
- Effect of subsea dispersant application on deepwater oil spill in the South China Sea*
- Geochemical characteristics of cold-seep carbonates in Shenhu area, South China Sea*
- Examination of seasonal variation of the equatorial undercurrent termination in the Eastern Pacific diagnosed by ECCO2*
- Deviation of the Lagrangian particle tracing method in the evaluation of the Southern Hemisphere annual subduction rate*
- Immunostimulatory effect of quaternary degree and acetyl group of quaternized chitosan on macrophages RAW 264.7*
