In Vivo Development of Fetal Pig Kidneys in Mature Monkeys under Clinically Approved Immunosuppressant Drugs
Tsuyoshi Tkmur, Knji Mtsui, Noto Mtsumoto, Ytsumu Sito, Toshinri Fujimoto,Susumu Tjiri, Shuihiro Ymnk, Ki Mtsumoto, Akimitsu Koyshi, Izumi Ymmoto,Hiroshi Sski, Hruyuki Hirym, Hitomi Mtsunri, Kzuki Nkno, Hiroshi Ngshim,Akihiko Kiyoshi, Tko Kuro, Mkoto Inou, Tkshi Miywki, Tkshi Yokoo, Eiji Koyshi*
a Division of Nephrology and Hypertension, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo 105-8461, Japan
b Department of Urology, The Jikei University School of Medicine, Tokyo 105-8461, Japan
c Department of Plastic and Reconstructive Surgery, The Jikei University School of Medicine, Tokyo 105-8461, Japan
d Meiji University International Institute for Bio-Resource Research, Kanagawa 214-8571, Japan
e Sumitomo Dainippon Pharma Co., Ltd., Osaka-fu 564-0053, Japan
f Department of Kidney Regenerative Medicine, The Jikei University School of Medicine, Tokyo 105-8461, Japan
Keywords:Cynomolgus monkey Pig Kidney Fetal kidney Immunosuppression
ABSTRACT Controlling the immune response with only clinically approved immunosuppressant drugs is difficult in renal heterotransplantation from pigs to nonhuman primates.Moreover,to the best of our knowledge,no reports exist on the use of fetal pigs as kidney donors. This study aimed to compare the degree of transplant rejection between neonatal and fetal kidneys, with genetically unmodified pigs as donors and cynomolgus monkeys as recipients. The left kidneys of the recipient monkeys were removed, followed by transplantation of neonatal as well as fetal pig kidneys, which had undergone vascular anastomosis at the same site, into the retroperitoneum. Immunosuppression was performed with only US Food and Drug Administration-approved drugs. The fetal kidneys were transplanted into the omentum and paraaortic regions of cynomolgus monkeys. Consequently, the engraftment and development of the transplanted tissues were pathologically examined by sampling over time (twice in each experiment). An acute rejection was observed after a few weeks in neonatal renal grafts with vascular anastomosis.However, fetal pig kidneys were spared from rejection despite the administration of the same immunosuppressive protocol to the monkeys and the recipient blood vessels flowing into the fetal kidneys. The immunogenicity of fetal kidneys in pig–monkey renal heterotransplantation was lower than that of neonatal kidneys.
1. Introduction
Heterologous transplantation has captured attention as a means to avoid the problem of organ donor shortage since the first organ transplantation was performed.Consequently,there has been progress in advanced methods for the genetic engineering of pigs.Genetically manipulated pigs, useful for heterotransplantation,have been produced, and their verification in preclinical models with non-human primates as the recipients is in progress [1,2].In recent years, there have been several reports of long-term engraftment via control with extremely strong immunosuppressant drugs [3–5]. However, immunosuppression with only immunosuppressant drugs that are clinically approved in humans has shown limitations in its effectiveness in preclinical models of pigs and nonhuman primates [6].
Therefore, we aimed at developing a new therapeutic method for donor kidney fabrication that would use chimeric fetal kidneys produced using tissue engineering via in vitro injection of human-derived renal progenitor cells into pig fetal kidneys as xeno-regenerative medicine [7]. As a preliminary step toward this goal, this study examined the superiority of pig fetal kidneys over vascularized neonatal kidneys as grafts in a non-human primate model. Theoretically, the inflow blood vessels of the transplanted fetal kidneys are of the host,and vascular endothelial dysfunction,the first target of heterologous rejection, may be avoided in this method [8,9].
Although a few reports involving small animals are available,to the best of our knowledge, no report of a clear experimental history involving pig–monkey preclinical models currently exists[10]. Thus, we believe that this study is the first to compare and examine the engraftment degree of a model with genetically unmodified normal pigs and cynomolgus monkeys as donors and recipients, respectively, under an immunosuppressive protocol using clinically approved immunosuppressant drugs.
Initially, newly developed vascularized neonatal kidneys [2]and pig fetal cloacae comprising fetal kidneys,ureters,and bladder were transplanted into the same recipient monkeys,and the differences in the degree of development and immune reaction were verified in this study.Then,the pig fetal cloacae were transplanted around the aorta and omentum of the recipient monkeys and observed for a long period. Consequently, the degree of development was observed pathologically over time.
2. Materials and methods
2.1. Experimental animals and ethics
Wild pigs were used as donors,and cloacae of vitrified cryopreserved E28–30 were thawed and used for fetal kidneys. Furthermore, neonatal pig kidneys of 20–28-day-old pigs weighing 5.00–6.12 kg were used as donor kidneys. The experiments were conducted according to the appropriate guidelines for animal experiments.
Experiments on neonatal pig kidneys were conducted in the laboratory of IVTeC Co., Ltd. (Japan) with the approval of the Animal Experiment Ethics Committee of IVTeC Co., Ltd. (approval numbers: IVT20-26 and 20-84; experiment numbers: K-20-019 and K-20-051).The experiments on the recipients were conducted at the laboratory of Sumitomo Dainippon Pharma Co.,Ltd.,using 5-to 10-year-old cynomolgus monkeys weighing 6.5–7.9 kg with the approval of the Animal Ethics Committee of Sumitomo Dainippon Pharma Co., Ltd. (approval number: AN12843; experiment numbers: RD-AN12843-02, RD-AN12843-03, and RD-AN12843-04).All heterologous regenerated kidney experiments were approved by the Animal Ethics Committee of the Jikei University School of Medicine (approval number: 2020-055).
2.2. Experiment 1
2.2.1. Transplantation of neonatal kidneys and fetal cloacae
The transplantation of neonatal pig kidneys with vascular anastomosis was performed according to the method described by Takamura et al. [2]. Schematics of the experimental plans are shown in Fig. 1. The blood flow of the neonatal kidney donors was cut off following an abdominal midline incision.Subsequently,the kidney was removed along with the blood vessels and ureter after reflowing with a preservation solution consisting of 465 mL of Euro-Collins’ solution, 35 mL of 50% glucose solution, and 1 000 units of heparin.It was brought to the recipient facility to prepare for the transplantation (total storage time, 3 h).
All recipient monkeys fasted from the evening of the previous day until the surgery. Maintenance anesthesia was administered using isoflurane (0.5%–2.0%) inhalation following an induction of atropine(0.1 mg·kg-1,intramuscular injection(i.m.))and ketamine(10 mg·kg-1,i.m.)on the day of surgery.Butorphanol(0.1 mg·kg-1,i.m.) was used as an analgesic and 47 500 units per body of benzylpenicillin potassium was used as an antibacterial drug before and after surgery.
Laparotomy was performed on the pretreated recipient monkeys through an abdominal midline incision under general anesthesia. The left kidney was removed after ligation of the left renal artery/vein and left ureter; the pig neonatal kidney was placed orthotopically,and the blood vessels and ureter were anastomosed [2].
The retroperitoneum near the aorta and the left kidney was then bluntly exfoliated to form a pocket, and two cloacae were transplanted into the pocket of each animal using a spatula [2].The pocket was closed after the transplantation with LIGACLIP®(Johnson&Johnson,USA).Similarly,two cloacae were transplanted into the omentum,and the pocket was closed with LIGACLIP®.This prevented the cloacae from falling out, as they were covered by omental tissues. After the cloacae were transplanted to the paraaorta and omentum, the abdomen and wound were closed, and the surgery was completed.
2.2.2. Transplant tissue recovery
The abdomen was reopened via an abdominal midline incision 13 days after the transplantation.The omentum was exposed after laparotomy,and the transplanted fetal kidney was identified using the part clipped with LIGACLIP®as a guide. A part of the transplanted fetal kidney was dissected along with the surrounding omental tissues to avoid injury, and the tissues were recovered.
Subsequently, the intestines were manually moved, and the transplanted pig kidney was exposed.The area surrounding the left renal artery/vein and left ureter was exfoliated,and the abdominal aorta and inferior vena cava were exposed. After ligating the left renal artery, the left renal vein and the left ureter were ligated,and the transplanted kidney was dissected at the pig renal arteriovenous side and removed en masse. Finally, the abdomen and wound were closed,and the surgery was completed.The administration of immunosuppressant drugs was continued after surgery while observing the dietary intake status.
Furthermore, the remaining fetal kidney, transplanted to the omentum, was identified again using the same method 27 days after the transplantation. The animals were sacrificed by intrathoracic exsanguination,the transplanted tissues were recovered,and the experiment was completed.
2.3. Experiment 2
2.3.1. Fetal kidney transplantation
The preoperative and postoperative preparations of the recipient monkeys were similar to those performed in Experiment 1.The whole abdomen was disinfected with povidone iodine,incised in the middle, and opened after anesthesia administration. The omentum and intestines were manually moved to expose the retroperitoneum after laparotomy. The retroperitoneum near the aorta and the left kidney were bluntly exfoliated to form a pocket,and two cloacae were transplanted into the pocket of each animal using a spatula. Subsequently, the pocket was closed with LIGACLIP®after the transplantation. Next, three cloacae were similarly transplanted into the omentum, and the pocket was then closed with LIGACLIP®. To ensure that the cloacae did not fall out, they were covered with omental tissues.After transplanting the cloacae to the para-aorta and omentum, the abdomen and wound were closed, and the surgery was completed.
2.3.2.Recovery of omental fetal kidney/anastomosis of the para-aortic fetal bladder and urinary tract
The abdomen was reopened using an abdominal midline incision 33 days after the transplantation.After laparotomy,the omentum was exposed and the whole transplanted fetal kidney was identified, using the part clipped with LIGACLIP®as a guide. The fetal kidney was then dissected with the surrounding omental tissues to avoid damage, and the tissues were recovered.
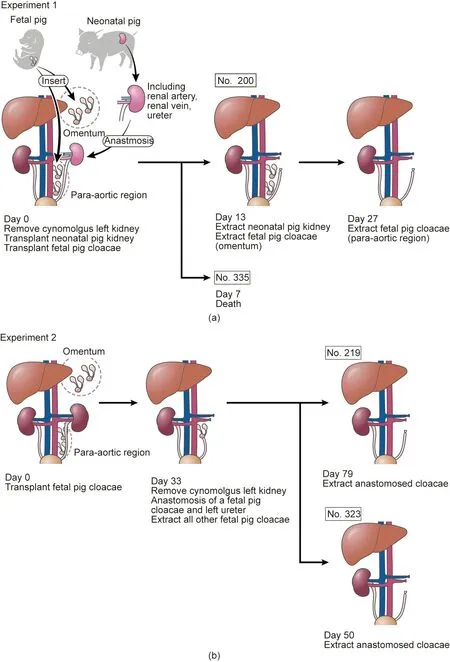
Fig. 1. Schematics of the experimental plans.
Similarly,the area surrounding the left renal artery/vein and the left ureter was exfoliated after the transplanted fetal kidney to the para-aortic region was identified, and the nearby abdominal aorta and inferior vena cava were exposed. After ligating the left renal artery, the left renal vein and left ureter were ligated, and the left kidney was removed. After the removal, urinary reconstruction was performed via end-to-end anastomosis of one of the fetal bladders and the left ureter of the recipient monkey by the parachute suture technique. Finally, after the urinary reconstruction, the abdomen and wound were closed,and the surgery was completed.
2.4. Para-aortic fetal kidney recovery after ureter formation
The abdomen was reopened using an abdominal midline incision 50 or 79 days after the transplantation. The omentum and intestines were moved manually after the laparotomy, and transplant tissues at the para-aortic region were identified. The right renal artery was ligated after identification, and approximately 100 mL of extracellular fluid(normal saline,glucose,and VITAMEDIN®(Alfresa Pharma,Japan))from the intravenous line along with furosemide (20 mg) and indigo carmine (20 mg) were administered. Following observation for >15 min, urine production from the transplanted tissues was evaluated. Subsequently, the transplanted tissues were dissected from the surrounding tissues and recovered, completing the experiment.
2.5. Immunosuppressive, anti-inflammatory, and supportive therapy
Immunosuppressive therapy was administered following a previous report, with some modifications [2,6], the details of which are provided in Table 1. The immunosuppressant drugs were allUS Food and Drug Administration (FDA)-approved, but the protocol was not clinically adopted.

Table 1 Immunosupresive, anti-inflammatory, and adjunctive drugs used in this study.
Specifically, this entailed tacrolimus (TAC) (0.02 mg·kg-1, i.m.,twice a day starting from day –9), abatacept (50 mg·kg-1, intravenous drip(div),once a week starting from day 7),and mycophenolate mofetil(MMF)(40 mg·kg-1,oral administration(p.o.),twice a day starting from day 5). Methylprednisolone (10 mg·kg-1, div)was also administered starting on day 0 and switched to p.o.from the next day, before tapering off. Thymoglobulin (10 mg·kg-1,intravenous injection (i.v.), on day –3), rituximab (10 mg·kg-1,i.v., on day –2), tocilizumab (10 mg·kg-1, s.c., on days –1, 7, and 14),and etanercept(0.5 mg·kg-1,s.c.,on days 0,3,6,and 10)were also administered.
The concentration of TAC and MMF in the blood was measured twice a week,and the dosage was adjusted as appropriate with the target trough level as the standard(TAC trough was days–9–2,15–20 ng·mL-1; after day 3, 9–12 ng·mL-1; and MMF trough was 4–6 μg·mL-1).
2.6. Monitoring of recipient monkeys
The daily dietary status and activity of the recipient monkeys were confirmed and supported through supplementation,if necessary. Moreover, their general condition was evaluated twice-aweek by weight measurement, body temperature, SpO2measurements,and blood sampling.In addition to TAC and MMF,red blood cell (RBC), hemoglobin (Hb), hematocrit (Ht) (mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC)), reticulocyte(RET), white blood cell (WBC) (neutrophils, lymphocytes, monocytes,eosinophils,and basophils),platelets(Plt),prothrombin time(PT), activated partial thromboplastin time (APTT), fasting blood glucose (Fbg), total protein (TP), albumin (Alb), total bilirubin (TBil), lactate dehydrogenase (LDH), aspartate aminotransferase(AST), alanine aminotransferase (ALT), alkaline phosphatase(ALP), γ glutamyl transferase (γGTP), creatine kinase (CK), blood urea nitrogen (BUN), creatinine (Cr), sodium (Na), potassium (K),chlorine (Cl), calcium (Ca), phosphorus (P), glucose (Glu), total cholesterol (T-Cho), triglyceride (TG), and C-reactive protein(CRP) were also measured via blood tests.
2.7. Histological and immunohistochemical evaluations
The recovered specimens were fixed with 4%paraformaldehyde and embedded in paraffin. They were then separated into 4 μm thick sections and stained with hematoxylin and eosin (HE), periodic acid–Schiff, and Masson’s trichrome stains.
For immunohistochemistry,4 μm-thick serial sections from the formalin-fixed specimens were deparaffinized and hydrated using graded concentrations of ethanol and deionized water. The tissue sections for the staining of CD3 and CD31 were subjected to antigen retrieval using 0.01 mol·L–1sodium citrate buffer in a pressure cooker for 10 s (preheat buffer for 30 s). Following antigen retrieval, the sections were gently washed with phosphate-buffered saline (PBS). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 20 min.After washing with PBS,all sections were blocked with 5% skim milk for 30 min. All slides were then incubated at 4 °C overnight with an anti-CD3 antibody (MA5-12577;Invitrogen, USA) or anti-CD31 antibodies (ab28364 and JC70/A;Abcam, UK). After washing with PBS, the sections were incubated with Histofine®Simple Stain (424151; Japan) for 30 min.Diaminobenzidine was used as the chromogen and hematoxylin as the counterstain.
2.8. Evaluation of erythropoietin (EPO) gene expression
Some cloacae collected on day 50 of Experiment 2 were stored in RNAlater (R0901; Sigma-Aldrich, USA). RNA was isolated from the collected samples using the RNeasy Micro Kit(74134;QIAGEN,Netherlands). The polymerase chain reaction (PCR) conditions were as follows: 94 °C for 2 min; followed by 40 cycles at 98 °C for 10 s, 60 °C for 15 s, and 68 °C for 1 min. The primers used are listed in Table 2.
3. Results
3.1. Experiment 1: Immunological benefits of fetal kidney against xenogeneic rejection reaction
The dosage of immunosuppressant drugs was increased or decreased based on the general condition and blood biochemical test results. The resulting pharmacokinetics are shown in Fig. 2(Experiment 2 is also shown here). In addition, a temporary increase in hepatic enzymes was observed after the initiation of immunosuppressant drug administration in all cases. Postoperative anemia was also observed.
One subject died on postoperative day 7 due to sudden worsening of its condition. The other subject was relatively more stable.However,its abdomen was reopened 13 days after transplantation to extract the vascularized pig kidney and perform a cloacae biopsydue to the worsening of its condition and the transition of blood platelets. The extracted kidney was significantly more swollen compared to when it was transplanted (weight, 21–124 g; maximum diameter, 5.2–10 cm) and the color also changed to dark red, whereas the cloacae were visually preserved (Figs. 3 and 4).Pathological evaluation revealed that the tissues were mostly destroyed.Although no obvious glomerular,tubular,or peritubular inflammatory findings could be detected due to the effects of severe interstitial hemorrhage and cortical necrosis, severe arteritis with fibrin thrombus and luminal obstruction were observed.Polynuclear cells deeply stained with HE and CD3-positive cells were observed in large amounts (Figs. 5(a)–(e)).
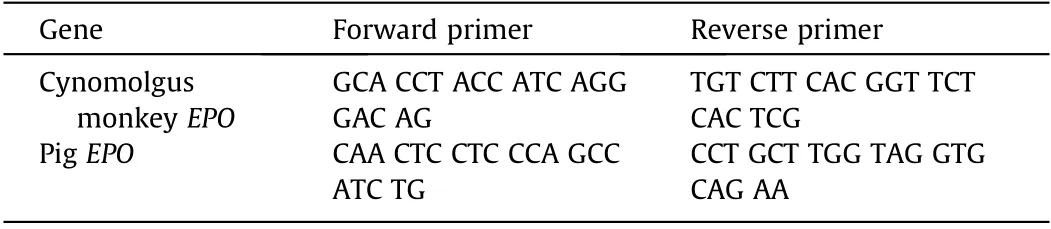
Table 2 The primers of pig and monkey EPO.
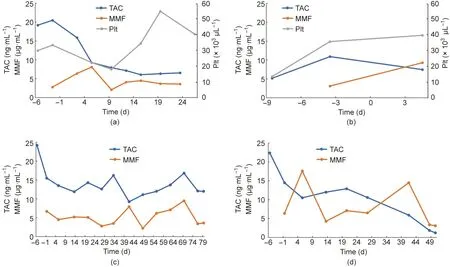
Fig. 2. Blood test data for each animal. (a) No. 200 (Experiment 1); (b) No. 335 (Experiment 1); (c) No. 323 (Experiment 2); (d) No. 219 (Experiment 2).
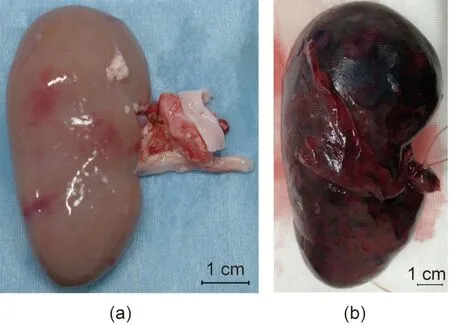
Fig. 3. Transplanted neonatal kidney in Experiment 1. (a) Before transplantation;(b) 13 days after transplantation.
The fetal kidney that was recovered at the same time retained almost all tissue structures, such as the glomerulus and renal tubules. Moreover, no infiltration of dyskaryotic and CD3-positive cells was observed (Figs. 5(f)–(h)).
We continued the administration of immunosuppressant drugs to the same monkey after the abdomen was closed again.It recovered after the transplanted pig kidney was removed and was relatively stable until sacrificial death, 27 days after transplantation.The two recovered fetal kidneys are shown in Fig. 4. Tissue structures were preserved 13 days after transplantation; infiltration of CD3-positive cells was observed in some parts (Figs. 5(i)–(k)).The Banff scores for each sample are shown in Table 3. We excluded endarteritis from the cloacae evaluation because no evaluable vessels for vasculitis could be confirmed in the transplanted cloacae. However, the presence of vessels was evaluated using two types of CD31 antibodies with different staining properties in pigs and monkeys (Fig. 6).
3.2. Experiment 2: Generation of fetal cloacae under FDA-approved immunosuppressive drugs
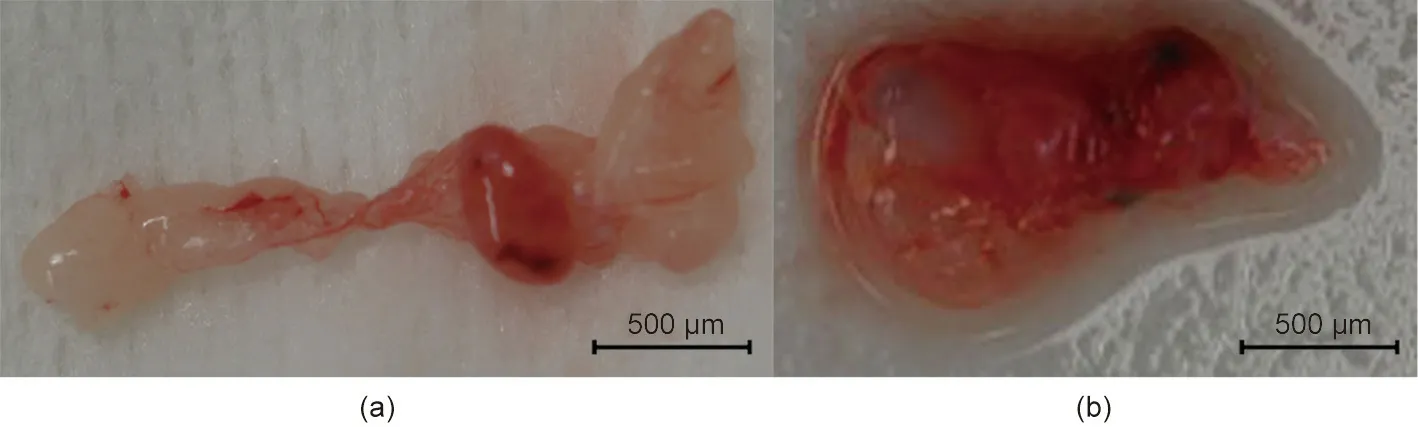
Fig. 4. Transplanted fetal cloacae in Experiment 1. (a) 13 days after transplantation; (b) 27 days after transplantation.
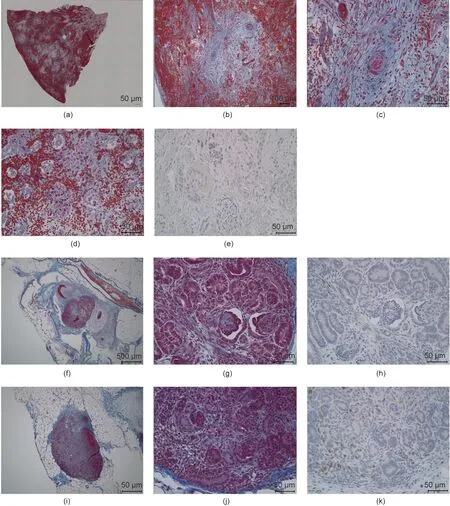
Fig. 5. Representative figures comparing the histopathological features of a neonatal kidney and fetal cloacae of Experiment 1. (a–e) Neonatal kidney; (f–h) fetal cloacae(13 days after transplantation); (i–k) fetal cloacae (27 days after transplantation). (a–d, f, g, i, j) Masson’s trichrome stain; (e, h, k) CD3 (MA5-12577).
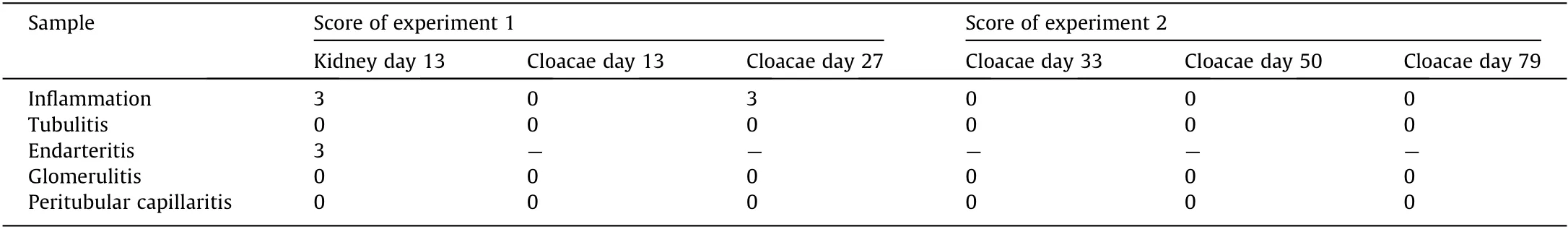
Table 3 Banff lesion scores of each sample.
Urinary tract anastomosis was performed on day 33 after tissue grafting for approximately four weeks. As a standard, the graft tissues were initially scheduled for evaluation six weeks after urinary tract anastomosis. However, the condition of one subject gradually worsened after the anastomosis.Thus,it was euthanized after the graft tissues were removed 50 days posttransplantation.The other subject was relatively more stable.Thus,the tissues were recovered 46 days after anastomosis(79 days posttransplantation),as scheduled.
The longest diameter of the largest fetal kidney recovered from the omentum, 33 days after transplantation, was approximately 800 μm (Fig. 7(a)). Tissue structures, such as the glomerulus and renal tubules, were mostly preserved, as in Experiment 1, and cell infiltration was not observed. Bowman’s space appeared slightly enlarged on the basis of visual examination.
The longest diameter of the largest fetal kidney recovered from the para-aortic region 50 days after transplantation was approximately 1.5 mm. In addition, the longest diameter of the largest fetal kidney recovered from the para-aortic region 79 days after transplantation was approximately 2 mm (Figs. 7(b) and (c)). The fetal bladders were punctured prior to tissue removal. Consequently, the liquid could not be aspirated from any, and no dye migration to the urinary tract could be confirmed, following the intravenous injection of indigo carmine to the recipients.Histopathological evaluation did not reveal any significant changes in the degree of cell infiltration in the fetal kidney 33, 50, and 79 days after transplantation (Fig. 8); however, the glomerulus appeared to be atrophied and Bowman’s space enlarged, suggesting the presence of hydronephrosis. In addition, the anastomotic part was open and cavitation that appeared to be liquid retention was observed in the fetal bladder in fetal kidneys where the fetal bladder and ureter of the recipient monkeys were anastomosed.PCR was also performed on the RNA extracted from cloacae collected on day 50. RNA amplification was observed in the primer corresponding to EPO mRNA of cynomolgus monkeys, suggesting the possible production of EPO (Fig. 9).
4. Discussion
This study, to the best of our knowledge, is the first to directly compare the immunogenicity of neonatal and fetal kidneys in xenotransplantation between genetically unmodified pigs and monkeys and to observe the long-term course following urinary tract anastomosis of fetal kidneys.These experiments verified that fetal kidneys may work more advantageously than neonatal kidneys in xenotransplantation and that long-term engraftment of fetal kidneys is possible.
Long-term engraftment is difficult even with genetically modified pigs under the administration of only FDA-approved immunosuppressant drugs when transplanting mature pig kidneys to monkeys [6]. The transplantation of pig neonatal kidneys in this study led to the worsening of one of the recipient monkey’s conditions,and the rejection of the transplanted kidneys was consistent with the results of previous reports. However, inflammatory cell infiltration and tissue destruction characteristics of the rejection reaction were hardly observed in fetal kidneys during the same period.Interestingly,vessels and EPO from monkeys were detected in transplanted fetal pig cloacae.This finding suggests that not only vessels but also cells of the recipient animals can flow into the fetal kidney and be chimerized. There have been reports on the transplantation of fetal mouse, pig, and human kidneys to xenogeneic recipient rodents[7,9,11].It has been established that fetal kidneys mature into urine-producing renal tissues without differentiating into other tissues in the case of fetal pig kidneys,if an appropriate period is selected, such as a period of four weeks. In addition, the blood vessels of xenogeneic recipients flow into fetal kidneys during the transplantation process, [7,9] and the expression of donor antigens is reduced in the kidneys during the developmental stage[9,12].This suggests that the rejection of cloacae may be less likely to occur in xenotransplantation than the rejection of neonatal kidneys. However, in Experiment 1, in which neonatal kidneys and fetal kidneys were transplanted at the same time, inflammatory cell infiltration was observed in fetal kidneys despite a shorter course in Experiment 1 than in Experiment 2. Thus, the presence of factors that strongly induce inflammatory reactions, such as the neonatal kidney,can also spread inflammation to the fetal kidneys, which are considered to have low immunogenicity.Moreover, natural antibodies, such as anti-pig immunoglobulin E(IgE)/IgA antibodies, may be involved in the immune response of recipient monkeys [13]. However, it is believed that they may not have a significant influence on the development of fetal kidneys.
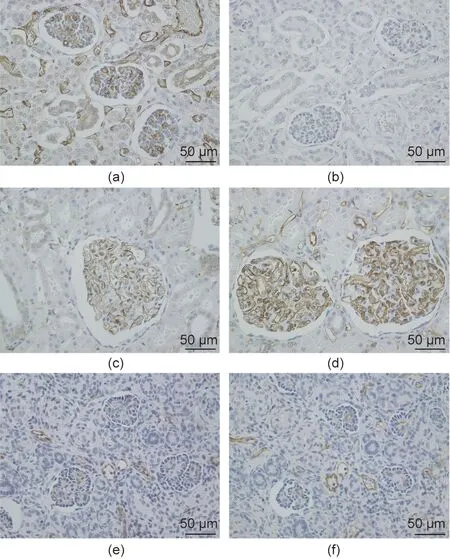
Fig.6. Evaluation of blood vessels of day 27 cloacae of Experiment 1.(a,b)Adult pig kidney; (c, d) adult monkey kidney; (e, f) fetal cloacae (27 days after transplantation). (a, c, e) CD31 (ab28364); (b, d, f) CD31 (JC70/A).
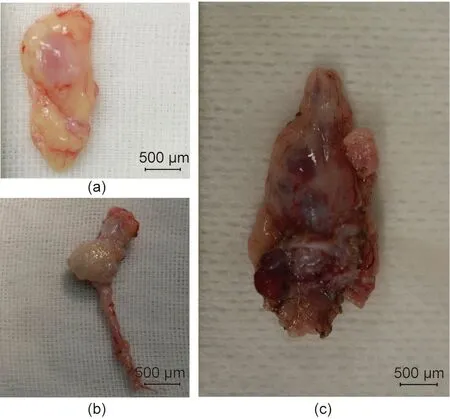
Fig. 7. Transplanted fetal cloacae in Experiment 2. (a) 33 days after the transplantation. (b) 50 days after the transplantation; (c) 79 days after the transplantation.
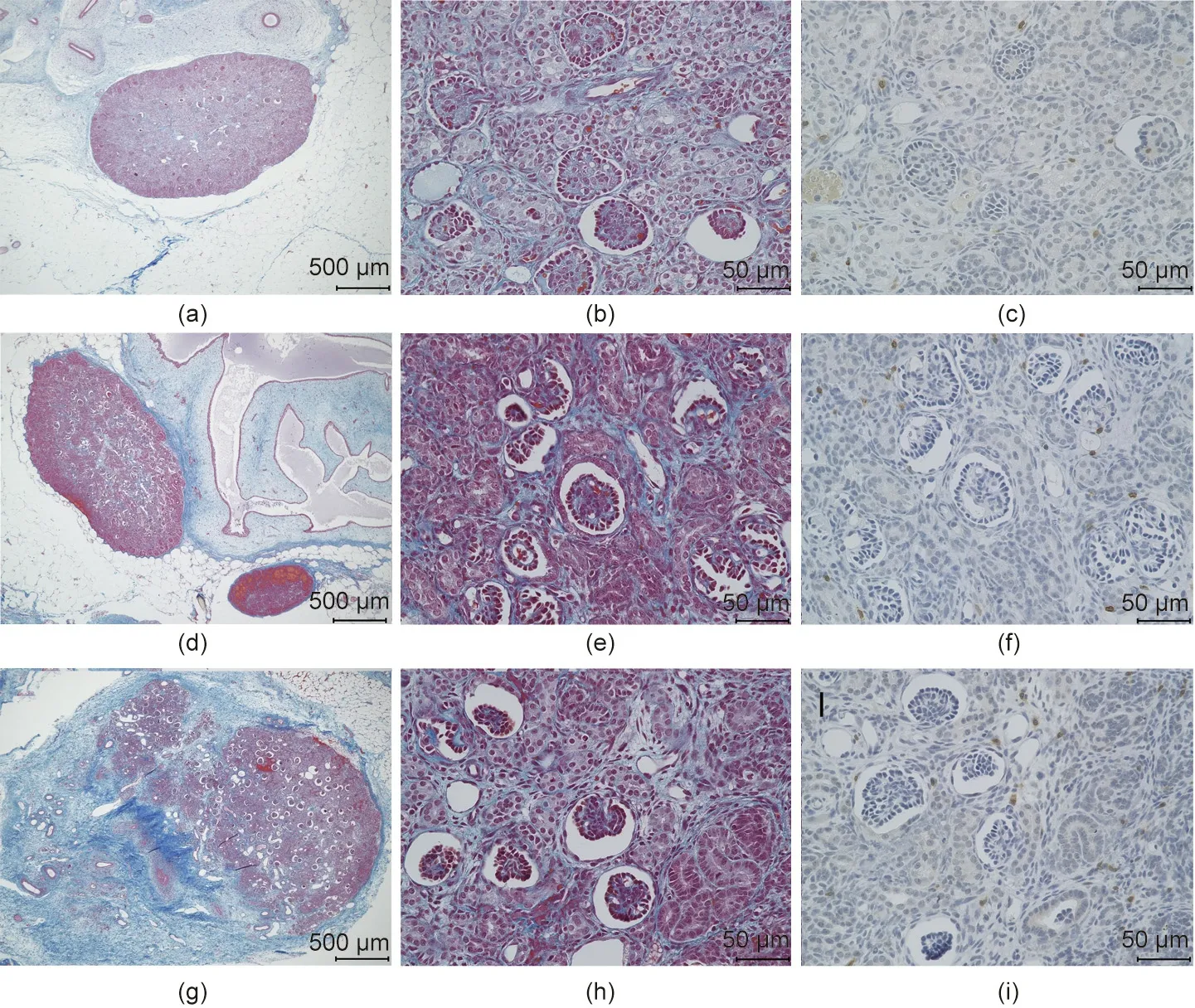
Fig. 8. Representative figures comparing histopathological features of fetal cloacae in Experiment 2. (a–c) 33 days after the transplantation; (d–f) 50 days after the transplantation (g–i) 79 days after the transplantation. (a, b, d, e, g, h) Masson’s trichrome stain; (c, f, i) CD3 (MA5-12577).
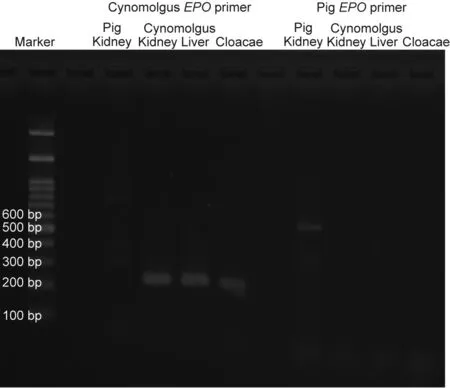
Fig. 9. EPO PCR of day 50 cloacae of Experiment 2.
Long-term engraftment is possible only under immunosuppressant drugs. However, long-term engraftment may even be expected by reducing the type and dosage of immunosuppressant drugs in the transplantation of fetal kidneys only (Experiment 2).
Both rejection control and urinary excretion system establishment are required to develop fetal kidneys into more mature kidneys in recipients [14]. In this study, as in previous reports, a urinary excretion system was established. However, clear urine production and discharge could not be confirmed, suggesting the possibility of urinary retention. Consequently, monkeys that are small and not quadruped may be prone to vascular insufficiency of transplanted vessels [2], and the urinary tract may also be impaired. The use of stents to prevent urinary obstruction and identification of an optimal anastomotic site that is not easily occluded is necessary to establish a reliable urinary excretion system in the future.
5. Conclusions
This study demonstrated the successful transplantation and long-term engraftment of fetal kidneys with a pig–monkey preclinical model without using genetically modified animals. Moreover,the study is also important in the development of chimeric fetal kidneys infused with human-derived renal progenitor cells to achieve humanized kidneys.
Acknowledgments
This work was supported by the Japan Agency for Medical Research and Development (AMED; 21bk0104094h0003) and by a grant from Sumitomo Dainippon Pharma Co., Ltd.
We thank Mr. Atsushi Kobayashi, Mr. Atsushi Matsumoto, Mr.Yuji Ogi (Sumitomo Dainippon Pharma Co., Ltd.), Ayuko Uchikura,Shiori Yonamine,Koki Hasegawa, and Shunichiro Kamida(Laboratory of Medical Bioengineering,Department of Life Sciences,School of Agriculture, Meiji University) for their technical assistance. We also thank Drs. T. Kimura, K. Fujimori, Y. Iwamura, A. Kishino,T. Yoshikawa, K. Adachi, R. Yamaguchi, K. Yoshida, and A. Ikeda(Sumitomo Dainippon Pharma Co., Ltd.) for their support in conducting the animal study.
Compliance with ethics guidelines
Tsuyoshi Takamura, Kenji Matsui, Naoto Matsumoto, Yatsumu Saito, Toshinari Fujimoto, Susumu Tajiri, Shuichiro Yamanaka, Kei Matsumoto,Akimitsu Kobayashi,Izumi Yamamoto,Hiroshi Sasaki,Haruyuki Hirayama, Hitomi Matsunari, Kazuaki Nakano, Hiroshi Nagashima,Akihiko Kiyoshi,Takao Kuroda,Makoto Inoue,Takeshi Miyawaki, Takashi Yokoo, and Eiji Kobayashi declare that they have no conflict of interest or financial conflicts to disclose.
The funders had no role in the study design,data collection and analysis, decision to publish, or preparation of the manuscript.
- Engineering的其它文章
- Supersonic Travel Seeks an Encore
- The Extremely Low Frequency Engineering Project for Underground Exploration
- The Dual Regulatory Roles of Macrophages in Acute Allogeneic Organ Graft Rejection
- Role of Intrahepatic Regional Immunity in Post-Transplant Cancer Recurrence
- Two-Layer High-Throughput: Effective Mass Calculations Including Warping
- Machine Learning-Assisted High-Throughput Virtual Screening for On-De mand Customization of Advanced Energetic Materials

