Serotonin type 3 receptor subunit gene polymorphisms associated with psychosomatic symptoms in irritable bowel syndrome: A multicenter retrospective study
Sabrina Berens, Yuanjun Dong,Nikola Fritz,Jutta Walstab, Mauro D'Amato, Tenghao Zheng, Verena Wahl,Felix Boekstegers, Justo Lorenzo Bermejo, Cristina Martinez, Stefanie Schmitteckert,Egbert Clevers, FelicitasEngel,Annika Gauss, Wolfgang Herzog, Robin Spiller, Miriam Goebel-Stengel,Hubert Monnikes Viola Andresen,Frieling Thomas, Jutta Keller, Christian Pehl, Christoph Stein-Thoringer,Gerard Clarke, Timothy GDinan, Eamonn M Quigley, Gregory Sayuk,Magnus Simren, Jonas Tesarz, Gudrun Rappold, Lukas van Oudenhove,Rainer Schaefert,Beate Niesler
Abstract
Key Words: Irritable bowel syndrome; 5 -HT3 receptor subunit gene polymorphisms; Single-nucleotide polymorphism score; Depression; Anxiety; Somatization
lNTRODUCTlON
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits[1 -4 ]. The pathophysiology of IBS has not entirely been resolved, but is understood to be biopsychosocial and affected by an impaired function of the central and enteric nervous systems and their crosstalkviathe brain-gut axis[5 ,6 ]. IBS patients often present with increased comorbid depressive and anxiety symptoms[7 -11 ], highlighting the complex relationship between visceral sensitivity and subjective psychological perceptions[12 ,13 ]. Nevertheless, about 50 % of IBS patients report GI symptoms but show no comorbid affective symptoms[14 ].
There is evidence that disturbances of the serotonergic system are important in GI disorders such as IBS and in mental disorders, both of which interactviathe brain-gut axis[15 ,16 ]. The serotonin type 3 receptors (5 -HT3 R) modulate key functions in the GI tract[17 ,18 ]. In line with such functions, 5 -HT3 R antagonists are beneficial in the treatment of diarrhea-predominant IBS (IBS-D)[19 -22 ]. 5 -HT3 Rs are also involved in emotional processing, mood regulation, and visceral perception and have been associated with depressive and anxiety symptoms that represent comorbid phenotypes in IBS[23 ]. Singlenucleotide polymorphisms (SNPs) in the 5 -HT3 R subunit genes (HTR3 ), namely HTR3 A c.-42 C>T(rs1062613 ), HTR3 B c.386 A>C (rs1176744 ), HTR3 C c.489 C>A (rs6766410 ), and HTR3 E c.*76 G>A(rs56109847 ), are associated with IBS according to studies investigating the effects of sex or IBS subtypes[12 ,24 -30 ]. However, whether HTR3polymorphisms are associated with IBS and comorbid depressive and anxiety symptoms has not been determined because existing studies have missing phenotypic data on comorbidities and small sample sizes. These studies had case-control designs and investigated associations between these polymorphisms in individuals with IBS phenotypes or mental behavioral conditions and controls rather than combining genetic data with specific psychosocial characteristics of IBS patients.
This multicenter observational study focused on a large IBS patient cohort comprising 768 participants from centers in Germany, Sweden, the United States, the United Kingdom, and Ireland with the aim of meeting three objectives: (1 ) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden (i.e., depressive, anxiety, and somatization symptoms)within an IBS population; (2 ) To investigate the impact of the HTR3 SNP score (computed as the number of minor alleles) on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3 ) To perform a functional analysis of variant 5 -HT3 AC receptors.
MATERlALS AND METHODS
Subjects
The study population was pooled from five different tertiary care expert centers. German participants were recruited from the Specialty Clinic for Functional GI Disorders at the Department of General Internal Medicine and Psychosomatics of Heidelberg University Hospital[31 ] and from our clinical partners in the IBS-Net in Hamburg, Krefeld, Berlin, Vilsbiburg, and Munich (www.ibs.uni-hd.de).Swedish participants were recruited at the specialized unit for patients with functional GI disorders at Sahlgrenska University Hospital in Gothenburg. United States participants were recruited at Washington University, Barnes-Jewish Hospital in St. Louis, Missouri. United Kingdom participants were recruited at the Nottingham Digestive Diseases Center and participants from Ireland from a specialty clinic at Cork University Hospital. Participant recruitment is shown in Figure 1 .
Written informed consent was obtained from all participants and the experiments were in accordance with the principles of the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. All studies were approved by the following local Ethics Committees:Heidelberg, Germany: Ethical Committee, Medical Faculty of the Heidelberg University Hospital(S067 /2010 ); Cork, Ireland: Clinical Research Ethics Committee (APC024 ); Gothenburg, Sweden:Regional Ethical Review Board in Gothenburg (S489 -02 and 731 -09 ); Nottingham, United Kingdom:registered at clinical trial clinicaltrials.gov (identifier NCT00745004 ) and approved by Nottingham Research Ethics Committee 2 (REC reference number 08 /H0408 /134 )[21 ]; and St-Louis, United States:Washington University St. Louis, Human Research Protection Office (IRB ID #: 201103220 ).
Inclusion/exclusion criteria
Only patients diagnosed with IBS according to the ROME III criteria were included in the analysis. All participants were of Caucasian ancestry and had comparable population stratification. Patients under 18 years of age or without SNP test results were excluded.
Genotyping
Genomic DNA was isolated from IBS patient blood samples using ethylenediaminetetraacetic acid according to standard protocols[32 ]. Four polymorphic HTR3 loci, namely HTR3 A c.-42 C>T (rs1062613 ),HTR3B c.386 A>C (rs1176744 ), HTR3 C c.489 C>A (rs6766410 ), and HTR3 E c.*76 G>A (rs56109847 ) were selected as target SNPs for this study. The corresponding primers were designed and synthesized using AssayDesigner 3 .1 software. Genotyping was performed at the Department of Human Molecular Genetics at Heidelberg University Hospital using the KASPar®SNP Genotyping System (KBiosciences,Ltd, Hoddesdon, United Kingdom). To analyzeHTR3 SNPs, the fluorescence plate reader of the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, California, United States) was used as recommended. About 10 % of the samples were repeat tested to ensure genotyping accuracy.
Data collection
In addition to sociodemographic characteristics and IBS diagnosis, we also collected data on depressive,anxiety, and somatization symptoms[33 ] and genetic markers of the serotonergic system.
IBS diagnosis:The diagnostic classification of IBS was based on the Scoring Algorithm for Rome III Diagnostic Questionnaire for Adult Functional GI Disorders (SA for Rome III-DQ)[34 ,35 ] in all five centers. Percentages of the different IBS subtypes,i.e., constipation-predominant IBS, IBS-D, IBS with mixed bowel habits, and unclassified IBS, were also calculated.

Figure 1 Recruitment strategy. IBS: Irritable bowel syndrome.
Depressive symptoms:The nine-item depression module from the Patient Health Questionnaire (PHQ-9 )[36 ,37 ] was used to measure depressive symptoms in the German cohort. The Hospital Anxiety and Depression Scale depression subscale[38 ] was used to identify depressive symptoms in participants from Sweden, the United Kingdom, and Ireland and the Beck Depression Inventory[39 ] was used to measure the severity of depressive symptoms in United States participants.
Anxiety symptoms:In the German cohort, symptoms of generalized anxiety were assessed using the brief measurement for generalized anxiety disorder (GAD-7 )[40 ]. In the cohorts from Sweden, the United Kingdom, and Ireland, the HADS anxiety subscale was used to identify anxiety symptoms. The Beck Anxiety Inventory[41 ] was used to assess anxiety in the United States cohort.
Somatization symptoms:In the cohorts from Germany, Sweden, and the United States, the 15 -item somatization module from the PHQ-15 [42 ] was used to identify somatization symptoms.
Genetic markers of the serotonergic system:The four functional SNPsHTR3A c.-42 C>T (rs1062613 ),HTR3B c.386 A>C (rs1176744 ), HTR3 C c.489 C>A (rs6766410 ), and HTR3 E c.*76 G>A (rs56109847 ) were selected for validation based on previous reports as outlined above[25 ].
Ligand binding and calcium influx assays
These procedures are described in the Supplementary material.
Statistical analysis
All statistical procedures were carried out using IBM SPSS Statistics 22 .0 for Windows. Variables with a skewed distribution were log-transformed prior to further analysis. If different measurements had been collected in the five centers,zvalues were calculated to enable pooling. The Hardy-Weinberg equilibrium (HWE) of genotype frequency distribution was tested using SHEsis[43 ]. Genome-wide SNP data were generated by the Bellygenes team of D’Amato M using the Illumina Global Screening array and platform[44 ,45 ]. We used the multidimensional scaling approach to correct for population stratification in PLINK[46 ]. Following the guidance provided at https://github.com/MareesAT/GWA_tutorial/, our data were anchored by data of the 1000 Genomes project (http://www.1000 genomes.org/). The 10 main components were used as covariates in the association tests to correct for population stratification[46 ] and exclude outliers. Polymorphisms were analyzed separately using the dominant and the recessive models. Also, stratified analyses based on sex and IBS subtypes were carried out. ANOVA was used to analyze group differences and to check for linear trends in depressive,anxiety, and somatization symptoms. For the independent variable, a SNP score was computed based on the number of minor alleles (i.e., continuous from 0 to 8 for the four SNPs). Based on the first humanHTR3locus-specific variant database (www.htr3 .uni-hd.de)[25 ], scoring criteria were as follows: major allele homozygous variant gene = 0 ; heterozygous variant gene = 1 ; and minor allele homozygous variant gene = 2 (for details see Table 1 ). Statistical comparisons were made between the two groupsusing theχ2test or Fisher’s exact test for frequencies andttests or Mann-WhitneyUtests for metric variables. Normal distribution and variance homogeneity were checked as conditions. Statistical tests were two-sided based on an alpha error of 0 .05 %. All analyses were explorative and not confirmatory.False discovery rates (FDRs) were calculated based on overallPvalues using the Benjamini-Hochberg method[47 ]. Significant values that were no longer significant after FDR multiple testing correction were named “nominally significant”.

Table 1 Strategy for computing the single-nucleotide polymorphism score
RESULTS
Sociodemographic and symptomatic characteristics
In total, 623 participants from five independent expert centers were included in this study (45 .1 % from Germany, 18 .3 % from Sweden, 19 .6 % from the United States, 12 .4 % from the United Kingdom, and 4 .7 %from Ireland). We excluded 76 Swedish participants who did not meet the population stratification criteria (Supplementary Figure 1 ). Participants from the United Kingdom and Ireland were excluded from the main analysis because the sample size was small. Table 2 presents the sociodemographic characteristics, IBS subtypes, and psychosomatic symptoms of the included participants. Participants had a mean ± SD age of 41 .7 ± 16 .1 years and 69 .5 % were female. Overall, IBS patients showed minimal to mild levels of depressive and anxiety symptoms, moderate levels of IBS symptoms, and moderate levels of somatization symptoms.
HTR3 SNP genotypes and allele frequencies
Genotype and allele frequencies of the functionalHTR3A c.-42 C>T, HTR3 B c.386 A>, HTR3 C c.489 C>A,andHTR3E c.*76 G>A polymorphisms were calculated. No significant differences in genotype frequency were observed between sexes or IBS subtypes. ForHTR3A c.-42 C>T, the frequency of the minorTallele was 21 .5 %; for HTR3 B c.386 A>C, the frequency of the minor C allele was 29 .6 %; for HTR3 C c.489 C>A,the frequency of the minorAallele was 41 .6 %; and for HTR3 E c.*76 G>A, the frequency of minorAallele was 6 .1 %. The genotypic distribution of the four polymorphic loci of rs1062613 , rs1176744 , rs6766410 ,and rs56109847 were in accordance with HWE (all P > 0 .05 ). The results are shown in Supplementary Tables 1 -3 .
HTR3 SNP analysis using the dominant and the recessive model
HTR3SNPs were separately analyzed using the dominant model and the recessive model stratified for sex and IBS subtypes. Depressive and anxiety symptoms worsened significantly with increasing numbers of minor alleles ofHTR3C c.489 C>A in the dominant model (Fdepressive = 7 .475 , Pdepressive = 0 .006 ;F anxiety= 6 .535 , Panxiety = 0 .011 ). This seemed to be driven by female sex (Fdepressive = 7 .040 , Pdepressive = 0 .008 ;Fanxiety= 7 .550 , Panxiety = 0 .006 ) and IBS-D (Fdepressive = 5 .670 , Pdepressive = 0 .018 ; Fanxiety = 13 .444 , Panxiety < 0 .001 ). The same trend was also found forHTR3A c.-42 C>T in male participants with depressive symptoms in the dominant model (Fdepressive= 4 .149 , Pdepressive = 0 .043 ). For the recessive model, depressive and somatization symptoms worsened with increasing numbers of minor alleles ofHTR3C c.489 C>A (Fdepressive = 6 .190 ,P depressive= 0 .014 ) and HTR3 B c.386 A>C (Fdepressive = 6 .482 , Pdepressive = 0 .011 ), respectively in IBS-D participants.Fvalues from the ANOVA are shown in Table 3 . As mentioned above, the analyses of participants from the United Kingdom and Ireland are presented separately in the Supplementary Table 4 .
Effect of SNP score on psychosomatic symptoms
SNP scores ranged from 0 to 6 ; 37 .1 % had one or zero minor alleles of the analyzed HTR3 SNPs and 30 .8 % had three or more minor alleles of the analyzed HTR3 SNPs. No significant differences in SNP scores were observed between sexes (F= 3 .550 , P = 0 .060 ) or IBS subtypes (F = 1 .485 , P = 0 .227 ).ANOVAs were conducted and linear trends were checked to analyze the effect of SNP scores on depressive, anxiety, and somatization symptoms. Overall, an increasing number of minor alleles was linked to worsening depressive symptoms (F= 7 .710 , P-linear trend = 0 .006 ). However, material trends did not reveal a link between more minor alleles and worsening anxiety or somatization symptoms. By stratifying analyses for sex, an increasing number of minor alleles was linked to worsened depressivesymptoms in female participants (F= 5 .770 , P-linear trend = 0 .017 ). There was no significant association between SNP score and depressive, anxiety, or somatization symptoms when looking at IBS subtypes separately (Table 4 ). As mentioned above, the analyses of participants from the United Kingdom and Ireland are presented separately in the Supplementary Table 5 .
Functional analysis of variant 5 -HT3 AC receptors
TheHTR3C SNP encodes the amino acid exchange p.Asn163 Lys (p.N163 K), and recombinantly expressed 5 -HT3 AC receptors harboring variant 5 -HT3 C subunits that mimic the homozygous minor allele and the heterozygous state presented with increased cell surface expression and enhanced 5 -HT maximum response. Radioligand-binding assays revealed higher Bmaxvalues of 117 .1 % ± 4 .38 % and 111 .9 % ± 1 .79 %. Calcium influx assays showed increased 5 -HT-induced maximum effects of 137 .2 % ±9 .0 % and 151 .9 % ± 17 .3 % for the minor allele 5 -HT3 AC 163 K or combined 5 -HT3 AC 163 N/5 -HT3 AC 163 K receptors compared with the major allele representing 5 -HT3 AC 163 N receptors, respectively. The affinity of the specific 5 -HT3 receptor antagonist [3 H]GR65630 , as reflected by the Kd values, and the potency of 5 -HT, as reflected by the EC50 values, did not differ between the receptor variants (Figure 2 ).
DlSCUSSlON
Main findings
The 5 -HT3 receptors modulate essential functions in the GI tract such as GI motility as well as mood and emotions[19 ], and HTR3 SNPs have been associated with depression, anxiety, and IBS[25 ]. In this study,we showed that: (1 ) In the dominant model, HTR3 C c.489C>Awas correlated with depressive and anxiety symptoms in IBS; (2 ) A higher number of minor alleles (i.e., a higher SNP score, which was computed by combining the individual SNP status ofHTR3A c.-42 C>T, HTR3 B c.386 A>C, HTR3Cc.489 C>A, and HTR3 E c.*76 G>A) was linked to more severe depressive symptoms in IBS; and (3 ) The potential relevance of theHTR3CSNP was corroborated in functional assays showing changes in the expression level of 5 -HT3 AC variant receptors. These findings are discussed in more detail below.

Table 3 Differences between single-nucleotide polymorphisms in depressive and anxiety symptoms, separately analyzed with ANOVA using the dominant model and the recessive model
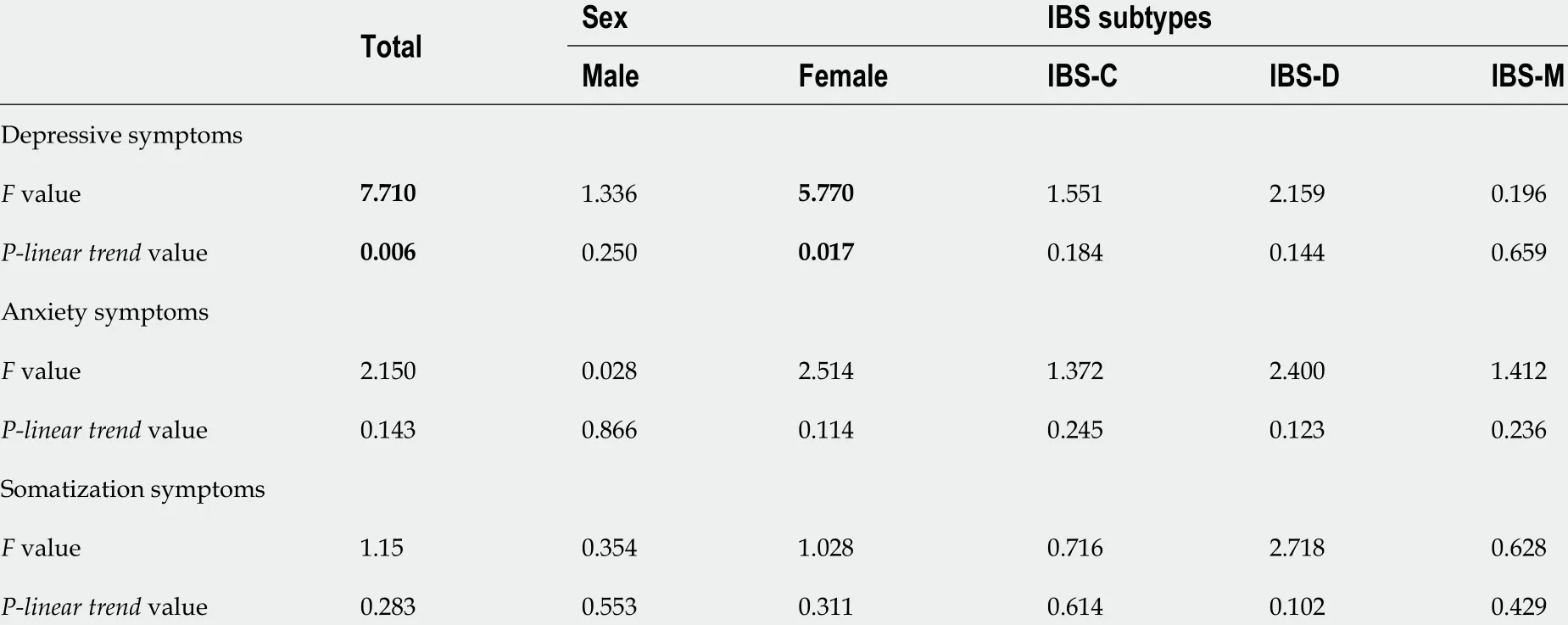
Table 4 Association of depressive, anxiety, and somatization symptoms with the single-nucleotide polymorphism score of the four tested polymorphisms
Sample characteristics
Participants with IBS were more frequently female than male, in line with previous findings[48 ,49 ] that IBS is more prevalent in young and middle-aged females. Most participants with IBS were only mildly affected by depression and anxiety symptoms. Of note, German participants only visited the Specialty Clinic for Functional GI Disorders at Heidelberg University Hospital after a long history of dealing with IBS[31 ]; therefore, these participants reported more severe depressive symptoms. However, causal relationships between IBS symptoms, depression, and anxiety are still controversial.
The investigated SNPs rs1062613 , rs1176744 , rs6766410 , and rs56109847 were in accordance with HWE. There was no population stratification, and the sample was representative and excluded genotyping errors. Although participants were recruited from various centers in different countries,there were no obvious selective differences.
Connections between HTR3 SNPs and mental symptoms
HTR3A c.-42 C>T (rs1062613):Depressive symptoms were “nominally significantly” more severe with increasing numbers of minorHTR3A c.-42 C>T alleles in male participants according to the dominant model. This SNP has been associated with bipolar affective disorder[50 ], IBS symptom severity,amygdaloid activity[29 ,51 ], early life trauma[52 ], altered emotional networks in the human brain, and the onset of depression[53 ]. However, these studies only included single sex participants and did not include subgroup analyses.
HTR3B c.386 A>C (rs1176744):Somatization symptoms worsened significantly with increasing numbers of minorHTR3B c.386 A>C alleles in the dominant model. The HTR3 B variant p.Tyr129 Ser (rs1176744 )has been associated with bipolar affective disorder in males and with major depression in females as well as with pain catastrophizing, a coping style characterized by excessively negative thoughts and emotions related to pain[30 ,54 -56 ]. This discrepancy may be due to an enhancement or weakening of this association by polymorphic interactions in the serotonin pathways[56 ].
HTR3C c.489 C>A (rs6766410):Depressive and anxiety symptoms worsened significantly with increasing numbers of minorHTR3C c.489 C>A alleles in the dominant model. This effect seemed to be driven by female sex and IBS-D.HTR3C c.489 C>A was previously associated with IBS-D in female patients[57 ], but the proportion of male patients was small in this study, which may limit the applicability of these findings. In the recessive model, depressive and anxiety symptoms “nominally significantly” worsened with increasing numbers of minor alleles ofHTR3C c.489 C>A in Irish participants. However, these results should be interpreted with caution because the Irish sample size was low. As far as we are aware,HTR3C c.489 C>A has not been analyzed in individuals with affective disorders before.

Figure 2 Comparative analysis of 5 -HT3 AC 163 N (major allele homozygous), 5 -HT3 AC 163 K (minor allele homozygous), and 5 -HT3 AC 163 K/163 N (heterozygous) receptors. A: Radioligand-binding studies Saturation experiments were performed in membranes of HEK293 cells transiently expressing different 5 -HT3 subunit combinations in triplicate with six increasing concentrations of [3 H]GR65630 (0 .02 -1 .5 nmol/L); Kd values were 0 .07 ± 0 .01 for 5 -HT3 AC 163 N; 0 .08 ± 0 .01 for 5 -HT3 AC 163 K, and 0 .07 ± 0 .01 for 5 -HT3 AC 163 K/163 N. n = 6 experiments; B: Concentration response curves were assessed in a calcium influx assay (aequorin assay) in HEK293 cells transiently transfected with different 5 -HT3 subunit combinations in quadruplicate with seven increasing concentrations of 5 -HT. pEC50 values were as follows: 68 ± 0 .03 for 5 -HT3 AC 163 N 5 and 5 .66 ± 0 .02 for 5 -HT3 AC 163 K; C: Respective maximal calcium influx values (Emax) evoked by 5 -HT (10 μmol/L). Data are expressed as percentages of the Emax of the heteromeric 5 -HT3 AC 163 N receptor (% of control), n = 8 experiments. Bars represent mean ± SE. bP < 0 .01 from ANOVA followed by Dunnett’s post-test or unpaired Student’s test for comparison of only two groups.
HTR3E c.*76 G>A (rs56109847 ): HTR3 E is restrictedly and robustly expressed in the GI tract[58 ,59 ],suggesting that it plays a special role in 5 -HT3 receptor function in the gut. In this study, we did not find a relationship between functional polymorphisms ofHTR3Eand depressive and anxiety symptoms in IBS patients. This may be attributed to a floor effect because depressive and anxiety symptoms were minimal to mild in our sample[60 ].
The SNP score and its impact on depressive symptoms
A single gene variant is not sufficient to explain all symptoms shaping the clinical phenotype of a complex disorder like IBS[61 ]. By computing SNP scores based on the number of minor alleles of rs1062613 , rs1176744 , rs6766410 , and rs56109847 , our study revealed that an increasing number of minor alleles is linked to increasing severity of depressive symptoms. However, there was no obvious association between an increasing number of minor alleles and the severity of anxiety or somatization symptoms. Stratification for sex revealed a correlation between increasing numbers of minor alleles and worsening depressive symptoms in female participants.
Functional properties of variant 5 -HT3 AC receptors
HTR3genes encode different 5 -HT3 subunits to make up heteromeric receptors. The 5 -HT3 A subunits play a major role in these receptors because they can form functional receptors on their own. The other subunits can only form functional receptors with 5 -HT3 A and seem to modulate the function and properties of the receptors[62 ]. How these native receptors might contribute to the pathogenesis of IBS,particularly regarding co-expression patterns ofHTR3, has not been established yet. The HTR3AandHTR3Evariants reside within untranslated regions and the respective SNPs correlate with increased expression levels, whereas theHTR3B variant changes the channel properties[25 ]. To gain insight into the pathophysiological relevance of the associatedHTR3C variant c.489 C>A (rs6766410 ), we characterized the pharmacological and functional properties of those 5 -HT3 AC receptors that altered the 5 -HTmediated maximum response and expression of variant 5 -HT3 AC receptors. However, how structural modifications in these receptors affect their functionin vivoand how they modulate the serotonergic system to influence mood, emotional processing, and the manifestation of IBS and comorbid conditions remains to be determined.
Limitations and strengths
Our study has some limitations. First, different instruments were used by different centers to assess phenotypic features. To correct for this, scale scores were converted intozstandard scores. However,given that the participants reported no severe psychosomatic symptoms, the discovery chances might be limited. Second, there is no sufficient evidence to show the relationship between risk alleles and respective major/minor alleles as patients and healthy controls were not compared in this study.Similarly, the relative strength of the cumulative effect represented by the SNP score was also affected to a certain extent. Third, our participants were all Caucasian, so the results may not apply to other ethnic groups.
Despite these limitations, our study has some strengths. First, this was a multicenter study so had a large sample size. Large, well-characterized samples like ours are necessary to identify molecular causes of IBS and comorbid conditions[12 ]. Second, this study investigated the association between polymorphisms inHTR3genes and comorbid psychosomatic symptoms for the first time. We conducted population stratification tests to ensure that the included populations were comparable. We also performed stratified analyses of sex and IBS subtypes and a more stringent multiple testing correction by FDR. Third, SNP scores have higher power and are better suited to testing multiple instead of single variants. This is useful because the pathogenesis of IBS is complex with multiple factors contributing to the manifestation of various subtypes. Also, individual genes may only play a minor role[12 ].
Clinical implications and further research
IBS is a complex condition. The continuous improvement of the allelic variation database forHTR3[25 ]and deep phenotyping combined with gene information (also in other datasets) may help to identify disease subgroups accurately and consistently, thereby facilitating future treatment[33 ,63 ]. This will be an important step towards standardization and unification of IBS genetic research strategies.
CONCLUSlON
Our results provide the first evidence that the accumulation ofHTR3SNPs (reflected by the SNP score computed byHTR3A c.-42 C>T, HTR3 B c.386 A>C, HTR3 C c.489 C>A, and HTR3 E c.*76 G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS. This study has revealed that depressive and anxiety symptoms significantly worsened from the major to the minor allele ofHTR3Cc.489 C>A in the dominant model and an increasing number of minor alleles are linked to more severe depressive symptoms in IBS.
ARTlCLE HlGHLlGHTS

Research objectives
The objectives are: (1 ) To explore the associations between functional HTR3 polymorphisms and psychosomatic burden within an IBS population; (2 ) To investigate the impact of the HTR3 SNP score on psychosomatic burden, based on our hypothesis that the observed number of minor alleles was associated with specific mental characteristics in IBS patients; and (3 ) To perform a functional analysis of variant 5 -HT3 AC receptors.
Research methods
In this retrospective study, 623 participants with IBS were recruited from five specialty centers in Germany, Sweden, the United States, the United Kingdom, and Ireland. Depressive, anxiety, and somatization symptoms and sociodemographic characteristics were collected. Four functional SNPs —HTR3A c.-42 C>T, HTR3 B c.386 A>C, HTR3 C c.489 C>A, and HTR3 E c.*76 G>A — were genotyped and analyzed using the dominant and recessive models. We also performed separate analyses for sex and IBS subtypes. SNP scores were calculated as the number of minor alleles of the SNPs above. The impact ofHTR3C c.489 C>A was tested by radioligand-binding and calcium influx assays.
Research results
Bringing together high quality data as well as methodological expertise, our results show that: (1 ) In the dominant model,HTR3C c.489 C>A was correlated with depressive and anxiety symptoms in IBS; (2 ) A higher number of minor alleles (i.e., the higher the SNP score, which was computed by combining the individual SNP status ofHTR3A c.-42 C>T, HTR3 B c.386 A>C, HTR3 C c.489 C>A, and HTR3 E c.*76G>A)was linked to more severe depressive symptoms in IBS; and (3 ) The potential relevance of the HTR3CSNP was corroborated in functional assays showing changes in the expression level of 5 -HT3 AC variant receptors.
Research conclusions
Our results provide the first evidence that the accumulation ofHTR3SNPs (reflected by the SNP score computed byHTR3A c.-42 C>T, HTR3 B c.386 A>C, HTR3 C c.489 C>A, and HTR3 E c.*76 G>A) may play a role in the pathophysiology of depressive and anxiety symptoms in IBS.
Research perspectives
We are confident that these results are of interest to your readership, as they contribute substantially to update current knowledge regarding the role of accumulation ofHTR3SNPs in depressive and anxiety symptoms in IBS patients. In turn, our data will contribute towards standardization and harmonization of genetic research strategies in IBS.
ACKNOWLEDGEMENTS
We would like to thank all patients for their participation in this study and the supporting staff at each site. We acknowledge the kind support of Bartram CR and Hinderhofer K. We would also thank Startt B for proofreading and Bacon C for editing the manuscript. This manuscript results in part from collaboration and network activities promoted under the frame of the international network GENIEUR (Genes in Irritable Bowel Syndrome Research Network Europe), which has been funded by the COST program(BM1106 , www.GENIEUR.eu) and is currently supported by the European Society of Neurogastroenterology and Motility (ESNM, www.ESNM.eu).
FOOTNOTES
Author contributions:Mönnikes H, Keller J, Walstab J and Wahl V performed the experiments; Dong Y, Berens S,Schaefert R, Clevers E and Walstab J carried out data analyses; Berens S, Engel F, Gauss A, Herzog W, Spiller R,Clarke G, Dinan TG, Quigley EM, Sayuk G, Simrén M, Tesarz J, Sayuk G and IBS-Net Germany characterized and enrolled the patients; Dong Y, Berens S, and Schaefert R drafted the manuscript; all authors contributed to discussing and editing of the manuscript; Niesler B, Schaefert R and van Oudenhove L designed and supervised the study and revised and finalized the manuscript; Berens S and Dong Y equally contributed to the paper; Schaefert R and Niesler B shared last authorship.
lnstitutional review board statement:The study was reviewed and approved by the Ethical Committee, Medical Faculty of the Heidelberg University Hospital (approval No. S067 /2010 ).
lnformed consent statement:All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement:APC Microbiome Ireland has conducted studies in collaboration with several companies, including GSK, Pfizer, Cremo, Suntory, Wyeth, Mead Johnson, Nutricia, 4 D Pharma, and DuPont. Dinan TG has been an invited speaker at meetings organized by Servier, Lundbeck, Janssen, and AstraZeneca and has received research funding from Mead Johnson, Cremo, Suntory Wellness, Nutricia, and 4 D Pharma. Clarke G has been an invited speaker at meetings organized by Janssen and is receipt of research funding from Pharmavite. The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this report.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Germany
ORClD number:Sabrina Berens 0000 -0001 -8585 -5304 ; Yuanjun Dong 0000 -0002 -7817 -767 X; Nikola Fritz 0000 -0003 -2662 -4340 ; Jutta Walstab 0000 -0001 -9546 -2205 ; Mauro D'Amato 0000 -0003 -2743 -5197 ; Tenghao Zheng 0000 -0003 -1587 -7481 ;Verena Wahl 0000 -0001 -9887 -5873 ; Felix Boekstegers 0000 -0002 -0587 -7624 ; Justo Lorenzo Bermejo 0000 -0002 -6568 -5333 ;Cristina Martinez 0000 -0002 -9368 -1356 ; Stefanie Schmitteckert 0000 -0001 -9555 -2614 ; Egbert Clevers 0000 -0003 -1931 -4926 ;Felicitas Engel 0000 -0002 -9300 -5681 ; Annika Gauss 0000 -0001 -8661 -449 X; Wolfgang Herzog 0000 -0003 -2778 -4135 ; Robin Spiller 0000 -0001 -6371 -4500 ; Miriam Goebel-Stengel 0000 -0003 -0995 -3062 ; Hubert Mönnikes 0000 -0002 -7447 -6373 ; Viola Andresen 0000 -0002 -3695 -3327 ; Frieling Thomas 0000 -0001 -8270 -1975 ; Jutta Keller 0000 -0002 -5884 -1115 ; Christian Pehl 0000 -0002 -0205 -3582 ; Christoph Stein-Thöringer 0000 -0002 -8020 -6986 ; Gerard Clarke 0000 -0001 -9771 -3979 ; Timothy G Dinan 0000 -0002 -2316 -7220 ; Eamonn M Quigley 0000 -0003 -4151 -7180 ; Gregory Sayuk 0000 -0003 -1586 -199 X; Magnus Simrén 0000 -0002 -1155 -1313 ; Jonas Tesarz 0000 -0003 -3268 -7778 ; Gudrun Rappold 0000 -0002 -3126 -1508 ; Lukas van Oudenhove 0000 -0002 -6540 -3113 ; Rainer Schaefert 0000 -0002 -3077 -7289 ; Beate Niesler 0000 -0001 -7881 -6112 .
S-Editor:Gao CC
L-Editor:A
P-Editor:Gao CC
 World Journal of Gastroenterology2022年21期
World Journal of Gastroenterology2022年21期
- World Journal of Gastroenterology的其它文章
- Viral hepatitis and hepatocellular carcinoma: From molecular pathways to the role of clinical surveillance and antiviral treatment
- Updates in therapeutic drug monitoring in inflammatory bowel disease
- Serological biomarkers for management of primary sclerosing cholangitis
- Impact of radiotherapy on the immune landscape in oesophageal adenocarcinoma
- Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome
- Differentiating malignant and benign focal liver lesions in children using CEUS LI-RADS combined with serum alpha-fetoprotein
