Updates in therapeutic drug monitoring in inflammatory bowel disease
Nilesh Lodhia, Shanti Rao
Abstract Biologics and immunomodulators (IMM) are generally considered the most effective therapies for the treatment of ulcerative colitis and Crohn’s disease.However, despite the efficacy of these therapies, many patients either have a primary lack of response or a secondary loss of response to these medications.Therapeutic drug monitoring (TDM) is a systematic approach to managing such patients. In this review, we summarize the latest data on TDM, including reactive and proactive TDM, in patients with inflammatory bowel disease on biologics and/or IMM.
Key Words: Inflammatory bowel disease; Therapeutic drug monitoring; Crohn’s disease;Ulcerative colitis
lNTRODUCTlON
Since the approval of the first biologic for inflammatory bowel disease (IBD) in the 1990 s, treatment for IBD has evolved tremendously. In addition to tumor necrosis factor (TNF) inhibitors, thiopurines,natalizumab, vedolizumab, ustekinumab, and tofacitinib have all been approved for the treatment of IBD. Previously, patients were treated based on symptoms, but we have now discovered that utilizing more objective parameters such as clinical and endoscopic remission reduces complications and leads to better outcomes[1 ].
Despite having effective treatments for ulcerative colitis (UC) and Crohn’s disease (CD), one-third of patients (primary non-responders) will not respond to induction therapy after a biologic. Risk factors for primary non-response include long duration of disease, smoking, extensive small bowel disease, a normal C-reactive protein (CRP) at the start of therapy, and previous exposure to a biologic agent[2 ].
Secondary loss of response occurs when a patient initially had response to therapy but lost that benefit over time. This can occur in up to 50 % of patients and can lead to the need for either dose intensification, or the use of an alternate agent. The formation of anti-drug antibodies (ADA) and inadequate drug exposure are the main factors contributing to secondary loss of response in patients on biologic therapies[1 ].
Therapeutic drug monitoring (TDM) is a way to optimize the dose of biologics and immunomodulators (IMM) to optimize treatment outcomes. The levels or metabolites, as well as the development of antibodies, are used to help guide drug dosing in order to enhance drug efficacy and reduce disease complications[3 ]. Current AGA guidelines published in 2017 recommend reactive TDM for patients with active IBD. Reactive TDM occurs when dosing of a therapy is changed following either primary non-response or secondary loss of response. Proactive TDM involves routine monitoring of drug levels and antibodies at set intervals with dose adjustments based on drug levels. Many studies have shown that there is a correlation between positive clinical outcomes and therapeutic ranges of serum drug concentrations for each agent available to treat IBD[4 ]. This review aims to discuss TDM for biologics and thiopurines in treatment of active IBD.
TNF lNHlBlTORS
TNF inhibitors available for treating active IBD include infliximab, adalimumab, certolizumab, and golimumab. Studies have confirmed that there is a correlation between clinical response and drug concentrations of anti-TNF agents measuredviaserologic work-up.
Infliximab is a chimeric monoclonal anti-TNF agent approved for patients with active UC or CD.Studies have shown that higher infliximab concentrations lead to improved outcomes in patients with IBD. TAXIT, a prospective trial on patients with CD on infliximab, demonstrated a significant improvement in remission and lower rates of ADA with dose escalation[5 ]. The TAILORIX trial was a second prospective trial for patients with CD on infliximab that tried to assess whether increasing the dose of infliximab based upon a combination of symptoms, biomarkers, and serum drug concentrations leads to improved outcomes compared to dose intensification based purely upon symptoms. This trial did not reach its primary endpoint of sustained corticosteroid-free clinical remission from weeks 22 through 54 [6 ]. However, a post-hoc analysis of the TAILORIX trial demonstrated that infliximab drug concentrations were higher in patients that achieved endoscopic remission by week 12 compared to patients who did not achieve remission, which supports TDM is beneficial for patients on infliximab[7 ].Furthermore, the TAILORIX utilized an infliximab drug concentration of 3 μg/mL as a target, which is widely considered low based upon the results of several recent studies[8 -11 ]. The low target infliximab level could have limited the efficacy analysis of TDM in the trial. Patients with UC on infliximab maintenance therapy were examined in a retrospective study that utilized TDM and endoscopic evaluation. This study was able to demonstrate that patients with endoscopic and histologic remission had significantly higher serum drug levels[12 ]. A cost-analysis performed on TDM for infliximab suggested that proactive TDM led to fewer flares than the reactive method, and that more patients remained on therapy with proactive TDM. Fewer flares paired with a reduction in the cost of infliximab over time suggests that proactive TDM may be more suitable for patients with IBD on biologic agents[13 ].
Adalimumab is a human monoclonal immunoglobulin G (IgG0 anti-TNF agent used for the treatment of active CD and UC. Numerous studies have demonstrated improved outcomes in patients with higher drug concentrations of adalimumab. Parket al[14 ] observed that higher serum drug levels of adalimumab were associated with more quiescent disease and normal CRP. The patients in this study also had higher rates of endoscopic and radiologic remission with higher serum concentrations of adalimumab. The POETIC study was a prospective study on patients with CD on adalimumab designed to evaluate the evolution of ADA over time, and its correlation with clinical outcomes. Many patients developed ADA as early as week 2 , and the early development of antibodies correlated with primary non-response[15 ].
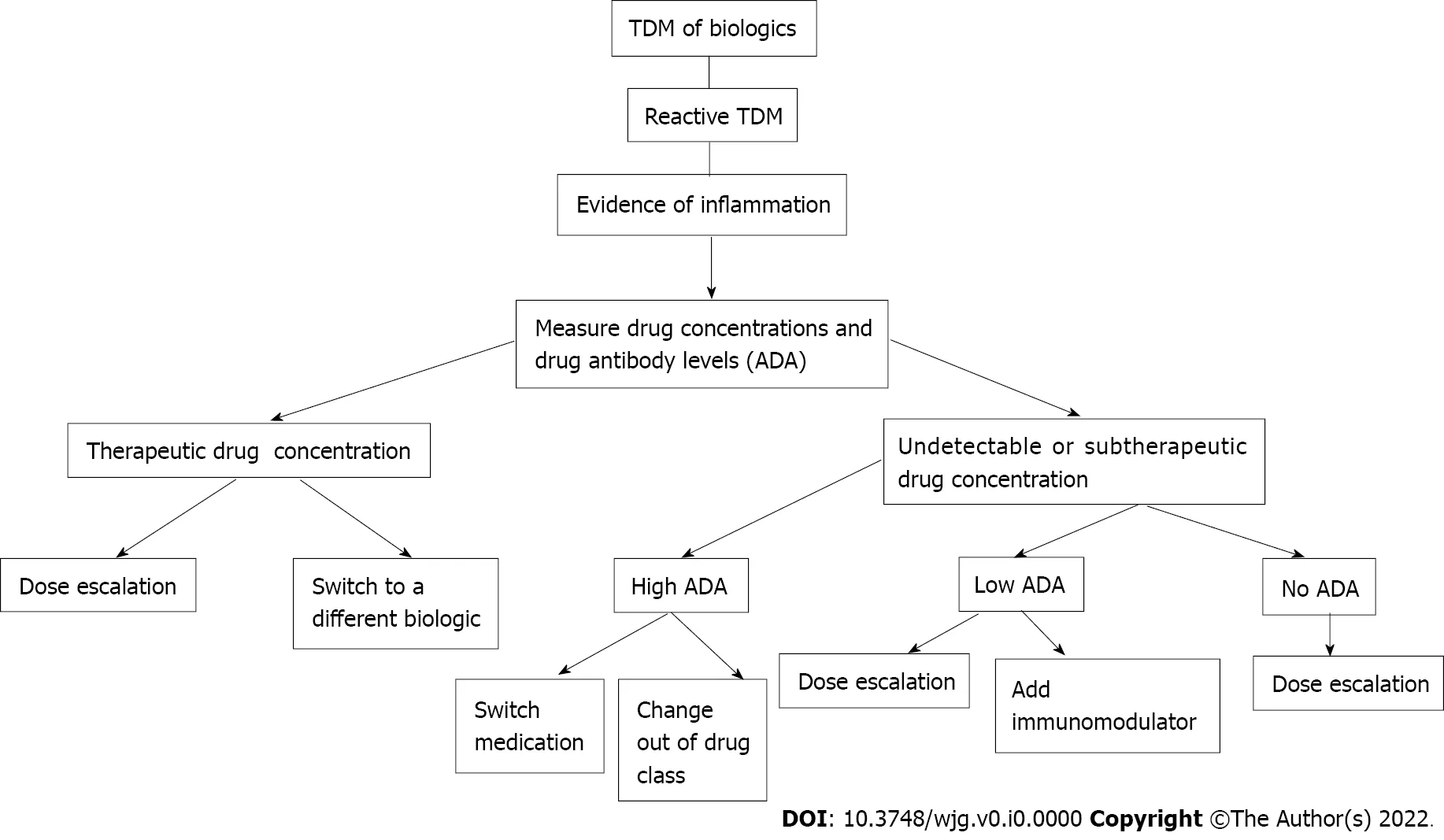
Figure 1 ln-depth algorithm for reactive therapeutic drug monitoring. TDM: Therapeutic drug monitoring; ADA: Anti-drug antibodies.
Golimumab is a human IgG1 kappa monoclonal anti-TNF therapy for patients with UC, which was approved based on the results from the PURSUIT trial. In the PURSUIT trial, serum golimumab concentrations and ADA were measured during induction as well as through maintenance therapy. An exposure-response relationship was noted; patients receiving the lowest dose of the drug had a higher incidence of ADA, as well as a higher fecal calprotectin and serum CRP[16 ]. Another study was able to demonstrate a positive correlation between golimumab concentrations and clinical and endoscopic outcomes[17 ].
Certolizumab pegol is a PEGylated Fab’ fragment of a humanized monoclonal antibody that binds to TNF. It is unique in that it lacks the Fc component that other TNF inhibitors have, making it incapable of fixing complement or binding Fc receptors[18 ]. Current studies show that higher certolizumab plasma concentrations lead to increased remission as well as decreased levels of CRP[19 ]. Patients with higher concentrations of certolizumab as early as week 2 had clinical remission by week 6 of induction, as well as continued positive outcomes during maintenance therapy[20 ].
VEDOLlZUMAB
Vedolizumab is a monoclonal antibody that binds α4β7 integrin for the treatment of moderate-to-severe UC and CD. A post hoc analyses of the GEMINI data confirmed the relationship between higher vedolizumab exposure and clinical remission in patents with IBD. Both patients with UC and CD showed higher rates of remission at week 6 with higher drug concentrations, which correlated with clinical response and mucosal healing, thus confirming an exposure-efficacy relationship[21 -23 ]. The LOVE-CD trial was a prospective trial in patients with active CD receiving vedolizumab that showed higher serum levels of vedolizumab correlated with higher rates of endoscopic and histologic remission at weeks 26 and 52 [24 ]. One single-center, cross-sectional, retrospective study showed higher serum levels of vedolizumab correlated with lower CRP levels. However, this study failed to demonstrate a correlation between vedolizumab concentrations and mucosal healing[25 ]. Notably, the rate of ADA to vedolizumab seems to be relatively low[25 ,26 ].
USTEKlNUMAB
Ustekinumab is a human monoclonal antibody that binds to the p40 subunit of interleukin (IL)-12 and IL-23 , thus preventing the interaction with the cell surface IL-12 RB1 receptor. This prevents IL-12 and IL-23 mediated cell signaling[27 ]. The efficacy of ustekinumab for the treatment of moderate to severe CD was demonstrated in the UNITI-1 and UNITI-2 studies[28 ]. An analysis of data from phase 3 studies of patients with active CD on ustekinumab demonstrated a dose response. Drug serum concentrations were positively associated with clinical remission and endoscopic improvement at week 44 ; there was also an inverse association with the CRP level. IMM were not found to have a significant effect on the serum concentration of ustekinumab[29 ,30 ]. The available data also suggest an extremely low rate of antibody formation in ustekinumab[28 ,29 ].
THlOPURlNES
IMM, including azathioprine (AZA) and 6 -mercaptopurine (6 -MP) have been used for the treatment of IBD for many years. AZA is converted to 6 -MP using a non-enzymatic pathway. 6 -MP is broken down in three different ways: Into 6 -thiouric acid by xanthine oxidase, activated to 6 -methyl-mercaptopurine(6 -MMP) by thiopurine methyltransferase (TPMT), or to 6 -thioguanine dehydrogenase (6 -TGN) by three different enzymes. TPMT has variants that can lead to a reduction in activity, therefore patients should have their TPMT phenotype checked prior to use. Once a thiopurine has been added to a regimen,thiopurine metabolites should be assessed, as patients have better outcomes with higher 6 -TGN levels and lower 6 -MMP levels[31 ]. Thiopurines play a role in reducing the risk of antibody formation, particularly to TNF inhibitors A study on patients with multiple different autoimmune diseases showed a reduction in ADA with the use of supplemental immunosuppressants[32 ].
The SONIC study is a landmark prospective trial that showed a combination therapy of AZA and infliximab for CD was more effective than infliximab monotherapy in induction and maintenance of steroid-free clinical remission at 26 and 52 wk. Combination therapy also led to lower rates of antibodies to infliximab and higher serum drug concentrations[33 ].
THE TRANSlENT NATURE OF SOME ADA
Although the formation of ADA can correlate to poor clinical outcomes, ADA levels may sometimes be transient[34 ,35 ]. Low levels of ADA may be overcome with higher serum drug concentrations and the addition of immunomodulators. However, if patients have sustained elevated levels of ADA,permanent loss of response is more likely to occur[36 ]. A small retrospective analysis of 5 patients investigated the addition of immunomodulators (thiopurines and methotrexate) to patients after the development of ADA to infliximab. All five patients had restoration of clinical response, and ADA levels gradually diminished over time[37 ]. It will be important for the future of IBD therapy to understand the role ADA formation plays in loss of response in patients on biologics, and the benefit of immunomodulators to recapturing response.
REACTlVE TDM
Reactive TDM is the current standard of care when treating IBD patients who have a loss of response to biologic therapy[4 ]. This approach can identify the subset of patients that would benefit from dose escalation of their current agentvstransitioning to a different therapy. Once a patient has a flare of their symptoms, drug concentrations and ADA levels are measured, and further management is based upon these results (Figure 1 ). A retrospective study of patients with suspected loss of response was performed that determined that the measurement of trough levels of anti-TNF agents or ADAs during a suspected loss of response led to improved interventions. Patients with high ADA levels benefited more from switching agents than from dose escalation, whereas patients with no or low ADA levels did benefit from dose intensification. This study also demonstrated that patients with adequate levels of infliximab or adalimumab with inadequate response would benefit from an agent that is out of the anti-TNF class[38 ]. A retrospective analysis of patients receiving infliximab who underwent dose escalation was examined, and clinical decisions with or without the use of TDM were compared. Patients for whom decisions were based upon TDM had improved endoscopic outcomes, higher rates of clinical remission,fewer hospitalizations, and less steroid use[39 ]. Reactive TDM has the benefit of cost savings, as less drug can be utilized, as well as the ability to try to optimize drug levels and increase the chance of recapturing response in order to prevent treatment failure[7 ].
PROACTlVE TDM
Proactive TDM differs from reactive TDM in that it aims to optimize drug concentrations by measuring serum drug concentrations and ADA levels at set intervals in order to prevent loss of response(Figure 2 ). Since the best therapeutic effect is obtained with the first biologic agent received, proponents of this approach consider it imperative to optimize the dose of the agent early in the treatment course[40 ].
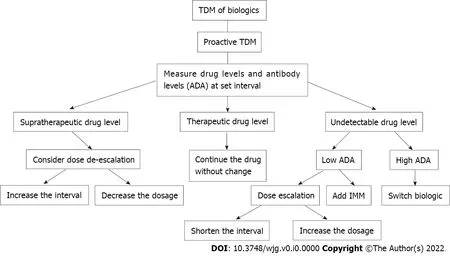
Figure 2 ln-depth algorithm for proactive therapeutic drug monitoring. TDM: Therapeutic drug monitoring; IMM: Immunomodulators; ADA: Anti-drug antibodies.
Fernandeset al[41 ] demonstrated in a prospective study on patients on infliximab therapy with CD and UC that proactive TDM had better outcomes than management without the use of TDM. Patients on infliximab underwent trough and ADA level measurements before the fourth infusion and at every 2 infusions, and dose-adjustment was made in order to keep a goal trough level between 3 and 7 μg/mL for CD and 5 and 10 μg/mL for UC. Compared to a retrospective cohort treated with infliximab without the use of TDM, the TDM group showed improved mucosal healing, fewer surgeries and hospitalizations, and less treatment discontinuation. A randomized, controlled trial of children with CD, the PAILOT trial, investigated proactive TDMvsreactive TDM. The primary endpoint was corticosteroidfree remission on adalimumab therapy. The results showed significantly higher rates of steroid-free remission in patients receiving proactive TDM than reactive TDM, as 31 children (82 %) in the proactive group reached the primary endpoint, meanwhile 19 children (48 %) reached the endpoint using reactive TDM[42 ]. Papamichael et al[43 ] performed a multicenter, retrospective study on patients receiving infliximab therapy for IBD. Proactive TDM had better clinical outcomes when compared to reactive TDM, including fewer surgeries, hospitalizations, lower ADA levels, longer time to treatment failure[44 -46 ]. Much of the current data for TDM seems to demonstrate that proactive TDM leads to better clinical outcomes compared to reactive TDM. However, a universal analysis on cost-effectiveness is much more difficult given varying degrees of coverage on biologics and TDM assays[47 ,48 ].
lMMUNOASSAY METHODS FOR THE DETECTlON OF ANTlBODlES
Although it is well known that patients with inflammatory bowel disease are at risk of developing antibodies to biologics, more attention should be paid toward the optimal methodology used to detect these antibodies. The various immunoassay methods for detection of drug antibodies are suspected to yield varying results when assessing immunogenicity of biologics due to the presence of drug and the potential underestimation of ADA[49 ]. Drug interference limits the detection of ADA due to the formation of ADA-drug complexes in the assay. Drug tolerant assays were developed that can detect free ADA and ADA bound in a complex. This assay can dissociate the ADA from the drug to estimate the quantity of ADA more accurately in a sample. Drug-sensitive antibody detection methods such as the antibody binding test (ABT) and bridging enzyme-linked immunosorbent assay (ELISA) preceded the drug-tolerant assays[50 ].
A study by Ruwaardet al[49 ] compared the efficacy of three different immunoassays to detect ADA,including ABT, ELISA, and drug-tolerant assays in 86 patients on adalimumab. There was a significant difference in the ability to detect ADA between the assays, with drug-tolerant assays detecting ADA in 69 % of patients, compared to 30 % in the ABT, and 2 % using the ELISA. This suggests that drug-tolerant assays should be the standard when detecting ADA in patients on adalimumab. A study by Wanget al[50 ] compared ELISA to a drug tolerant assay, the homogenous mobility shift assay (HMSA), in patients treated with infliximab. This study illustrated that the HMSA was significantly more sensitive in detecting ADA, especially in the presence of high serum drug concentrations. HMSA can overcome artifacts encountered using drug-sensitive assays, as it can dissociate the ADA from the drug. These studies suggest that future studies should consider using drug-tolerant assays as their method of detecting ADA to standardize the methodology and prevent inconsistent results between different studies.
It is still well-known that ADA formation leads to lower drug concentrations and worse outcomes in patients with IBD on biologics. The data suggest that drug-tolerant assays are ideal for detection of ADA in patients on adalimumab and infliximab. Standardization in detection of ADA would improve the variability amongst studies, thus improving clinicians’ ability to use and perform TDM. Unfortunately, the available data focus on TNF inhibitors, and the applicability to non-TNF inhibitor biologics is limited. Further studies with inclusion of all biologics could help lead to implementation of international standards and improve our understanding on the impact of ADA on clinical outcomes[51 ].
CONCLUSlON
TDM plays an important role in treatment outcomes for patients with IBD on biologic agents. TDM is useful for predicting loss of response and preventing treatment failure. Higher serum drug concentrations lead to improved outcomes, with fewer hospitalizations, surgeries, and treatment failures.Lower serum drug concentrations and the development of ADA lead to worse outcomes and loss of response. The addition of immunomodulators has not been standardized, but studies have shown that the addition of an immunomodulator to TNF inhibitors can lead to a reduction in the development of ADA as well as higher serum drug concentrations, thus eliminating the potential for failure of an agent.
Despite a multitude of studies, there are still limitations regarding the use of TDM in IBD patients.There is likely a fair amount of inter-individual variation regarding the appropriate serum concentration of various biologics, and the optimal target levels have therefore not been fully elucidated. Similarly,further research needs to be done regarding the significance of different levels of ADA. Different serum concentration and ADA assays can also complicate our interpretation of these values. Finally, analyses regarding cost-effectiveness of reactivevsproactive TDM in the setting of a variety of different health care settings are difficult to conduct.
FOOTNOTES
Author contributions:Rao S reviewed prior publications and composed the paper; Lodhia N revised the paper.
Conflict-of-interest statement:No potential conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:United States
ORClD number:Nilesh Lodhia 0000 -0003 -4177 -7548 ; Shanti Rao 0000 -0001 -9798 -711 X.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年21期
World Journal of Gastroenterology2022年21期
- World Journal of Gastroenterology的其它文章
- Endoscopic ultrasound-guided injectable therapy for pancreatic cancer: A systematic review
- Socioeconomics and attributable etiology of primary liver cancer, 1990 -2019
- Peripancreatic paraganglioma: Lesson from a round table
- Differentiating malignant and benign focal liver lesions in children using CEUS LI-RADS combined with serum alpha-fetoprotein
- Serotonin type 3 receptor subunit gene polymorphisms associated with psychosomatic symptoms in irritable bowel syndrome: A multicenter retrospective study
- Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome
