基于颗粒污泥的短程反硝化USB反应器启动和运行研究
马瑞婕,刘永红*,梁继东,王 宁,解凤霞
基于颗粒污泥的短程反硝化USB反应器启动和运行研究
马瑞婕1,刘永红1*,梁继东2,王 宁1,解凤霞1
(1.西安工程大学环境与化学工程学院,陕西 西安 710048;2.西安交通大学能源与动力工程学院,陕西 西安 710049)
在2个相同的USB反应器(R1无载体,R2采用多孔生物填料为载体)中构建了短程反硝化工艺,对R1和R2NO3--N→NO2--N转化性能、短程反硝化颗粒污泥物化特性、胞外聚合物(EPS)产生特性以及微生物功能菌群主要特征进行差异分析.结果表明,反应器运行81d,氮负荷(NLR)为1.2kg/(m3·d)时, NO3--N→NO2--N转化率(NTR)R2(85%)高于R1(80%);载体颗粒污泥(R2)沉降性能优于自固定化颗粒污泥(R1)且载体颗粒污泥(R2)更容易截留EPS,PN/PS值R1(1.29)>R2(1.15),污泥体积指数(SVI)R1(27.07mL/g MLSS)>R2(19.36mL/g MLSS);扫描电镜发现R1污泥表面聚集长杆菌,R2污泥表面聚集短杆菌和球菌,与R1相比R2颗粒污泥结构更加规则密实.微生物高通量测序结果表明,R2物种丰富度和多样性高于R1,变形菌门、拟杆菌门和绿弯菌门在短程反硝化系统中占主导地位,R1和R2主要NO2--N积累功能菌属均为属(R1-59.18%、R2-46.04%)和属(R1-6.81%、R2-5.99%).
短程反硝化(PDN);厌氧氨氧化(Anammox);亚硝酸盐积累;PDN颗粒污泥;生物载体
随着污水中氮素污染物增加,实现污水高效脱氮和达标排放是当前污水处理邻域研究重点之一.厌氧氨氧化(Anammox)工艺因无需外加碳源、污泥产量低、无需供氧,成为一种最经济有效的新型生物脱氮技术[1].然而,实际污水中Anammox电子受体(NO2--N)的匮乏使其工程应用存在局限性[2].短程反硝化(PDN)是将NO3--N还原至NO2--N的不完全生物反硝化过程[3],被认为是与Anammox耦合最具前景的NO2--N供给工艺[4].
微生物自固定化形成的颗粒污泥具有良好的沉降性能并能持留较高的生物量,可以满足高负荷生物反应器的运行需求[5].已有研究[6-7]在SBR反应器中以PDN污泥为种泥成功培养出PDN颗粒污泥,实现了较高的NO3--N→NO2--N转化率(NTR).然而污泥自固定化是一个漫长的过程,因此PDN颗粒污泥的快速培养技术是PDN工艺工程化应用的关键技术之一[8].
相关研究指出,高上升流速的USB反应器有利于颗粒污泥快速培养[9].此外,添加生物载体可加速污泥颗粒化以及持留功能菌.目前通过添加载体促进厌氧、好氧污泥颗粒化的研究较多[9-10],但在主流低氮素浓度污水条件下,添加载体促进PDN污泥颗粒化的研究较少.
因此,本研究在连续流态下,分别构建有无生物载体的PDN工艺,对比探究PDN工艺启动及稳定运行过程NO2--N积累性能,考察自固定化颗粒污泥和载体颗粒污泥培养过程中污泥物化特性差异,并基于高通量测序技术分析微生物菌群特征和功能菌属富集机制,旨在为颗粒污泥技术促进PDN工艺稳定运行及工程化应用提供借鉴.
1 材料与方法
1.1 实验装置与运行
采用2个相同的有机玻璃材质、双层夹套结构USB反应器(R1和R2).反应器有效容积为2.6L(内径6cm,高60cm),原水通过蠕动泵连续进入反应器底部.反应器设回流装置,从下至上平均设3个取样口,水浴维持反应器温度为25℃.
本实验分为2个阶段,阶段Ⅰ为PDN启动阶段(1~41d),阶段Ⅱ为负荷提升阶段(42~81d).R1和R2反应器不同阶段运行参数一致,如表1所示.
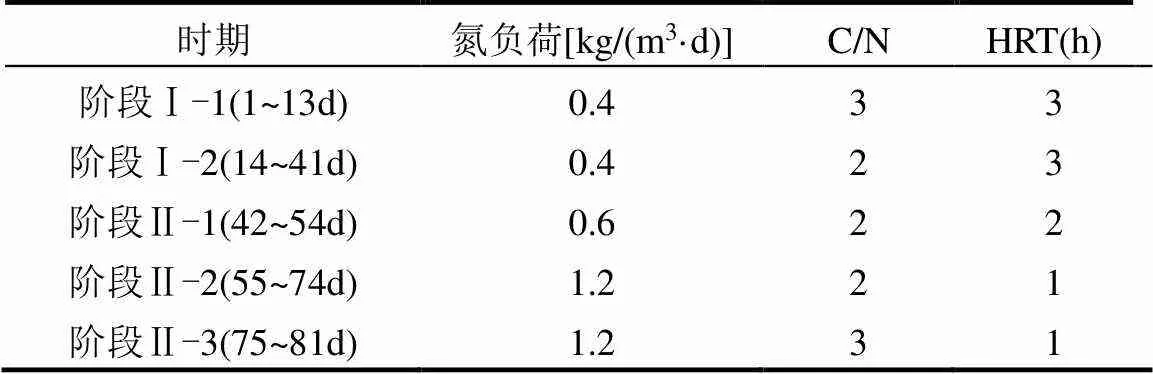
表1 R1和R2运行参数
1.2 进水水质
进水水质采用人工配水,NO3--N和COD由硝酸钠(NaNO3)和无水乙酸钠(CH3COONa)按比例配制,矿物质组成如下:0.15g/L MgSO4·7H2O, 0.14g/L CaCl2, 0.03g/L KH2PO4, 1.00mL/L微量元素Ⅰ和Ⅱ,微量元素Ⅰ的成分:EDTA·2Na 6.39g/L、FeSO45.00g/L;微量元素Ⅱ的成分:EDTA·2Na 19.11g/L、H3BO40.014g/L、MnCl2·4H2O 0.99g/L、CuSO4·5H2O 0.25g/L、ZnSO4·7H2O 0.43g/L、NiCl2· 6H2O 0.19g/L、CoCl2·6H2O 0.24g/L、NaMoO4·2H2O 0.22g/L[11].采用1mol/L NaOH溶液调节进水pH值.
1.3 多孔生物填料及接种污泥
本实验采用的亲水性多孔生物填料(HPBC)是一种具有多孔结构的有机高分子-无机复合材料,通体呈鳞片状及通孔状的亲水结构,大小为4mm左右,内部孔隙大小为5~10μm,比重略大于水[12].该载体结构与颗粒污泥较为相似,有利于微生物在其表面附着生长.
R1和R2接种泥源来源于西安某高校污水站沉淀池污泥,呈黄棕色絮状.污泥混合液悬浮固体质量浓度(MLSS)为11.26g/L,混合液挥发性悬浮固体质量浓度(MLVSS)为4.47g/L.R1接种1.0L污泥;R2接种1.0L污泥并投入30%生物填料.
1.4 分析项目及检测方法
水样经0.45μm滤纸过滤后,COD采用快速消解分光光度仪[连华5B-3 (C)]测定,COD数据为校正值,NO2--N对COD测定理论贡献为1.14g/g(以COD/NO2--N计)[13];NO2--N和NO3--N分别采用N-(1-萘基) -乙二胺比色法和紫外分光光度法; pH值采用PHS-3C型pH计直接测定法.采用热提取法[14]分层提取EPS,分别采用考马斯亮蓝(G-250)法[15]和蒽酮分光光度法[10]测定蛋白质(PN)和多糖(PS)含量.
1.5 微生物高通量测序分析
采集2个反应器运行第81d底端的少量污泥样品 (对应编号分别为R1和R2),由上海生工公司进行测序,测序方法见文献[6].
1.6 NTR计算方法

式中:NO2--Neff和NO2--Ninf分别为USB反应器出水和进水NO2--N浓度,mg/L;NO3--Neff和NO3--Ninf分别为USB反应器出水和进水NO3--N浓度,mg/L[16].
2 结果与讨论
2.1 PDN系统启动及运行特性
维持进水NO3--N浓度为50mg/L,R1和R2反应器PDN启动运行特性如图1所示.
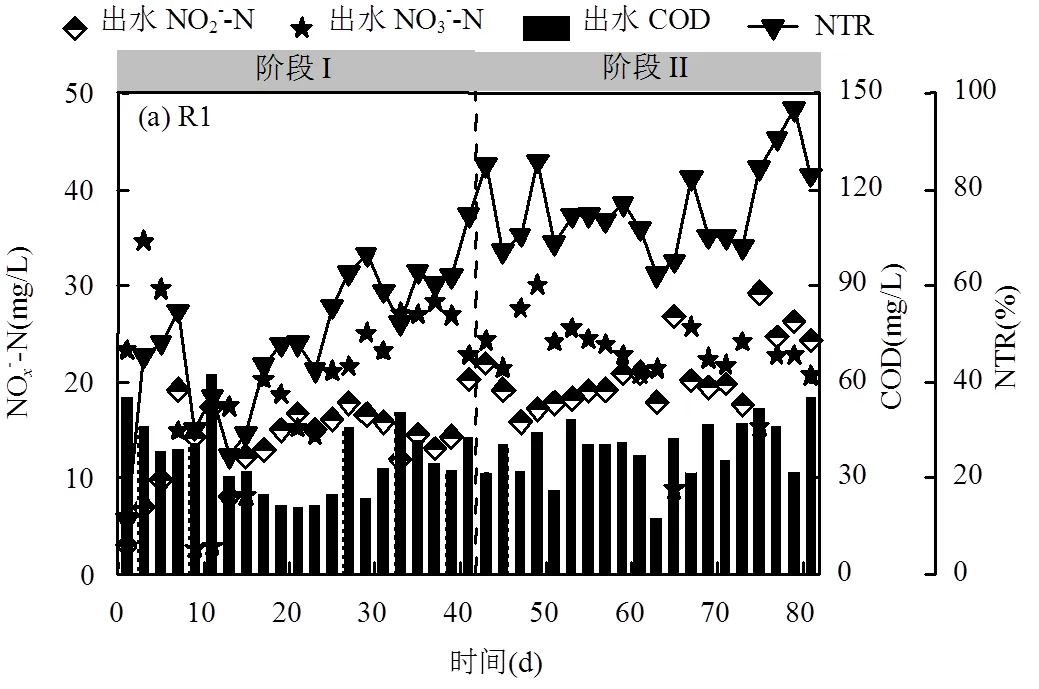
阶段Ⅰ-1(1~13d),以COD/NO3--N=3.0启动R1和R2反应器,启动初期,由于接种原泥中PDN功能菌活性较低,R1出水NO2--N和NO3--N浓度分别为3.00mg/L和23.27mg/L,COD去除率为65%,NTR仅为11.22%; R2出水NO2--N和NO3--N浓度分别为1.40mg/L和7.61mg/L,COD去除率为65%,NTR仅为3.3%.随着PDN污泥的驯化, R1和R2PDN活性均逐步上升,NTR不断增加.第7d后,由于系统碳源充足R1和R2全程反硝化菌利用有机物将NO2--N进一步还原为N2,R1和R2NTR均开始降低,R1NTR从54.65%(第7d)下降至24.56%(第13d), R2NTR从58.46%(第7d)下降至5.96%(第13d).
阶段Ⅰ-2(14~41d),以COD/NO3--N=2.0运行反应器,R1和R2NO2--N积累量从第13d的8.0mg/L和1.8mg/L分别提高到第23d的20.3mg/L和20.9mg/L.表明低COD/NO3--N比使PDN功能菌占据优势地位,抑制NO2--N还原菌属增殖[17].第24d进水pH值提高为9,导致NO2--N积累量增加.高pH值会降低有机物的氧化速率, 导致反硝化酶之间竞争电子[18],且高pH值抑制NO2--N还原酶活性,而对NO3--N还原酶活性没有显著影响,有利于选择富集PDN功能菌[19-20].相关研究认为反应器出水NTR = 70%是PDN启动的一个标志[16],R1NTR在第41d达到74.71%, R2NTR在第39d达到74.50%,说明R1和R2均已成功启动PDN.
阶段Ⅱ-1(42~54d),R1和R2水力停留时间(HRT)由3h降为2h,氮负荷(NLR)达到0.6kg/(m3·d), R1和R2平均NTR分别为75.30%、78.57%.阶段Ⅱ-2(55~74d),R1和R2HRT降为1h,NLR达到1.2kg/ (m3·d),R1和R2NO2--N积累量均先下降后上升.阶段Ⅱ-3(75~81d),为进一步提高NTR和NO2--N积累量,以COD/NO3--N=3.0运行反应器,R1和R2的NTR分别增加为84.46%、89.31%,NO2--N积累量分别为29.3mg/L、29.4mg/L.R1 和R2NTR分别稳定维持在80%、85%以上,与R1自固定化颗粒污泥相比,R2载体颗粒污泥在PDN启动运行性能方面具有优势,R2内添加亲水性多孔生物载体有利于微生物附着并维持系统稳定运行.
2.2 PDN系统污泥特性分析
2.2.1 污泥表观形态与微生物相观察 R1和R2运行过程污泥表观形态变化如图2所示.接种的普通絮状活性污泥(图2a,d),经过38d的培养, R1内污泥表现出颗粒特征但小颗粒数量较多(图2b);R2内絮状污泥附着在亲水性多孔生物载体表面,呈黄棕色颗粒(图2e).随着反应器运行,颗粒污泥粒径增大,第81d观察到R1自固定化颗粒污泥结构较松散(图2c);R2载体颗粒污泥结构更加规则密实(图2f).
通过SEM进一步观察污泥的微观结构,R1和R2运行第81d反应器底端污泥SEM图如图3所示.R1和R2颗粒污泥培养完成后,R1自固定化颗粒污泥表面杆状微生物占优势地位(图3a,b);R2 载体颗粒污泥表面聚集大量短杆菌和球菌(图3c,d).R1和R2微生物周围均含有大量的黏性物质,其可能是细菌分泌的胞外聚合物(EPS),这些物质将细菌互相黏连在一起,促进PDN污泥颗粒化.与自固定化颗粒污泥(R1)相比,载体颗粒污泥(R2)表面微生物排列更加紧密.

图3 R1和R2第81d 污泥 SEM观察
2.2.2 污泥浓度与沉降性能分析 由图4可知, R1和R2的MLSS在运行过程中均先缓慢增长(0~ 40d)后迅速增加(41~80d),第80d,R1和R2的MLSS分别为25.86g/L、41.33g/L.由于接种的污泥活性较低,前期增长缓慢,污泥经培养驯化后活性提高,在R2中采用多孔生物填料为载体有助于微生物聚集生长.R1的MLVSS在运行过程中缓慢增长,最大值为7.69g/L.R2的MLVSS初期增长较慢,末期(60~ 80d)迅速增长,最大值达到15.87g/L.在运行末期, R2的MLSS和MLVSS均大于R1,表明载体颗粒污泥(R2)较自固定化颗粒污泥(R1)更利于生物量的聚集.
R1和R2MLVSS/MLSS比值均先升高后降低趋于稳定.在运行末期,R1和R2 MLVSS/MLSS比值分别从最高值53.77%、62.04%下降到29.75%、38.39%.R1和R2污泥中非挥发性物质增加,由于进水中的金属离子(Ca2+、Mg2+等)在PDN过程形成无机盐,无机盐在颗粒污泥内部沉淀导致颗粒内惰性组分增加[21].已有研究报道,非挥发性物质的积累可促进污泥颗粒化[22].

污泥体积指数(SVI)能较好地反映出活性污泥的松散程度和凝聚沉降性能,R1和R2运行过程SVI变化如表2所示.

表2 R1和R2 SVI变化
R1和R2SVI小于普通良好的活性污泥SVI (50~120mL/g MLSS之间)[11],表明在R1和R2内培养的PDN颗粒污泥均具有良好的沉降性能.在负荷提升阶段(42~81d),R2SVI小于R1SVI,载体颗粒污泥沉降性能优于自固定化颗粒污泥.
2.2.3 污泥中EPS含量变化 EPS能够促进细胞聚集且维持稳定,主要由(PN)和(PS)组成,EPS与颗粒污泥的形成密切相关[23].分层提取溶解型EPS (S-EPS)、松散附着型EPS(L-EPS)和紧密粘附型EPS(T-EPS),R1和R2颗粒污泥EPS含量变化如图5所示.

由图5可知,在阶段Ⅰ(1~41d),R2EPS含量高于R1,表明多孔生物载体颗粒污泥更容易截留EPS, EPS有助于微生物聚集生长以及污泥颗粒化,使R2污泥颗粒化程度较R1明显. 在阶段Ⅱ(42~81d),由于反应器运行后期颗粒污泥成熟导致微生物分泌EPS含量减少且异养菌利用部分EPS作为有机碳源进行内源反硝化, R1和R2EPS含量均逐渐降低且R2EPS含量低于R1.第80d,R1和R2EPS含量分别降低至24.10 和17.57mg/gVSS.在整个运行过程中EPS含量T层最高,表明T-EPS对PDN颗粒污泥的稳定性具有重要作用,这与大多数研究结果一致[24].
此外,在阶段Ⅰ(1~41d),R2EPS 中PN和PS含量均高于R1,研究表明,PN具有较高黏结强度且有助于微生物维持自身生长和代谢活性[25].粘性和亲水性的 PS能够促使生物群体通过桥联作用形成交叉的网状结构,从而促进污泥形成颗粒状结构[26]. PN/PS一般作为评价污泥沉降性能的重要指标,相关研究报道颗粒污泥PN/PS在4.0以下时具有良好的沉降性能,过高的比例会导致沉降性能差[27].本研究第80d R1和R2颗粒污泥的PN/PS分别为1.29、1.15,再次表明载体颗粒污泥(R2)沉降性能优于自固定化颗粒污泥(R1).
2.3 PDN系统微生物群落多样性分析
2.3.1 物种丰富性和多样性 R1和R2运行第81d污泥样品丰富性和多样性指数如表3所示.R1和R2污泥样品中共产生46518和47643个有效序列,OTUs分别为415和425.R1和R2的覆盖率估计值均在0.99以上,表明测序结果能够全面分析微生物群落结构.ACE和Chao值代表物种丰富度, Shannon和Simpson值估算样品中物种的多样性.由表1可知R2的物种丰富度和多样性比R1高,这与SEM观察到的结果一致.

表3 R1和R2污泥丰富度和多样性
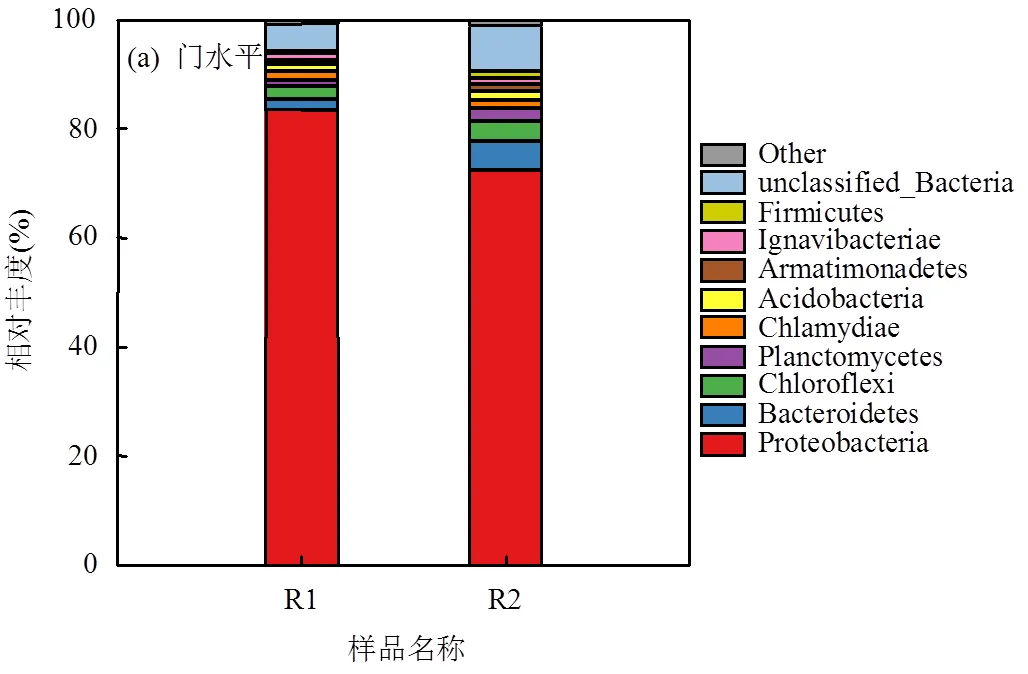
2.3.2 门水平微生物群落结构 R1和R2运行第81d污泥样品门水平微生物群落结构如图6(a)所示.按门水平相对丰度高低排序,R1主要菌门分别为变形菌门(Proteobacteria)(84.00%)、绿弯菌门(Chloroflexi) (2.36%)、拟杆菌门(Bacteroidetes) (2.14%)和衣原体门(Chlamydiae)(1.80%);R2依次为变形菌门(Proteobacteria)(73.34%)、拟杆菌门(Bacteroidetes) (5.49%)、绿弯菌门(Chloroflexi) (3.50%)和浮霉菌门(Planctomycetes)(2.58%).
变形菌门在氮素循环和有机物利用过程中发挥重要作用,研究表明变形菌门在PDN系统中占比很高[28].R1和R2的绝对优势菌门是变形菌门,高丰度的变形菌门是高效积累NO2--N的保证[29].拟杆菌门具有降解有机物和促进污泥颗粒化的功能,绿弯菌门可以提供絮体形成的丝状支架并具有降解碳水化合物的功能[6-7],R2拟杆菌门和绿弯菌门占比均高于R1.
2.3.3 属水平微生物群落结构 R1和R2运行第81d污泥样品属水平微生物群落结构如图6(b)所示.按属水平相对丰度高低排序,R1主要菌属分别为(59.18%)、(6.81%)、(2.68)和(1.58);R2主要菌属分别为(46.04%)、(5.99%)、(2.13%)、(1.69%).
属于变形菌门的属和属均是两个反应器的优势菌属.属在污水处理系统发挥高效的反硝化性能,张星星等[16]研究发现在PDN-SBR反应器中属丰度达40.6%,推测属在PDN系统具有重要的作用.属是广泛报道的 PDN反应器中实现NO2--N积累的功能菌属[30].本研究培养驯化的自固定化颗粒污泥和载体颗粒污泥均成功富集PDN功能菌属,R1和R2 反应器具有良好的NO2--N积累性能.
3 结论
3.1 在25℃, pH=9.0和COD/NO3--N为2~3条件下, R1和R2均成功启动PDN.NLR达到1.2kg/ (m3·d),R1和R2NTR分别高于80%、85%,添加多孔生物填料有助于提高 NO2--N积累性能.
3.2 以絮状普通活性污泥为接种物连续运行USB反应器81d均成功培养PDN自固定化颗粒污泥和载体颗粒污泥.添加亲水性多孔生物填料促进了污泥颗粒化进程,与PDN自固定化颗粒污泥(R1)相比, PDN载体颗粒污泥(R2)结构规则紧密、沉降性能优且污泥浓度高.
3.3 R1和R2的主要菌门均是变形菌门、拟杆菌门和绿弯菌门,两个反应器均成功富集PDN功能菌属属和属,保证了系统高效稳定积累NO2--N.R2载体颗粒污泥的物种丰富度和多样性高于R1自固定化颗粒污泥,添加亲水性多孔生物填料有利于微生物聚集生长.
[1] 赵鑫磊,邢嘉伟,付 雪,等.NO2--N/NH4+-N及COD/NH4+-N对厌氧氨氧化耦合反硝化脱氮除碳的影响[J]. 中国环境科学, 2021,41(6): 2586-2594.
Zhao X L, Xing J W, Fu X, et al. Effect of NO2--N/NH4+-N and COD/NH4+-N on anaerobic ammonia oxidation coupled denitrification nitrogen and carbon removal [J]. China Environmental Science, 2021, 41(6):2586-2594.
[2] 毛佩玥,付 雪,赵鑫磊,等.短程反硝化的启动及多参数优化下NO2--N积累特性[J]. 中国环境科学, 2021,41(3):1189-1198.
Mao P Y, Fu X, Zhao X L, et al.Start-up of partial denitrification and characteristics of nitrite accumulation by multiple factors [J]. China Environmental Science, 2021,41(3):1189-1198.
[3] Cao S B, Wang S Y, Peng Y Z, et al. Achieving partial denitrification with sludge fermentation liquid as carbon source: the effect of seeding sludge [J]. Bioresource Technology, 2013,149:570-574.
[4] 陈凯琦,张 亮,孙事昊,等.新型PD/A工艺同步处理低C/N生活原水和二级出水[J]. 中国环境科学, 2020,40(4):1515-1522.
Chen K Q, Zhang L, Sun S H, et al. Novel PD/A process to treat low C/N domestic wastewater and secondary effluent simultaneously [J]. China Environmental Science, 2020,40(4):1515-1522.
[5] Wang J X, Liang J D, Sun L, et al. Achieving reliable partial nitrification and anammox process using polyvinyl alcohol gel beads to treat low-strength ammonia wastewater [J]. Bioresource Technology, 2021,324:124669.
[6] Cao S B, Peng Y Z, Du R, et al. Characterization of partial denitrification (PD) granular sludge producing nitrite: effect of loading rates and particle size [J]. Science of the Total Environment, 2019, 671:510-518.
[7] Du R, Cao S B, Zhang H Y, et al. Formation of partial-denitrification (PD) granular sludge from low-strength nitrate wastewater: The influence of loading rates [J]. Journal of Hazardous Materials, 2020, 384:121273.
[8] 周 锋,刘勇弟,厉 巍.同步短程硝化-厌氧氨氧化-短程反硝化颗粒污泥培育过程及其性能[J]. 环境科学, 2021,42(10):4864-4871.
Zhou F, Liu Y D, Li W. Cultivation and performance analysis of simultaneous partial nitrification, ANAMMOX, and denitratation granular sludge [J]. Environmental Science, 2021,42(10):4864-4871.
[9] 梁家豪,王庆宏,傅达理,等.加速厌氧污泥颗粒化研究进展[J]. 工业水处理, 2017,37(1):18-22.
Liang J H, Wang Q H, Fu D L, et al. Research progress in the acceleration of anaerobic sludge granulation process [J]. Industrial Water Treatment, 2017,37(1):18-22.
[10] 郝 伟,刘永军,刘 喆,等.低有机负荷下不同载体对好氧污泥颗粒化的影响[J]. 化工进展, 2018,37(8):3222-3230.
Hao W, Liu Y J, Liu Z, et al. Effects of different carriers on the process of aerobic activated sludge granulation with low-strength wastewater [J]. Chemical Industry and Engineering Progress, 2018, 37(8):3222-3230.
[11] 操沈彬.基于短程反硝化的厌氧氨氧化脱氮工艺与菌群特性[D]. 哈尔滨:哈尔滨工业大学, 2018.
Cao S B. Nitrogen removal via ANAMMOX process based on partial- denitrification and microbial characteristics [D]. Harbin: Harbin Institute of Technology, 2018.
[12] 刘永红,党 康,王 宁,等.基于新型载体AMC/UASB-SCMBBR生物工艺处理印染废水的中试研究[J]. 环境科学学报, 2019,39(10): 3350-3355.
Liu Y H, Dang K, Wang N, et al.Pilot study on dyeing wastewater treatment by using AMC /UASB-SCMBBR biological process based on novel carriers [J]. Acta Scientiae Circumstantiae, 2019,39(10): 3350-3355.
[13] Qian W T, Ma B, Li X Y, et al. Long-term effect of pH on denitrification: High pH benefits achieving partial-denitrification [J]. Bioresource Technology, 2019,278:444-449.
[14] Wang J X, Liang J D, Sun L, et al. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads [J]. Bioresource Technology, 2020,309:123448.
[15] 陈茂霞,周后珍,朱晓华,等.考马斯亮蓝法检测活性污泥中蛋白质含量优化[J]. 环境科学与技术, 2015,38(4):1-5.
Chen M X, Zhou H Z, Zhu X H, et al. Optimization of determination of protein in activated sludge by bradford method [J]. Environmental Science & Technology, 2015,38(4):1-5.
[16] 张星星,王超超,王 垚,等.基于不同废污泥源的短程反硝化快速启动及稳定性[J]. 环境科学, 2020,41(8):3715-3724.
Zhang X X, Wang C C, Wang Y, et al. Rapid start-up and stability of partial denitrification based on different waste sludge sources [J]. Environmental Science, 2020,41(8):3715-3724.
[17] Zhang Z Z, Zhang Y, Chen Y G, et al. Recent advances in partial denitrification in biological nitrogen removal: from enrichment to application [J]. Bioresource Technology, 2020,298:122444.
[18] Pan Y T, Ye L, Ni B J, et al. Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers [J]. Water Research, 2012,46(15):4832-4840.
[19] Si Z, Peng Y Z, Yang A M, et al. Rapid nitrite production viapartial denitrification: pilot-scale operation and microbial community analysis [J]. Environmental Science: Water Research & Technology, 2018,4(1):80-86.
[20] Qian W T, Ma B, Li X L, et al. Long-term effect of pH on denitrification: High pH benefits achieving partial denitrification [J]. Bioresource Technology, 2019,278:444-449.
[21] Suja E, Nancharaiah Y V, Mohan T V K, et al. Denitrification accelerates granular sludge formation in sequencing batch reactors [J]. Bioresource Technology, 2015,176:32–37.
[22] Zhang P, Fang F, Chen Y P, et al. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation [J]. Chemosphere, 2014,117:59-65.
[23] 彭永臻,王锦程,李翔晨,等.氮负荷对短程反硝化耦合厌氧氨氧化生物膜系统脱氮性能的影响[J]. 北京工业大学学报, 2021,47(12): 1367-1376.
Peng Y Z, Wang J C, Li X C, et al. Effect of nitrogen loading rates on nitrogen removal performance of partial-denitrification coupling with Anammox biofilm system [J]. Journal of Beijing University of Technology, 2021,47(12):1367-1376.
[24] 李 鸿,张立秋,张绍青,等.Cd2+胁迫对短程反硝化的影响与微生物群落变化[J]. 中国给水排水, 2021,37(5):10-16,25.
Li H, Zhang L Q, Zhang S Q, et al. Effects of Cd2+stress on partial denitrification and change of microbial community [J]. China Water & Wastewater, 2021,37(5):10-16,25.
[25] Sheng G P, Yu H Q, Li X Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review [J]. Biotechnology Advances, 2010,28(6):882-894.
[26] Yan L, Liu Y, Wen Y, et al. Role and significance of extracellular polymeric substances from granular sludge for simultaneous removal of organic matter and ammonia nitrogen [J]. Bioresource Technology, 2015,179:460-466.
[27] Liu C S, Li W Fi, Li X C, et al. Nitrite accumulation in continuous- flow partial autotrophic denitrification reactor using sulfide as electron donor [J]. Bioresource Technology, 2017,243:1237–1240.
[28] 张小玲,张 萌,陈紫薇,等.内碳源短程反硝化启动及EPD- ANAMMOX耦合工艺性能[J]. 中国环境科学, 2022,42(2): 601-611.
Zhang X L, Zhang M, Chen Z W, et al. Start-up of endogenous partial denitrification and performance of EPD-ANAMMOX coupling process [J]. China Environmental Science, 2022,42(2):601-611.
[29] Cao S B, Du R, Li B K, et al. Nitrite production from partial- denitrification process fed with low carbon /nitrogen(C/N) domestic wastewater: performance, kinetics and microbial community [J]. Chemical Engineering Journal, 2017,326:1186-1196.
[30] 张星星,张 钰,王超超,等.短程反硝化耦合厌氧氨氧化工艺及其应用前景研究进展[J]. 化工进展, 2020,39(5):1981-1991.
Zhang X X, Zhang Y, Wang C C, et al. Research advances in application prospect of partial denitrification coupled with anammox: a review [J]. Chemical Industry and Engineering Progress, 2020,39 (5):1981-1991.
Study on start-up and operation of USB reactor for partial denitrification based on granular sludge.
MA Rui-jie1, LIU Yong-hong1*, LIANG Ji-dong2, WANG Ning1, XIE Feng-xia1
(1.School of Environment and Chemical Engineering, Xi′an Polytechnic University, Xi′an 710048, China;2.School of Energy and Power Engineering, Xi′an Jiaotong University, Xi′an 710049, China)., 2022,42(5):2129~2135
In this study, the partial denitrification process was constructed in two identical Up-flow Sludge Bed reactors (R1 without the carrier, R2 with porous biological carrier). The transformation performance of nitrate-to-nitrite, the physicochemical characteristics of partial denitrification granular sludge, the production characteristics of extracellular polymeric substances (EPS), and the dominant characteristics of functional bacteria generawere compared and analyzed. The NO3--N→NO2--N transformation ratio(NTR) in R2 (85%) was higher than that of R1(80%) when the reactors ran for 81 days and the nitrogen loading rates (NLR) was 1.2kg/(m3·d). The sedimentation performance of carrier granular sludge (R2) was better than that of self-immobilized granular sludge (R1), and the carrier granular sludge in R2 was easier to capture EPS. The PN/PS value of R1 (1.29) was greater than that of R2 (1.15), and the sludge volume index (SVI) of R1(27.07mL/g MLSS) was also greater than that of R2 (19.36mL/g MLSS). Under scanning electron microscopy, it was found that Long bacillus gathered on the surface of R1 sludge, while short-bacillus and coccus were observed on the surface of R2 sludge. In comparison, the structure of granular sludge in R2 was more regular and dense than that in R1. Microbial high throughput sequencing showed that the species richness and diversity of R2 were higher than that of R1.,, andwere dominant in the partial denitrification system. The main NO2--N accumulating genera in both R1 and R2 were(R1 accounted for 59.18% and R2 accounted for 46.04%) and(R1 accounted for 6.81% and R2 accounted for 5.99%).
partial denitrification(PDN);anaerobic ammonia oxidation(anammox);nitrite accumulation;PDN granular sludge;biological carrier
X703
A
1000-6923(2022)05-2129-07
马瑞婕(1996-),女,山西大同人,西安工程大学硕士研究生,主要从事污水处理技术研究.
2021-10-15
国家自然科学基金青年科学基金资助项目(22008188);陕西省重点研发计划项目(2018ZDXM-SF-023,2019GY-154,2020SF-413);西安工程大学2021年度研究生创新基金资助项目(chx2021023)
* 责任作者, 教授, liuyhxa@hotmail.com

