丁香蒲桃果实母丁香化学成分研究
谭培艺,王春杰,于 欢,刘 芳,唐生安
丁香蒲桃果实母丁香化学成分研究
谭培艺1,王春杰2, 3,于 欢4,刘 芳1,唐生安4*
1. 天津中医药大学第一附属医院药学部,国家中医针灸临床医学研究中心 天津 300073 2. 天津医学高等专科学校,天津 300222 3. 天津天药药业股份有限公司,天津 300462 4. 天津医科大学药学院,天津市临床药物关键技术重点实验室,天津 300070
研究丁香蒲桃的果实母丁香的化学成分。采用硅胶柱色谱、SephadexLH-20凝胶柱色谱及半制备高效液相色谱法分离纯化,采用MS、1H-NMR、13C-NMR、DEPT、HSQC、HMBC、COESY等波谱学方法鉴定化合物的结构。从母丁香乙醇提取物中分离得到了15个化合物,分别鉴定为1,3,5-三羟基-3-甲基苯乙酮- 1--β-D-(6'--没食子酰基)葡萄糖苷(1)、triterpenoster(2)、积雪草酸(3)、齐墩果酸(4)、山楂酸(5)、熊果酸(6)、花椒油素(7)、methylxanthoxylin(8)、母丁香酚(9)、没食子酸(10)、没食子酸甲酯(11)、丁香酚(12)、萎叶醇(13)、β-氧化石竹烯(14)、去氢双丁香酚B(15)。化合物1为新化合物,命名为母丁香酚A;化合物2为新天然产物,化合物7~8、11首次从该植物中分离得到。化合物1、7~9为苯乙酮类成分,2~3为乌苏烷型三萜类成分,4~5为齐墩果烷型三萜类成分,12~13、15为苯丙素类成分,14为倍半萜类成分。
丁香蒲桃;母丁香酚A;triterpenoster;花椒油素;没食子酸甲酯;去氢双丁香酚B
丁香蒲桃(L.) Merr. & L. M. Perry为一种常用草本植物,隶属于桃金娘科、蒲桃属。母丁香是丁香蒲桃的成熟果实,呈椭圆形,形似鸡舌,又称鸡舌香。该植物广泛分布在我国海南、广东、福建、浙江和云南省等地[1],果实及花蕾作为一种药食同源食物,在民间有广泛应用。文献报道,丁香蒲桃中含有苯乙酮类、色原酮类、黄酮类、苯丙素类、倍半萜类、和三萜类成分,具有抗炎、抗菌、抗病毒等广泛的药理活性[2-4],其丁香酚类的挥发油成分具有抗菌活性[5-7]。
近年来对于丁香蒲桃的研究主要集中在公丁香(花蕾),对母丁香(果实)的研究相对较少。为进一步明确母丁香的活性成分,便于对其进行后续研究和开发利用,本实验采用硅胶柱色谱、SephadexLH-20凝胶柱色谱及半制备高效液相色谱等色谱分离方法分离纯化,采用核磁共振波谱和质谱等波谱学方法鉴定单体化合物的结构。从母丁香中分离得到15个化合物(图1),分别鉴定为1,3,5-三羟基-3-甲基苯乙酮-1--β--(6'--没食子酰基)葡萄糖苷(1)、triterpenoster(2)、积雪草酸(asiatic acid,3)、齐墩果酸(oleanolic acid,4)、山楂酸(maslinic acid,5)、熊果酸(ursolic acid,6)、花椒油素(xanthoxylin,7)、methylxanthoxylin(8)、母丁香酚(bancroftione,9)、没食子酸(gallic acid,10)、没食子酸甲酯(methyl gallate,11)、丁香酚(eugenol,12)、萎叶醇(chavibetol,13)、β-氧化石竹烯(β-caryophyllene oxide,14)、去氢双丁香酚B(dehydrodieugend B,15)。其中,化合物1为未见文献报道的新化合物,命名为母丁香酚A;化合物2为新天然产物,化合物7~8、11首次从该植物中分离得到。化合物1、7~9为苯乙酮类成分,2~3为乌苏烷型三萜类成分,4~5为齐墩果烷型三萜类成分,12~13、15为苯丙素类成分,14为倍半萜类成分。
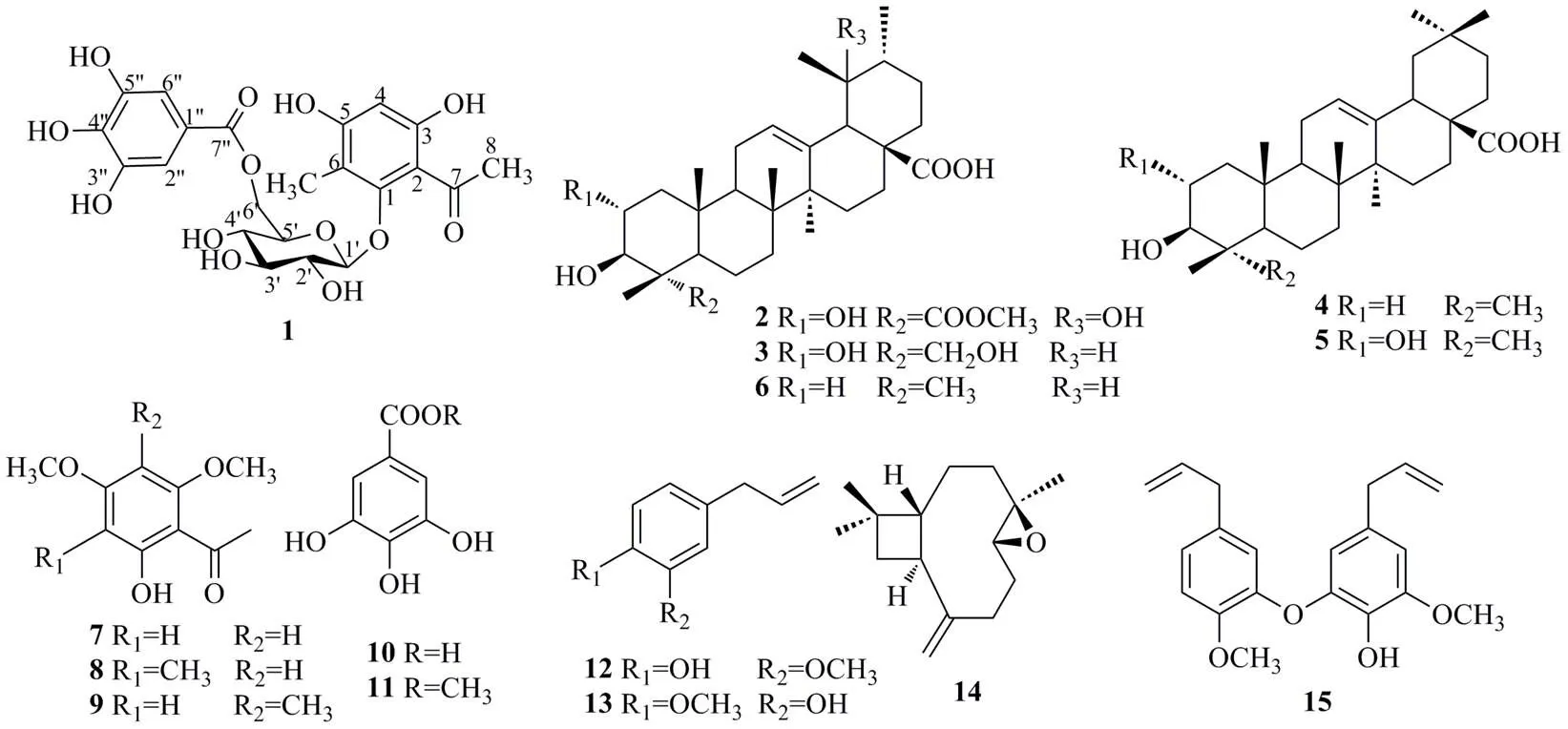
图1 化合物1~15的结构
1 仪器与材料
核磁共振波谱由布鲁克核磁共振波谱仪Bruker AVANCE 400(1H-NMR,400 MHz;13C-NMR,100 MHz)采集,1H-1H COSY,HSQC,HMBC和ROESY均为在标准测试序列下完成,四甲基硅烷为内标。高分辨质谱由Varian 7.0 T ESI-MS(Varian Inc.,CA,美国)采集。LC3000半制备型高效液相色谱仪,色谱柱为YMC-Pack ODS-A 柱(250 mm×20 mm,5 μm;YMC Co. Ltd.,Kyoto,日本)。薄层硅胶GF254和柱色谱硅胶(青岛海洋化工厂),Sephadex LH-20填料(Amersham Biosciences公司),反相ODS填料(Merck公司)。实验所用试剂均为分析纯有机试剂,为天津市津东天正精细化学试剂厂产品;氘代试剂均为Cambridge Isotope Laboratories,Inc. USA生产。
药材采集自中国广西壮族自治区,由天津医科大学唐生安副教授鉴定为(L.) Merr. & L. M. Perry。标本(T20171021)保存于天津医科大学药学院。
2 提取与分离
母丁香(0.5 kg)打磨成粉,用95%乙醇回流提取,每次1 L回流2 h,共提取3次。合并提取液浓缩后得到51.5 g提取物。提取物用水混悬,依次用石油醚、醋酸乙酯、正丁醇萃取。石油醚层萃取物(8.2 g)经Sephadex LH-20凝胶柱色谱纯化,流动相为二氯甲烷-甲醇(4∶1),得到9个组分Fr-PE.1~9,其中Fr-PE.7为化合物12(90.1 mg)。组分Fr-PE.3(2.4 g)和Fr-PE.4(3.5 g)合并后经Sephadex LH-20凝胶柱色谱进一步纯化,流动相为二氯甲烷-甲醇(100∶1、50∶1、10∶1)和甲醇,得到14个组分Fr-PE.3.1~3.14,其中Fr-PE.3.9为化合物5(13.0 mg)。组分Fr-PE.3.1(4.4 g)经硅胶柱色谱分离,流动相为石油醚-醋酸乙酯(100∶1),得到17个组分Fr-PE.3.1.1~3.1.17。Fr-PE.3.1.3(257.8 mg)经半制备高效液相色谱法纯化,石油醚-醋酸乙酯为流动相(50∶1,5 mL/min,210 nm),得到化合物14(17.7 mg,R=60.2 min)。组分Fr-PE. 3.1.7与Fr-PE. 3.1.8合并后经半制备高效液相色谱法纯化,石油醚-醋酸乙酯为流动相(30∶1,5 mL/min,210 nm),得到化合物13(26.3 mg,R=50.6 min)、7(2.8 mg,R=64.1 min)、9(80.1 mg,R=40.2 min)。组分Fr-PE.3.1.9和Fr-PE.3.1.10合并后经半制备高效液相色谱法纯化,石油醚-醋酸乙酯为流动相(10∶1,5 mL/min,210 nm),得到化合物8(4.6 mg,R=123.9 min)和15(2.0 mg,R=40.3 min)。醋酸乙酯层萃取物(18.1 g)经Sephadex LH-20凝胶柱色谱纯化,二氯甲烷-甲醇(4∶1)为流动相洗脱得到16个组分Fr-EA.1~16,其中Fr-EA.7为化合物9(349.3 mg)。组分Fr-EA.4(0.2 g)和Fr-EA.5(1.7 g)合并经硅胶柱色谱分离,石油醚-醋酸乙酯(2∶1)为流动相洗脱得到化合物2(39.8 mg)、4(23.2 mg)、5(56.3 mg)、3(102.5 mg)和6(262.3 mg)。Fr-EA.8(1.0 g)经反相硅胶柱色谱分离,甲醇-水(1∶1)为流动相,得到9个组分Fr-EA.8.1~8.9,其中Fr-EA.8.4为化合物10(41.4 mg)。组分Fr-EA.8.2(0.8 g)经硅胶柱色谱分离,流动相为二氯甲烷-甲醇(15∶1,加0.1%冰醋酸)得到13个组分Fr-EA.8.2.1~8.2.13,其中Fr-EA.8.2.3为化合物11(9.5 mg)。Fr-EA.8.2.11(61.6 mg)经硅胶柱色谱分离,流动相为二氯甲烷-甲醇(11∶1,加0.1%冰醋酸)得到化合物1(22.7 mg)。
3 酸水解实验
依据文献报道进行新化合物的酸水解实验[8]。将化合物1(2.0 mg)溶解于5 mL浓度为2 mol/L的盐酸溶液中,95 ℃下加热2 h,然后用5 mL氯仿萃取3次。水层溶液用氮吹仪浓缩干燥,后用2 mL无水吡啶溶解,再加入2.5 mg-半胱氨酸甲酯盐酸盐。混合物溶液在60 ℃下加热2 h后加入醋酸酐,然后在90 ℃下继续加热0.5 h,溶液随后用氮吹仪吹干得供试品。供试品用安捷伦HP-5MS毛细管电泳仪进行分析。进样温度为100 ℃,以10 ℃/min的速度升温至150 ℃,再以15 ℃/min的速度升温至280 ℃。以氦气为载气,体积流量1.0 mL/min,入口温度为260 ℃,电离源温度为230 ℃。
4 结构鉴定
表1 化合物1的1H-HMR和13C-NMR 数据(400/100 MHz, CD3OD)
Table 1 1Hand 13C-NMR spectral data of compound 1 (400/100 MHz, CD3OD)
碳位δHδC 1 157.9 2 111.6 3 163.1 46.02 (1H, s)100.3 5 164.4 6 112.6 7 206.3 82.50 (3H, s)32.8 1′4.52 (1H, d, J = 7.6 Hz)105.5 2′3.51 (1H, m)75.7 3′3.35 (1H, m)77.5 4′3.47 (1H, m)71.2 5′3.29 (1H, m)75.5 6′4.25 (2H, m)63.7 1″ 120.0 2″6.96 (1H, s)110.3 3″ 141.8 4″ 146.5 5″ 141.8 6″6.96 (1H, s)110.3 7″ 168.3 6-CH32.04 (3H, s)9.3
从HMBC图谱(图2)中可以看到,H2.50 (CH) 同C206. 3 (C-8), 111.6 (C-2) 存在HMBC相关信号,这表明乙酰基同C-2相连;H2.04 (6-CH3) 同C157.9 (C-1), 112.6 (C-6), 164.4 (C-5) 存在HMBC相关信号,这提示另一个甲基与苯环C-6相连。通过1H-1H COSY谱,可以鉴别出2组偶合系统,分别是H-4/6-CH3、H-1′/2′/3′/4′/5′/6′。糖的端基质子H-1′信号H4.52和C157.9 (C-1)碳信号存在相关,这表明在糖是连接在C-1位成苷的。糖的C-6′位质子信号H4.25与C168.3 (C-7″) 碳信号存在相关峰,这表明在没食子酰基应该连接在化合物1的C-6′位。化合物1的酸水解实验结果表明,1的单糖乙酰化衍生物的保留时间(R=13.052 min),与标准品-葡萄糖的乙酰化物的保留时间(R=13.056 min)基本一致,结合分析2D NMR谱的相关信号确定了1的糖基为β--葡萄糖。综上所述,化合物1为未见文献报道的新化合物,命名为母丁香酚A。

图2 化合物1主要的HMBC及1H-1H COSY相关
化合物2:白色固体。ESI-MS/: 531 [M-H]−,分子式为C31H48O7。1H-NMR (400 MHz, pyridine-5): 5.56 (1H, s, H-11), 4.40 (1H, d,= 13.2 Hz, H-3), 4.20 (1H, m, H-2), 3.64 (3H, s, OCH3), 3.10 (1H, m, H-18), 1.66 (3H, s, H-24), 1.56 (3H, s, H-27), 1.42 (3H, s, H-29), 1.11 (3H, d,= 6.8 Hz, H-30), 1.07 (3H, s, H-25), 1.02 (3H, s, H-26);13C-NMR (100 MHz, pyridine-5): 48.5 (CH2, C-1), 68.8 (CH, C-2), 81.2 (CH, C-3), 55.7 (C, C-4), 52.9 (CH, C-5), 21.9 (CH2, C-6), 33.6 (CH2, C-7), 40.9 (C, C-8), 48.5 (CH, C-9), 38.9 (C, C-10), 24.5 (CH2, C-11), 128.1 (CH, C-12), 140.4 (C, C-13), 42.5 (C, C-14), 29.7 (CH2, C-15), 26.8 (CH2, C-16), 48.7 (C, C-17), 55.0 (CH, C-18), 73.1 (C, C-19), 42.8 (CH, C-20), 27.4 (CH2, C-21), 38.9 (CH2, C-22), 178.6, (C=O, C-23), 13.6 (CH3, C-24), 17.5 (CH3, C-25), 17.3 (CH3, C-26), 25.1 (CH3, C-27), 181.2 (COOH, C-28), 27.5 (CH3, C-29), 17.7 (CH3, C-30)。以上数据与文献报道的人工合成的化合物基本一致[9],为皂苷的水解产物,故鉴定化合物2为新天然产物triterpenoster。
化合物3:白色固体。ESI-MS/: 487 [M-H]−,分子式为C30H48O5。1H-NMR (400 MHz, pyridine-5): 5.51 (1H, brs, H-12), 4.27-4.18 (3H, m, H-2, 23), 3.73-3.70 (1H, m, H-3), 1.12 (3H, s, H-27), 1.10 (3H, s, H-24), 1.08 (3H, s, H-25), 1.06 (3H, d,= 6.6 Hz, H-30), 0.99 (3H, s, H-26), 0.94 (3H, d,= 6.4 Hz, H-29);13C-NMR (100 MHz, pyridine-5): 48.0 (CH2, C-1), 68.5 (CH, C-2), 82.1 (CH, C-3), 41.8 (C, C-4), 50.3 (CH, C-5), 16.7 (CH2, C-6), 33.0 (CH2, C-7), 39.6 (C, C-8), 48.0 (CH, C-9), 37.8 (C, C-10), 23.7 (CH2, C-11), 125.5 (CH, C-12), 139.0 (C, C-13), 42.5 (C, C-14), 28.6 (CH2, C-15), 24.5 (CH2, C-16), 47.1 (C, C-17), 52.9 (CH, C-18), 39.1 (CH, C-19), 39.3 (CH, C-20), 31.0 (CH2, C-21), 37.8 (CH2, C-22), 65.0 (CH2, C-23), 12.5 (CH3, C-24), 16.2 (CH3, C-25), 17.0 (CH3, C-25), 24.2 (CH3, C-27), 180.0 (C, C-28), 17.3 (CH3, C-29), 21.2 (CH3, C-30).以上数据与文献报道基本一致[10],故鉴定化合物3为积雪草酸。
化合物4:白色固体。ESI-MS/: 455 [M-H]−,分子式为C30H48O3。1H-NMR (400 MHz, CDCl3): 5.27 (1H, s, H-12), 3.21 (IH, brd,= 7.2 Hz, H-3), 2.83 (1H, brd,= 1.0 Hz, H-12), 1.97 (2H, m, H-2), 0.91 (3H, s, H-23), 0.72 (3H, s, CH3-24), 0.76 (3H, s, CH3-25), 0.88 (3H, s, CH3-26), 1.14 (3H, s, CH3-27), 0.92 (3H, s, CH3-29), 0.96 (3H, s, CH3-30);13C-NMR (100 MHz, CDCl3): 38.8 (CH2, C-1), 27.8 (CH2, C-2), 79.1 (CH, C-3), 38.3 (C, C-4), 55.2 (CH, C-5), 18.3 (CH2, C-6), 32.5 (CH2, C-7), 39.1 (C, C-8), 47.6 (CH, C-9), 37.1 (C, C-10), 22.9 (CH2, C-11), 122.6 (CH, C-12), 143.6 (C, C-13), 41.6 (C, C-14), 27.9 (CH2, C-15), 23.4 (CH2, C-16), 46.5 (C, C-17), 40.9 (CH, C-18), 45.7 (CH2, C-19), 30.6 (C, C-20), 33.8 (CH2, C-21), 32.5 (CH2, C-22), 28.7 (CH3, C-23), 15.3 (CH3, C-24), 15.5 (CH3, C-25), 17.3 (CH3, C-26), 25.5 (CH3, C-27), 183.2 (C, C-28), 33.1 (CH3, C-29), 23.8 (CH3, C-30)。以上数据与文献报道基本一致[11],故鉴定化合物4为齐墩果酸。
化合物5:白色固体。ESI-MS/: 471 [M-H]−,分子式为C30H48O4。1H-NMR (400 MHz, pyridine-5): 5.45 (1H, brs, H-12), 4.09 (1H, ddd,= 4.5, 9.5, 11.0 Hz, H-2), 3.38 (1H, d,= 9.5 Hz, H-3), 1.26, 1.25, 1.06, 1.00, 0.98, 0.97, 0.92 (3H×7, s, CH3×7);13C-NMR (100 MHz, pyridine-d): 47.8 (C-1), 68.6 (C-2), 83.8 (C-3), 39.8 (C-4), 55.9 (C-5), 18.9 (C-6), 33.2 (C-7), 48.2 (2C, C-8, 9), 38.6 (C-10), 23.8 (C-11), 122.5 (C-12), 144.9 (C-13), 42.2 (C-14), 28.3 (C-15), 23.7 (C-16), 46.7 (C-17), 23.0 (C-18), 42.0 (C-19), 31.0 (C-20), 34.3 (C-21), 33.3 (C-22, 29), 29.4 (C-23), 16.9 (C-24), 17.5 (C-25), 17.7 (C-26), 26.2 (C-27), 180.2 (C-28), 24.0 (C-30)。以上数据与文献报道基本一致[12-13],故鉴定化合物5为山楂酸。
化合物6:白色固体。ESI-MS/: 455 [M-H]−,分子式为C30H48O3。1H-NMR (400 MHz, pyridine-5): 5.40 (1H, t,= 3.2 Hz, H-12), 3.39 (1H, dd,= 9.6, 5.2 Hz, H-3), 1.30 (3H, s, H-23), 1.22 (6H, s, H-26, 27), 0.98 (3H, s, H-24), 0.92 (3H, d,= 6.4 Hz, H-30), 0.80 (3H, d,= 6.0 Hz, H-29), 0.74 (3H, s, H-25);13C-NMR (100 MHz, pyridine-5): 39.3 (CH2, C-1), 28.4 (CH2, C-2), 78.4 (CH, C-3), 39.6 (C, C-4), 56.1 (CH, C-5), 19.0 (CH2, C-6), 33.8 (CH2, C-7), 40.2 (C, C-8), 48.3 (CH, C-9), 37.5 (C, C-10), 23.8 (CH2, C-11), 125.9 (CH, C-12), 139.5 (C, C-13), 42.7 (C, C-14), 28.9 (CH2, C-15), 25.2 (CH2, C-16), 48.3 (C, C-17), 53.8 (CH, C-18), 39.7 (CH, C-19), 39.7 (CH, C-20), 31.3 (CH2, C-21), 37.7 (CH2, C-22), 29.1 (CH3, C-23), 16.8 (CH3, C-24), 15.9 (CH3, C-25), 17.7 (CH3, C-26), 24.2 (CH3, C-27), 180.1 (C, C-28), 17.7 (CH3, C-29), 21.7 (CH3, C-30)。以上数据与文献报道基本一致[14],故鉴定化合物6为熊果酸。
化合物7:黄色固体。ESI-MS/: 197 [M+H]+,分子式为C10H12O4。1H-NMR (400 MHz, CDCl3): 14.1 (1H, s, OH), 6.06 (1H, d,= 2.0 Hz, H-3), 5.92 (1H, d,= 2.0 Hz, H-5), 3.86 (3H, s, 6′-OCH3), 3.82 (3H, s, 4′-OCH3), 2.62 (3H, s, 7-CH3C=O);13C-NMR (100 MHz, CDCl3): 106.0 (C, C-1), 162.9 (C, C-2), 93.4 (CH, C-3), 166.1 (C, C-4), 90.7 (CH, C-5), 167.6 (C, C-6), 203.2 (C, C-7), 32.3 (CH3, C-8), 55.6 (6′, 4′-OCH3)。以上数据与文献报道基本一致[15],故鉴定化合物7为花椒油素。
化合物8:黄色固体。ESI-MS/: 211 [M+H]+,分子式为C11H14O4。1H-NMR (400 MHz, CDCl3): 14.0 (1H, s, 1-OH), 5.93 (1H, s, H-4), 3.89 (3H, s, 3/5-OCH3), 3.88 (3H, s, 3/5-OCH3), 2.61 (3H, s, H-8), 2.00 (3H,s, 6-CH3);13C-NMR (100 MHz, CDCl3): 163.7 (C, C-1), 105.3 (C, C-2), 161.5 (C, C-3), 85.6 (CH, C-4), 163.5 (C, C-5), 105.7 (C, C-6), 203.4 (C, C-7), 33.2 (CH3, C-8), 7.15 (CH3, 6-CH3), 55.5 (3/ 5-OCH3), 55.4 (3/5-OCH3)。以上数据与文献报道基本一致[16],故鉴定化合物8为methylxanthoxylin。
化合物9:黄色油状物。ESI-MS/: 211 [M+H]+,分子式为C11H14O4。1H-NMR (400 MHz, CDCl3): 13.5 (1H, s, 1-OH), 6.24 (1H, s, H-6), 3.84 (3H, s, 3/5-OCH3), 3.73 (3H, s, 3/5-OCH3), 2.69 (3H, s, H-8), 2.06 (3H, s, 4-CH3)。以上数据与文献报道基本一致[17],故鉴定化合物9为母丁香酚。
化合物10:白色结晶。ESI-MS/: 171 [M+H]+,分子式为C7H6O5。1H-NMR (400 MHz, MeOD-4): 6.96 (2H, s, Ar-H)。以上数据与文献报道基本一致[18],故鉴定化合物10为没食子酸。
化合物11:白色固体(甲醇)。ESI-MS/: 185 [M+H]+,分子式为C8H8O5。1H-NMR (400 MHz, CD3OD): 7.06 (2H, s, Ar-H), 3.83 (3H, s, COOCH3)。以上数据与文献报道基本一致[19],故鉴定化合物11为没食子酸甲酯。
化合物12:淡黄色油状物。ESI-MS/: 165 [M+H]+,分子式为C10H12O2。1H-NMR (400 MHz, CDCl3): 6.82 (1H, m, H-6), 6.62 (2H, m, H-2, 5), 5.88 (1H, m, H-8), 5.02 (2H, m, H-9a, 9b), 3.72 (3H, s, 3-OCH3), 3.25 (2H, d= 6.4 Hz, H-7);13C-NMR (100 MHz, CDCl3): 132.0 (C, C-1), 111.5 (CH, C-2), 146.8 (C, C-3), 144.1 (C, C-4), 114.7 (CH, C-5), 121.3 (CH, C-6), 40.0 (CH2, C-7), 138.1 (CH, C-8), 115.6 (CH2, C-9), 55.9 (3-OCH3)。以上数据与文献报道基本一致[20],故鉴定化合物12为丁香酚。
化合物13:淡黄色油状物。ESI-MS/: 165 [M+H]+,分子式为C10H12O2。1H-NMR (400 MHz, CDCl3): 6.83 (1H, d,= 8.5 Hz, H-6), 6.68 (2H, m, H-2, 5), 5.95 (1H, m, H-8), 5.09 (2H, m, H-9), 3.86 (3H, s, 4-OCH3), 3.32 (2H, d,= 6.4 Hz, H-7);13C- NMR (100 MHz, CDCl3): 146.4 (C, C-4), 143.8 (C, C-3), 137.8 (CH, C-8), 131.9 (C, C-1), 121.1 (CH, C-6), 111.1 (CH, C-2), 40.0 (CH2, C-7), 114.2 (CH, C-5), 115.5 (CH2, C-9), 55.8 (4-OCH3)。以上数据与文献报道基本一致[21],故鉴定化合物13为萎叶醇。
化合物14:透明油状物。ESI-MS/: 219 [M-H]−,分子式为C15H24O。1H-NMR (400 MHz, CDCl3): 4.98 (1H, s, H-15a), 4.86 (1H, s, H-15b), 2.88 (1H, dd,= 4.4, 10.8 Hz, H-5), 2.62 (1H, m, H-9), 1.25 (3H, s, H-14), 1.01 (3H, s, H-12), 0.99 (3H, s, H-13);13C-NMR (100 MHz, CDCl3): 50.7 (CH, C-1), 27.2 (CH2, C-2), 39.1 (CH2, C-3), 59.6 (C, C-4), 63.8 (CH, C-5), 30.2 (CH2, C-6), 29.9 (CH2, C-7), 151.8 (C, C-8), 48.7 (CH, C-9), 39.7 (CH2, C-10), 34.0 (C, C-11), 21.6 (CH3, C-12), 31.3 (CH3, C-13), 17.0 (CH3, C-14), 112.8 (CH2, C-15)。以上数据与文献报道基本一致[22],故鉴定化合物14为β-氧化石竹烯。
化合物15:棕色油状物。ESI-MS/: 327 [M+H]+,分子式为C20H22O4。1H-NMR (400 MHz, CDCl3): 6.89 (1H, d,= 8.4 Hz, H-5′), 6.79 (1H, d,= 2.0 Hz, H-2′), 6.70 (1H, dd= 8.4, 2.0 Hz, H-6′), 6.49 (1H,= 1.8 Hz, H-2), 6.40 (1H, d,= 1.8 Hz, H-6), 6.00~5.93 (2H, m, H-8, 8′), 5.12~5.05 (2H, m, H-9a, 9′), 3.89 (3H, s, 5-OCH3), 3.86 (3H, s, 3′-OCH3), 3.36 (1H, d,= 6.6 Hz, H-7′), 3.24 (1H, d,= 6.6 Hz, H-7);13C-NMR (100 MHz, CDCl3): 150.3 (C, C-3′), 147.8 (C, C-5), 144.3 (C, C-3), 144.2 (C, C-4′), 137.4 (CH, C-8′), 137.3 (CH, C-8), 136.4 (C, C-1′), 135.1 (C, C-4), 131.0 (C, C-1), 120.8 (CH, C-6′), 119.4 (C, C-5′), 116.0 (CH2, C-9′), 115.7 (CH2, C-9), 112.8 (CH, C-2′), 111.9 (CH, C-6), 107.2 (CH, C-2), 56.2 (CH3, 5-OCH3), 56.0 (CH3, 3′-OCH3), 40.0 (CH2, C-7), 39.9 (CH2, C-7′)。以上数据与文献报道基本一致[23],故鉴定化合物15为去氢双丁香酚B。
利益冲突 所有作者均声明不存在利益冲突
[1] 韩群鑫, 黄寿山, 陈杰林. 丁香应用技术的研究进展 [J]. 江西植保, 2006, 29(1): 24-26.
[2] Dorman H J, Deans S G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils [J]., 2000, 88(2): 308-316.
[3] Ryu B, Kim H M, Woo J H,. A new acetophenone glycoside from the flower buds of(cloves) [J]., 2016, 115: 46-51.
[4] Cai L, Wu C D. Compounds frompossessing growth inhibitory activity against oral pathogens [J]., 1996, 59(10): 987-990.
[5] Pinto E, Vale-Silva L, Cavaleiro C,. Antifungal activity of the clove essential oil fromon,and dermatophyte species [J]., 2009, 58(Pt 11): 1454-1462.
[6] Rattanachaikunsopon P, Phumkhachorn P. Protective effect of clove oil-supplemented fish diets on experimentalinfection in tilapia [J]., 2009, 73(9): 2085-2089.
[7] 鲁杰, 马国祥, 江健, 等. 龙脑樟挥发油对大鼠离体颈总动脉环的作用[J]. 世界中医药, 2020, 15(13): 1934-1938.
[8] Shen S, Chen C J, Bu R,. Three new steroidal saponins from[J]., 2011, 13(11): 1014-1022.
[9] Gao F, Chen F H, Tanaka T,. 19.ALPHA.- hydroxyursane-type triterpene glucosyl esters from the roots ofS. Lee [J]., 1985, 33(1): 37-40.
[10] 张蕾磊, 王海生, 姚庆强, 等. 积雪草化学成分研究 [J]. 中草药, 2005, 36(12): 1761-1763.
[11] 刘书霞, 李振麟, 兰太进, 等. 石崖茶的化学成分研究 [J]. 中草药, 2016, 47(14): 2436-2440.
[12] 黄丽刚, 魏冬梅, 李若恒, 等. 隐脉假卫矛中三萜类化学成分研究 [J]. 广东化工, 2016, 43(11): 18-19.
[13] 冯萌萌, 张艳侠, 夏兵, 等. 滇虎榛叶的化学成分及其抗氧化活性研究 [J]. 中草药, 2013, 44(19): 2650-2656.
[14] 桂明玉, 金永日, 王宝珍. 蓝萼香茶菜化学成分研究 [J]. 中国药学杂志, 1999, 34(8): 516-518.
[15] Lee W, Lee D, Lee Y R,. Isolation, synthesis, and antisepsis effects of a C-methylcoumarinochromone isolated fromcell culture [J]., 2018, 81(5): 1173-1182.
[16] Morgenstern T, Bittner M, Silva M,. Diterpenes and phloracetophenones from[J]., 1996, 41(4): 1149-1153.
[17] Nakagawa-Goto K, Lee K H. Anti-AIDS agents 68. The first total synthesis of a unique potent anti-HIVfrom genus[J]., 2006, 47(47): 8263-8266.
[18] 杨素珍, 蒋建勤. 新疆芍药的化学成分研究 [J]. 中草药, 2014, 45(6): 760-764.
[19] 朱春玲. 蜜柑草正丁醇部位化学成分研究 [J]. 中药材, 2014, 37(4): 608-610.
[20] Yoshimura M, Amakura Y, Yoshida T. Polyphenolic compounds in clove and pimento and their antioxidative activities [J]., 2011, 75(11): 2207-2212.
[21] Momin R A, Ramsewak R S, Nair M G. Bioactive compounds and 1, 3-di [()-9-octadecenoyl]-2-[(cis,)-9, 12-octadecadienoyl]glycerol fromL. seeds [J]., 2000, 48(9): 3785-3788.
[22] Barrero A F, Molina J, Oltra J E,. Stereochemistry of 14-hydroxy-β-caryophyllene and related compounds [J]., 1995, 51(13): 3813-3822.
[23] Costa-Silva T A, Grecco S S, Sousa F SImmunomodulatory and Antileishmanial Activity of Phenylpropanoid Dimers Isolated from[J]., 2015, 78(4): 653-657.
Chemical constituents from fruits of
TAN Pei-yi1, WANG Chen-jie2, 3, YU Huan4, LIU Fang1, TANG Sheng-an4
1. National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Department of Pharmacy, the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300073, China 2. Tianjin Medical College, Tianjin 300222, China 3. Tianjin TIANYAO Pharmaceutical Company Limited, Tianjin 300462, China 4.Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy, Tianjin Medical University, Tianjin 300070, China
To study on the chemical constituents from the fruits of.The chemical constituents were separated and purified by Sephadex LH-20, silica gel, semi-prepared HPLC and other chromatography techniques. Their structures were elucidated by MS and NMR data.Fifteen compound were isolated, and were identified as 1,3,5-trihydroxy-3-methylacetophenone-1--β--(6'--galloxyl)glucoside (1), triterpenoster (2), asiatic acid (3), oleanolic acid (4), maslinic acid (5), ursolic acid (6), xanthoxylin (7), methylxanthoxylin (8), bancroftione (9), gallic acid (10), methyl gallate (11), eugenol (12), chavibetol (13), β-caryophyllene oxide (14), dehydrodieugend B (15).Compound 1 is a new compound named syzygiumol A. Compounds 2 is isolated as a new natural compound. Compounds 7—8 and 11 are isolated from this plant for the first time. Compounds 1, 7—9 are acetophenone; Compounds 2—3 are ursane-type triterpenoids;Compounds 4—5 are oleanane-type triterpenoids; Compounds 12—13, 15 are phenylpropanoids; Compounds 14 is sesquiterpenoid.
(L.) Merr. & L. M. Perry; syzygiumol A; triterpenoster; xanthoxylin; methyl gallate; dehydrodieugend B
R284.1
A
0253 - 2670(2022)11 - 3280 - 06
10.7501/j.issn.0253-2670.2022.11.002
2022-03-18
天津市教委科研计划项目(2019ZD031)
谭培艺(1984—),女,主管药师,硕士,研究方向为临床药学。E-mail: amelie_tan@qq.com
唐生安(1977—),男,副教授,研究方向为天然药物化学。Tel: (022)83336658 E-mail: tangshengan@tmu.edu.cn
[责任编辑 王文倩]

