Polymeric microneedle-mediated sustained release systems:Design strategies and promisingapplications for drug delivery
,Xiowei Zeng,
aInstitute of Pharmaceutics,School of Pharmaceutical Sciences(Shenzhen),Sun Yat-sen University,Shenzhen 518107,China
b Tianjin Key Laboratory of Biomedical Materials,Key Laboratory of Biomaterials and Nanotechnology for Cancer Immunotherapy,Institute of Biomedical Engineering,Chinese Academy of Medical Sciences&Peking Union Medical College,Tianjin 300192,China
Keywords:Transdermal drug delivery Microneedles Sustained release Long-term exposure therapy
ABSTRACT Parenteral sustained release drug formulations,acting as preferable platforms for longterm exposure therapy,have been wildly used in clinical practice.However,most of these delivery systems must be given by hypodermic injection.Therefore,issues including needle-phobic,needle-stick injuries and inappropriate reuse of needles would hamper the further applications of these delivery platforms.Microneedles (MNs) as a potential alternative system for hypodermic needles can benefit from minimally invasive and self-administration.Recently,polymeric microneedle-mediated sustained release systems(MN@SRS) have opened up a new way for treatment of many diseases.Here,we reviewed the recent researches in MN@SRS for transdermal delivery,and summed up its typical design strategies and applications in various diseases therapy,particularly focusing on the applications in contraception,infection,cancer,diabetes,and subcutaneous disease.An overview of the present clinical translation difficulties and future outlook of MN@SRS was also provided.
1.Introduction
Transdermal drug delivery (TDD),an important substitute for oral administration and hypodermic injections,has attracted great attention.It shows several advantages such as convenient self-administration,reduced systemic side effects,and avoidable hepatic first-pass elimination [1,2].However,the outermost layer of skin,stratum corneum (SC),known as the main obstacle,limits the transport of drug molecule and weakens its therapeutic efficacy [3].Notably,marketed TDD formulations can only effectively deliver drugs with lowmolecular-mass (≤400 Da) due to the SC barrier [4].In the past few decades,various ways have been employed in TDD to increase the permeability of SC,such as electroporation,iontophoresis,and ultrasound [5-7].However,even with stimulation of external conditions,there are still challenges for larger molecules delivery through the skin,including proteins and genes[8].
Microneedles (MNs),an emerging versatile TDD system to overcome the skin barrier that limits drug penetration efficacy,knock the door of TDD to some extent.They are used to painlessly pierce the SC to remarkably facilitate TDD by generating microchannels for molecules which cannot be delivered across the skin by passive diffusion alone[9].Since the first introduced of MNs in 1966,MNs have been widely explored to improve the effect of TDD [10].Until now,MNs can be divided into several categories:solid and hollow MNs,which are used as tools to pierce skin barrier to improve the drug delivery into dermal;coated MNs,which can implant coated drug layers into the skin;polymeric MNs,the cargos can be packaged and transported by host MNs [11,12].Among them,polymeric MNs are coming to the fore as excellent biocompatibility,outstanding biodegradability,and sufficient drug loading capacity [13].Nevertheless,initial studies of polymeric MNs mostly focused on rapid drugs delivery [14,15].There are some drawbacks for rapid release polymeric MNs delivery system:(1) Frequent use of MNs increases chemical burden and irritation to patients;(2) Poor efficacy for therapeutics that require long-term exposure;(3) The release profiles are uncontrollable.
With the rapid development of formulation technology,parenteral sustained release formulations are wildly used in clinical practice,such as microparticles (MPs),implants and drug depot [11].There are some advantages of these drug delivery system.They can reduce frequency of administration,present good patient compliance and show fewer side effects.Especially,taking advantages of sustained release of therapeutics is benefit for diseases that require longterm or repeated administration,such as contraceptives,vaccines and other chronic diseases.Due to its stable drug concentrations in blood and avoiding multiple doses can improve therapeutic effects [16-18] and patient compliance respectively.However,key issues still need to be solved.Parenteral sustained release formulations usually inject subcutaneously or intramuscularly.These injections lead to problems including pain,generating sharp medical waste,and needle abuse [19].More importantly,they need professionals to administration,limiting the drug accessibility to patients,especially in developing countries,where suffer severe lack of professional medical staff.In addition,organic solvents for injection can lead to toxicity,such as Eligard,an in situ-forming drug depot which was approved by the FDA[20].In particular,the uncontrollable shape and size of the depots formedin vivowould further affect drug release [21].An alternative delivery system to avoid these shortcomings is reshaping the landscape of parenteral sustained release formulations.
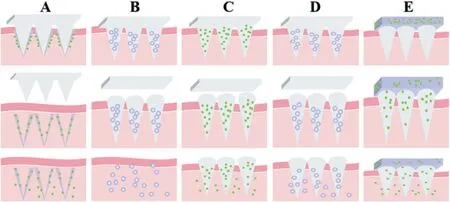
Fig.1-Design strategies of polymeric microneedle-mediated sustained release system(A)Long-acting coated MNs,(B)Long-acting encapsulated MNs,(C)Polymeric-based sustained release MNs,(D)Double-sustained release MNs,(E)Back layer-based depot MNs.
Polymeric microneedle-mediated sustained release systems (MN@SRS) integrate the advantages of polymeric MNs and sustained release technique,can address these mentioned issues.MN@SRS are minimally invasive,significantly avoid the needle-stick injuries and pain caused by subcutaneously injections.MN@SRS are designed as self-administration,that is,no requirement for skilled medical personnel.More importantly,MN@SRS with different release properties can be fabricated by polymers,which have different degradation manners [9].The purpose of this review is to highlight the superiorities of MN@SRS in terms of long-term and repeated administration diseases.Specifically,several typical strategies for constructing MN@SRS have been introduced.Furthermore,we summed up the applications of MN@SRS on various diseases therapy,particularly focusing on the applications in contraception,infection,cancer,diabetes,and subcutaneous disease.The clinical translation difficulties at present and development prospect in the future of MN@SRS were also discussed.
2.Design strategy and fabrication of MN@SRS
Four typical categories of MN@SRS have been developed(Fig.1).One is called long-acting encapsulated or coated MNs.These MNs are only employed as tools to pierce into skin and subsequently implant the pre-loaded cargos.Their sustained release property is acquired by packaged or coated drugs.Another is polymeric-based sustained release MNs,which was fabricated with long-acting polymer that acquire sustained release manner through host MN polymer.Double-sustained release MNs is the third strategy of MN@SRS.These MNs load long-acting packaged drugs in sustained release MNs,in order to obtain a longer sustained release period.Backing layer-based depot MNs is the fourth strategy to realize sustained release of MNs.In this design,drug was loaded in the backing layer as reservoir instead of needles.The needles act as tools to create channels for drug penetration as well as to provide a boost drug loading.
2.1.Long-acting encapsulated or coated MNs
MNs based on dissolving polymers such as dextran,sodium chondroitin sulfate,hyaluronic acid (HA),polyvinylpyrrolidone (PVP) are usually combined with nanoparticles (NPs) or MPs to achieve sustained release manner [22-24].These MNs dissolve within several seconds/minutes after being inserted into the skin [25,26],following with rapid release of loaded cargos (NPs or MPs),which functioned as drug depots for long-lasting release.For example,Tekko et al.constructed a composite MN patch based on water-soluble polymer to deliver methotrexate(MTX),which played the role of drug reservoirs in the form of nanocrystals(NC)in MNs.In vitrodrug release profile showed it could continuously release over 72 h [27].In another study,McCrudden et al.prepared the dissolving polymeric MNs containing a long-acting NP loaded with rilpivirine (RPV),to provide a more acceptable choice for treatment of HIV.Based on the results ofin vivostudies,approximately 28 cm2of these MNs could maintain effective RPV plasma levels over 7 d in humans by conservative estimate [28].Another form is long-acting coated MNs.In this design,drug reservoir coated on the MNs surface.Once the MNs was inserted into the skin,they could be taken out completely from skin after successfully implant coating layer into skin as reservoir.For example,DeMuth’s group constructed the polyelectrolyte multilayers(PEMs)coated poly(l-lactide)(PLLA)MNs to deliver DNA.By changing the composition of the coating film,sustained release period of cargos last a few days to weeks can be achieved[29].
2.2.Polymeric-based sustained release MNs
Biodegradable polymers hardly dissolve or swell in the skin interstitial fluid (SIF),but can slowly degrade over months.Biodegradable polymers that are appropriate for developing MNs can be divided into two categories:synthetic biodegradable polymers and nature biodegradable polymers.The synthetic ones include poly (lacticco-glycolic) acid(PLGA) and poly (lactic acid) (PLA) [30,31].The natural biodegradable polymers include chitosan,silk firbroin,and chitin [32-34].Drug release rate and duration of this type of polymers are primarily dominated by degradation rate of the biodegradable polymers rather than drug diffusion [35].Based on the attractive chemical and biological properties,biodegradable polymers are employed to prepare sustained release MNs for multiple drug delivery.Li et al.reported a rapidly separable biodegradable polymeric MN patch with PLGA/PLA needles used for the sustained release of a contraceptive hormone,levonorgestrel (LNG).In rats,the MN patch kept the plasma concentrations of LNG over the human therapeutic level for one month [36].Chen et al.prepared MNs composed of vaccine-loaded chitosan needle tips and a water-soluble supporting part that gave enough length to insert into the skin.Vaccine-loaded chitosan needle tips can be quickly and entirely implanted into the skin to serve as a reservoir for durable release of vaccines over 16 weeks[37].
2.3.Double-sustained release MNs
In order to obtain a longer sustained release period than long-acting encapsulated MNs,the third strategy of MN@SRS has been designed.These MNs superimposes the sustained release ability of the host MNs and the long-acting packaged cargos.For example,the sustained release biodegradable polymers-based MNs loaded with long-acting NPs or MPs own potential application value on therapeutics that require longterm exposure for several months or years.Recently,Zhu et al.fabricated a composite LNG-loaded silk MN patch.100 d of sustained drug release was achieved when the drug was freely loaded into the MNs,while over one year of sustained drug release was achieved when the drug was pre-encapsulated in silk MPs prior to be encapsulated into the MN patches.These long-acting MNs open up a new world for women contraception[38].
2.4.Backing layer-based depot MNs
The limited drug loading of the MNs may not be able to meet the drug amount required for long-term release.Fortunately,encapsulating cargos into backing layer can greatly enhance drug loading capacity in MNs [1].In this approach,MNs are usually prepared by swellable or dissolvable polymers.Upon applied into the skin,they are swelled or dissolved after absorb SIF and created channels between drug-loaded backing layer and dermal microenvironment.These MNs act as host to control drug release from baseplate,occasionally,as well as increased drug dose and rapid drug delivery parts [39,40].Lee et al.firstly demonstrated the feasibility of MNs backing layer as drug reservoirs to achieve durable release based on water-soluble polymers.The MNs,function as delivering initial drug and boosting dose,completely dissolved within 1 h after administration,leaving channels in the skin.Drug-loading backing layer swelled by absorbing SIF,and then cargos diffused from the backing layer depot via micropores produced by the dissolved MNs,exhibiting continuous release of drugs for several hours to days[41].
2.5.Fabrication of MN@SRS
There are many methods for preparing polymer-MNs,such as micromolding [42],droplet-born air blowing(DAB) [43],drawing lithography [44],electro-drawing [45],photolithography[46],continuous liquid interface production(CLIP) [47] (Table 1).Among them,micromolding method is the most wildly used in fabrication of MN@SRS due to its easy production,cost-efficiency,and potential for industrialization.

Table 1-Different methods for fabrication of polymer-MN along with advantages and disadvantages of each method.
Micromolding method consists of six main steps:(1)fabricating MNs male mold;(2) preparing a female MNs mold;(3) casting polymers solution onto female molds and forcing polymers solution into caves by centrifugation or directly pumping polymers solution into caves of female molds;(4) removing bubbles by centrifugation or vacuum;(5) Curing MNs by drying or photocrosslinking;(6) peeling off the MNs from female molds.The most commonly used material for preparing the MN female mold in the step 2 is polydimethylsiloxane (PMDS) based on its excellent stability,good copying ability and low adhesion (beneficial to peel off the MNs from female mold).The drug can be loaded by mixing drug or drug-loaded MPs and NPs with polymer solution,and then forcing mixture into the mold.
3.The biomedical application of MN@SRS
3.1.The application of MN@SRS for contraception
Voluntary family planning has made a great progress in public health and contraceptive methods allow women to make decisions about the timing and spacing of pregnancies[48,49].Although great progresses in contraceptive methods,85 million pregnancies were unintended in 2012,accounting for 40% of all pregnancies worldwide [50].Many unintended pregnancies are terminated,bringing about 56 million abortions every year [51].The high incidence of accidental pregnancy leads to serious economic and emotional burden to women and society.Increasing contraceptive options,which better meet the needs of women at diverse stages of their reproductive life cycle,is the key to women’s safety and wellbeing.
Long-acting contraceptives can provide women with flexible contraceptive protection.To date,there are many long-acting contraceptives on the market,including injectable long-lasting hormonal contraceptives and implantable longacting contraceptives.Injectable long-lasting hormonal contraceptives require trained professionals who are limited in developing counties,and invasive administration,therefore limiting patient access and reducing patient compliance[52,53].Implantable long-acting hormonal contraceptives can last for years,such as subcutaneous implants and intrauterine devices (IUDs).However,they need administration by even greater trained health care professionals and are even more invasive compared to other methods.Therefore,options for self-administration long-acting contraceptives are limited[54,55].
To response these issues,MN@SRS have been employed to supply a novel option for women contraception.MN@SRS can be self-administrated and maintain long-acting to reduce frequent administration,which greatly increase patient acceptance and compliance.Moreover,MN@SRS are minimally invasive and biodegradable,avoiding needle-stick injury and generating no biohazardous sharps waste.These properties are particularly important in developing countries.
He et al.prepared a long-acting MN patch based PLGA for sustained release of etonogestrel (ENG),the third-generation progesterone.A novel preparation method called controllable casting mold technique was introduced to fabricate PLGA MNs and ENG crystals were evenly distributed in the needle tip.By optimization of MN formulations,this MN@SRS can successfully pierce the skin and deliver the entrapped drug,with a drug loading up to 153.0±13.5μg,achieving sustained release of cargos more than two weeks[18].In another design,Zhu et al.fabricated a composite LNG-loaded MN@SRS.In this approach,changing the properties of silk protein or formulation of loaded LNG enabled to modify the extendedrelease time of MNs.Specifically,sustained release of LNG maintained 100 d when the drug was directly encapsulated into silk MNs,while release reach up more than a year when the drug was pre-encapsulated in silk MPs prior to load into the MN patches[38].

Fig.2-(A)Schematic of rapidly separable microneedle patches,(B)The black arrows identify bubble structures at the interface of the microneedles and patch backing,(C)Cumulative release profile of encapsulated LNG[36].Copyright 2019,Springer Nature.
Despite the MN@SRS described above studies provided new options for long-acting contraception,there are still shortcomings.Obviously,these MNs need to be worn throughout the whole drug release period for several days,months or even years.Prolonged contact of MNs patch with skin may cause irritation,or MNs drop from skin by accident could lead to contraception failure [56,57].To address these problems,Li and coworkers described a rapidly separable MN platform for long-term delivery of LNG (Fig.2).These MNs were made of biodegradable polymers,PLGA,which controlled the release of LNG,and polymer PLA,which increased the mechanical strength of MNs.An air bubble structure was designed between the MN and patch backing,which allowed MNs to efficiently penetrate skin and snap off in five seconds,and successfully relized the rapid separation of MN from backing.Results showed that the MNs implanted into the dermis function as depots for the long-term and systemic delivery of LNG for more than one month [36].Li and coworkers also developed another rapid separation MN@SRS for long-acting contraception.In this study,rapid separation feature was achieved by an effervescent backing that designed between the MN and adhesive paper.Sodium bicarbonate and citric acid were introduced to the effervescent backing,which would generate carbon dioxide bubbles upon contacting with the SIF after MNs were inserted in the skin.The connection between MNs and backing layer was broken by carbon dioxide bubbles and separation occurred within 1 min.In vivorelease result showed that cargos released into medium,which simulated the environment in the body,over 30 d[58].
3.2.The application of MN@SRS for cancer therapy
According to the report published by the Global Cancer Statistics,about 313 million people suffer from superficial cancer.Among them,0.3 million and over 209 million patients are suffering from melanoma and breast cancer in 2018,respectively [59].Obviously,superficial tumors have seriously endangered human health,causing psychological burden on patients.Unfortunately,topical administration of drug faces limited drug delivery efficiency while systemic treatments are always accompanied by side effects,and monotherapies remains suboptimal clinical efficacy [60-66].Notably,superficial cancers are aggressive and recurrent,repeated treatments are necessary,as a result,chemical burden and the risk of toxicity were increased[67,68].
In terms of superficial tumor therapy,MN@SRS provide unique advantages.MN@SRS directly insert into lesion site of superficial tumor,significantly increase the drug accumulation,and reduce systemic side effects.Besides,sustained release property of MN@SRS avoids repeated treatments,improves convenience of patients and healthcare providers.Moreover,different function cargos loaded into MN@SRS provide synergistic or combined effects.
3.2.1.The application of MN@SRS for chemotherapy
Chemotherapy is one of the most important arsenals to kill tumors [69].Various versatile carriers greatly improve the anti-tumor effect of chemotherapy drugs [70,71].For chemotherapy,MN@SRS have been employed for delivery of doxorubicin (Dox),dacarbazine,and other drugs for chemotherapy [72-74].Typically,Kim et al.reported a design of a biodegradable MN patch based on porous silicon (p-Si) with a novel water-soluble backing (Fig.3).Dox was covalently linked withp-Si MNs.The water-soluble backing was temporarily used to help insert the MNs into the skin and subsequently dissolved within 1 min,making MNs avoid discomfort and irritation to the patients caused by traditional flexible backing like PDMS backing.Consequently,the p-Si MNs remained implanted inside tissues,gradually hydrolyzed into biocompatible byproducts,continuously released Dox for several days with a controlled rate.The study results showed the MN@SRS exhibit superior anti-tumor efficacy without local skin lesions,which attributed to the minimally invasive and long-last effective drug concentration at the local site based on the sustained release property of MN@SRS[75].Luo et al.developed a MN@SRS prepared by gelatin methacryloyl (GelMA),an innovative biodegradable material for sustained delivery of Dox.Delivery rates of therapeutics and mechanical properties can be adjusted based on the crosslinking degree of GelMA.The results showed novel GelMA MNs successfully penetrated the skin,and a reverse correlation between sustained release period and crosslinking time could be observed[74].

Fig.3-(A)Schematic illustrations for the construction of MNs on a water-soluble backing,(B)Cumulative release of the covalently linked(amide and urea bonds)Dox,compared to the control phyically trapped Dox,(C)Measurement results of the tumor size for 12 d postinoculation[75].Copyright 2020,American Chemical Society.
3.2.2.The application of MN@SRS for immunotherapy
Cancer immunotherapy is considered as a potentially powerful approach for cancer management [76-78].Recently,two treatment pathways exhibit enormous potential in advanced cancer patients.One is adoptive cell therapy(ACT),which is based on directed transfer ofTcells into the body to stimulate the immune response against cancer [79,80].The other is checkpoint blockade,this method treatment with antibodies such as cytotoxic T lymphocyte antigen-4(CTLA-4)or programmed death-1 (PD-1,or its counter-receptors PDL1) that block the inhibitory effect [81,82].However,cancer immunotherapy not only obtains the remarkable effect but also brings non-negligible side effects.For instance,when patients treated with anti-CTLA-4,it might lead to serious adverse events reach up to grade 3 or 4 [83,84].Therefore,a looming challenge for cancer immunotherapy is avoiding systemic toxicities that prevent immunotherapies from successful clinical transformation.On the other hand,despite anti-PD-1 agents(αPD11)or anti-PD-L1 agents(αPDL1)have acquired impressive clinical responses in patients suffered from melanoma,only 30%-40% of patient response to antibodies blocking PD1/PDL1 because of the presence of intrinsic or acquired resistance of immunotherapies [85-87].Combination therapy of antibodies blocking PD1/PDL1 with other immune activation agent could achieve synergistic anti-tumor effect [88-90].As a result,a series of clinical trials of combination therapy are underway[91].Unfortunately,the protocol of most current clinical trials consists of repeated intratumoral injections,that may lead to the destruction of the tumor microenvironment and blood vessels,increasing the risk of tumor metastasis and penetration[92-95].
To response challenges mentioned above,immunotherapy mediated by MN@SRS shows broad prospects,including(1) directly local administration of MN@SRS into lesion sites,the drug is preferentially reserved at the injection site,which allow lower drug concentration than systemic administration;(2)MN@SRS can locally control the release of drug,confine therapeutics to target lesion sites,reaching full curative potential of drug by avoiding debilitating toxicities and multiple intratumoral injections;(3) Multiple drugs can be loaded into MN@SRS to obtain synergistic effect and enhance anti-cancer efficacy.(4) MN@SRS could overcome the SC barrier and insert into dermis,where are plenty of dermal dendritic cells (DCs) that play important role in immunomodulation.Therefore,MN@SRS are approaches to enhance local immunotherapy for superficial tumors.

Fig.4-(A)Schematic illustration of MN-based transdermal vaccination,(B)In vitro collective release of tumor lysate proteins from the MN patch,(C)Average tumor volumes in treated mice after tumor challenge[100].Copyright 2017,The American Association for the Advancement of Science.
Wang et al.loadedαPD1 into a MN patch for local and controlled delivery ofαPD1.αPD1 and glucose oxidase(GOx) were loaded into the pH-sensitive dextran NPs.GOx transformed the blood glucose to gluconic acid during MNs were inserted into skin.The acid environment promoted the dissolution of NPs and following release of packagedαPD1.Compared with control groups (MNs without GOx or intratumoral injection of freeαPD1),this MN patch produced strong immune responses[85].
However,tumor cells can catalyze a series of immunosuppressive molecules.For example,indoleamine 2,3-dioxygenase (IDO),which can restrictTcells functions[96,97].Thus,monotherapy with immunologic drugs obtains limited immunotherapy clinical benefit [98].To overcome this problem,Ye and coworkers employed MNs for sustained delivery ofαPD1 combined with an inhibitor of IDO,1-methyl-DL-tryptophan (1-MT).Both cargos achieved sustained release for over 48 h.The synergistic and sustained delivery of 1-MT andαPD1 by MNs exhibited potent anti-cancer potential and prolonged anti-tumor efficacy [99].Ye et al.developed a MN@SRS system encapsulated inactive B16F10 melanoma lysates and melanin (Fig.4).In this study,melanoma lysates were gradually release from MNs for over five days,subsequently facilitated immune response.At the same time,melanin generated immunogenic substrates through local release of heat,which was transferred via nearinfrared (NIR),further enhanced tumor immune activation.In vivoresults indicated that the MNs successfully hampered tumor engraftment in prophylactic models and achieved prolonged tumor suppressor efficacy in tumor-bearing mice[100].Zhou et al.designed a transfersome/MNs complex system to co-deliver pembrolizumab(αPD1),tumor associated antigen (TAA) and adjuvant poly I:C.In this study,HA-GMSαCD40 was introduced to modify the surface of transfersome to endue target capacity to DCs.The results showed the MN@SRS with controlled release of all cargos exhibited great potential in tumor immunotherapy[87].
3.2.3.The application of MN@SRS for gene therapy
Gene therapy has been wildly applied to treatment of disease,including cancer therapy [101-103].With the successful of LUXTURNA®,a FDA approved gene therapy for biallelic RPE65 mutation-associated retinal dystrophy [104],many strategies have been developed to explore effective gene delivery.Unfortunately,there are still some challenges.Appropriate delivery carriers and suitable delivery routes play important roles in gene delivery [105].Based on previous studies,MN@SRS provide a new platform of gene delivery [106,107].Typically,Qu et al.presented a MN patch based on GelMA for local and sustained delivery of plasmid DNA (pDNA)(Fig.5).Intracellular delivery of the pDNA was achieved by encapsulating it into poly (β-amino ester) (PBAE) NPs.The MN@SRS showed robust transfection effect of the nucleic acid cargo bothin vitroandin vivo[108].DeMuth’s group constructed a releasable PEM coated polymeric MNs patch.On this base,they loaded DNA and adjuvants into the MNs to achieve continuous release of cargos from day to weeks,strengthening immune response[29].
3.3.The application of MN@SRS for diabetes therapy
Diabetes is a chronic disease accompanied by disorders of blood glucose metabolism[109-111].According to the reports published from the International Diabetes Federation (IDF),approximately 463 million people suffered from diabetes in 2019,and it is estimated the number would reach up to 700 million by 2045.Obviously,the situation is worrisome[3].Diabetes can be divided into three categories:type 1 diabetes(T1D),type 2 diabetes(T2D)and gestational diabetes(GD).T1D is caused by less secretion of insulin while T2D is usually caused by insulin resistance [112].For T1D and advanced T2D,insulin is the most used drug to effectively control blood glucose levels (BGLs) [113-115].To date,there are several insulin formulations available on the market[116,117].Usually,these formulations are administrated by subcutaneous injection mediated by hypodermic needle or pump [115].Frequent injections lead to poorly patient compliance [118,119].In recent years,new choices have been emerged in treatment of T2D,including glucagon-like peptide-1(GLP-1)agonists/analogues and dipeptidyl peptidase-4(DPP-4) inhibitors [120].The most representative drug is longacting exenatide,Bydureon,which has been approved by FDA and European Medicines Agency (EMA).Bydureon is microsphere based on PLGA for once-weekly subcutaneous injection [121,122].Long-acting exenatide greatly reduces frequency of injection,but injection pain,needle phobia and adverse reactions at the injection site are still inevitable.
In response to these challenges,polymeric MNs as a dramatic delivery system have been wildly explored for insulin delivery [123-125].Based on the previous studies,insulin maintained biological activity during the preparation and storage period of polymeric MNs,which means insulin encapsulated into polymeric MNs do not require cold chain to transport and storage,and can be used conveniently by patients for long-term [126-128].Moreover,the BGLs in animal models were effective controlled by insulin MNs have been demonstrated [129-132].Nonetheless,these traditional polymeric MNs release insulin with uncontrolled manner,which is not conductive to manage patient’s BGLs [133,134].The limited drug loading efficacy of traditional polymeric MNs would fail to meet the requirement of long-term exposure drug dose[134].
To solve these problems,MN@SRS were designed for sustained delivery of insulin with a controlled manner.In particular,the integrated system based on MN@SRS can deliver cargos for initial quick release and subsequent sustained release,with flexible application for diabetes patients.Moreover,backing layer-based MN@SRS significantly increase drug loading capacity of MNs,and successfully avoid limited loading efficacy of drugs which need longterm exposure.Donnelly et al.prepared hydrogel MNs to enhance transdermal delivery efficiency and drug loading of macromolecules like insulin.Interestingly,the MNs themselves contained with no drugs,but instead,attached patch acted as drug reservoirs.After application,the MNs rapidly absorbed skin SIF to produce continuous and un-blocked hydrogel conduits.These hydrogel conduits connected attached patch drug reservoirs with dermal microcirculation,resulting in controlled drug release from reservoirs.This MN@SRS platform significantly improved drug loading efficacy of insulin to 5 mg/cm2with a release period over 24 h [135].Similarly,Zhu and coworkers utilized backing layer as depot for sustained release of insulin.The MN@SRS showed approximative relative pharmacological availability and more lasting and stable hypoglycemic effect compare to injection [40].Che et al.constructed smart MNs combined boronate-containing hydrogel with silk fibroin.Acute,long-lasting,and glucoseresponsive insulin delivery properties were integrated into the smart MNs(Fig.6).Silk fibroin was introduced to enhance mechanical strength while the boronate-containing hydrogel possessed glucose-responsive ability,providing insulin with diffusion-controlled release for days.Thus,the smart MNs provided a potential choice to solve a series of unsatisfied medical needs in the treatment of diabetes patients[136].

Fig.6-(A)Glucose-dependent equilibria of PBA derivatives,(B)Schematic representation of formation of semi-IPN hydrogel,(C)“Skin-layer”controlled glucose-responsive insulin release from the MN-array patch[136].Copyright 2018,John Wiley and Sons.
3.4.The application of MN@SRS for infectious disease therapy
In the past decades,millions of people from all over the world are threatened by infectious diseases,causing an enormous burden on public health and global economies[137-139].In fact,the emerging coronavirus disease (COVID-19)clearly revealed the huge challenges of pandemic to public health [140-142].To date,vaccination has been the main method of effective prevention and treatment of infectious diseases.According to the data estimated by the world health organization,vaccination protected 2.5 million children from infectious deaths each year[143].Skin is an attractive immune organ for delivering vaccine because of its easily accessible character and rich network of DCs such as langerhans cells (LCs) and macrophages [144-146].These specialized DCs possess strong antigen presentation capability.They transfer to the skin draining lymph nodes (DLNs) and then present processed antigens of T and B lymphocytes to induce pathogen-specific protective immunity [147-150].Unfortunately,most of vaccines are delivered by hypodermic needle injection because of skin barrier layer.Palpably,hypodermic needle injection brings a serious of problems including injection pain,needle stick injuries and time consuming [151].Importantly,traditional immunization causes rapid antigen clearance,and one-time injection can only activate the immune system but fails to elicit protective immunity [152,153].Based on the previous studies,repeated injections or sustained delivery of antigens via implanted miniosmotic pumps enhanced the humoral responses compared to traditional immunization,own to increased antigen availability [154-157].However,immunizations through osmotic pump implantation or multiple injections are impractical for prophylactic vaccination.Furthermore,cold chain transportation and storage increase the cost of vaccines.
Owing to these challenges,MN@SRS are wildly used as a pain-less and self-administration method for vaccines delivery.MN@SRS control vaccine kinetics,resulting in continuous exposure of lymphoid tissues to vaccine antigens with promotional humoral immunity.In addition,MN@SRS are dry formulations that improved the stability of vaccines,thereby eliminating the problems caused by cold chain requirements during vaccine transportation and storage.Moreover,the self-administration potential of MN@SRS is meaningful for prophylactic vaccination,especially for areas where there is lack of professional medical personnel.
Motivated by these merits of MN@SRS,Boopathy et al.designed a silk fibroin-based MN patch for continuous delivery of HIV vaccine.Poly (acrylic acid) (PAA) was chosen as the material of dissolving backing layer.Once the MN@SRS were applied into the skin,the backing layer made by PAA rapidly dissolved,allowing silk needle tips to separate from patch and implant into the skin.With the swelling and hydrolysis of silk matrix,the entrapped vaccine was released over two weeks.In vitrorelease studies showed the structure of antigen released from the MNs maintained structural integrity.Immune response by MNs was enhanced 1300-fold higher antibody titers than equivalent bolus injections at 1-month post-boost,which was due to sustained release vaccines from MNs [158].Interestingly,DeMuth et al.also prepared a silk fibroinbased separation MN for programmable vaccine release and enhanced immunogenicity in transcutaneous immunization(Fig.7).The PAA acted as a separation layer,both silk fibroin needle tips and PAA separation layer loaded a model drugvaccine ovalbumin(OVA).After a short application of the MN patch into the skin,the PAA layer quickly dissolved to provide a bolus of OVA.At the same time,a long-release silk fibroin reservoir was implanted into the skin to within 1-2 weeks low-level sustained skin vaccine release[159].Similarly,Chen et al.employed chitosan-PLA based rapidly detachable MNs to deliver OVA.After applied into skin,OVA-loaded chitosan needle tips were separated from the PLA sporting array by soluble PVP coated layer dissolving.The implanted needle tips allow the antigen to release constantly at insert site for at least 14 d,inducing a robust immune response for over 6 weeks[160].

Fig.7-(A)Schematic of silk/PAA MN preparation,(B)Optical image and confocal images of silk/PAA MN array encapsulating AF647-OVA(blue)in silk tips,and AF555-OVA(red)in PAA pedestals,(C)SEM images of silk/PAA MN,(D)OVA release from silk and PAA fractions of composite MNs over time[159].Copyright 2013,John Wiley and Sons.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
3.5.The application of MN@SRS for skin diseases therapy
Skin diseases caused by bacteria,fungi,and viruses have been wildly explored due to their high prevalence and serious consequence [161-164].As reported,they are the fourth leading cause of disability globally in 2013 excluding mortality,affecting over 3% of worldwide population [165,166].Until now,polymeric MNs have been diffusely employed to treat skin diseases such as hypertrophic scar,acne and actinic keratosis[167-169].For example,Lin et al.designed polymeric MNs based on HA to reduce abnormal scar [170].Zhang and coworker prepared poly (ionic liquids) (PILs) based MNs for acne infections treatments[171].
3.5.1.The application of MN@SRS for chronic skin diseases therapy
For chronic skin diseases,like psoriasis,an autoimmune disease needed long-term treatment,MN@SRS show unique advantages.For instance,Tekko’s group developed a dissolving MN patch for local and sustained delivery of MTX,a first-line treatment drug for psoriasis.A bottom-up technique was introduced to produce poorly water-soluble MTX NC,which performed as drug reservoirs with high drug loading efficacy.The MN@SRS possessed the sustained release period of MTX over three days and the therapeutic efficacy was significantly improved.Moreover,the MN@SRS reduced the side effects and avoided systemic exposure of MTX compared to the traditional systemic administration routes[27].
3.5.2.The application of MN@SRS for fungal skin infections therapy
Around 20-25% of the worldwide population affected by skin fungal infection,also known as mycosis,especially those patients with AIDS and cancer with compromised immune systems [172,173].Completely eliminating deep cutaneous mycosis is still a big challenge[174-177].The key point to cure deep cutaneous mycosis is effectively deliver localized and sustained release drugs into infected deep tissue,which can completely kill the fungi in chronic infection and successfully prevent the development of drug resistance[178,179].To tackle these issues,MN@SRS provide a promising choice for the treatment of deep fungal infection.Zan et al.prepared a MN patch consists with chitosan-polyethylenimine (CP) and Amphotericin B (AB) to treat deep cutaneous mycosis (Fig.8).CP acted as biocompatible polymer as well as antimicrobial,which produced synergistic antibacterial effect with AB.High permeability of therapeutics by piercing the barrier of skin,localized and sustained release of therapeutic contributed to superior antifungal infection effectiveness of the MN@SRS compared with conventional cream application[180].
3.5.3.The application of MN@SRS for chronic wound healing
Another type of skin disease is skin wounds caused by various reasons,among them,chronic wounds are a global healthcare challenge,causing serious personnel and economic burden[181].Chronic wounds mostly occur in diabetic and bedbound patients,they may maintain inflammation period for months or years,such skin damage can be life-threatening if it is not healed in time[182,183].One of the current challenges in the treatment of chronic wounds is bacterial infection.The use of antibacterial dressings or prophylactic antibiotics has been successful in reducing wound infections to some extent,however,they usually reduce the speed of wound healing and promote the development of drug resistance [184].The other challenge is chronic wounds are usually accompanied by eschar,therapeutic drugs need to pass through the eschar to reach the healthy cells below.It is well known that the localized delivery of exogenous growth factors and antiinflammatory factors can improve the quality of wound healing,such as vascular endothelial growth factor(VEGF)and platelet-derived growth factor(PDGF)[185,186].Unfortunately,these biological factors need the right concentration at specific time points to be effective.Therefore,smart drug delivery systems based on stimuli-responsive materials have been developed,such as hydrogel [187].These materials can response to various wound environments to release loaded drugs.But there is still a problem worthy of attention:whether these drug delivery systems can deliver drugs through eschar and effectively accumulate in healthy cells below the eschar.

Fig.8-(A)Illustration of the antimicrobial MN patch,(B)In vivo fluorescence imaging shows the release of Cy5 from MN at different times,(C)Quantitative in vivo release profiles of Cy5 from MN[180].Copyright 2019,John Wiley and Sons.
To address the issues,MN@SRS show the potential for chronic wound treatment.MN@SRS are micro-invasive,they can pierce the eschar and effectively deliver drugs to healthy cells.They can also destroy the biofilm formed in bacterial infections,which is a protective barrier for bacteria,obtain better antibacterial effects.More importantly,active and smart drug delivery strategies integrated with MN@SRS can easily comply with the dynamic pathophysiological conditions of chronic wounds and further enhance wound healing duo to their controlled drug release property.For example,Chi et al.developed a chitosan MN patch integrated with VEGF-loaded temperature sensitive hydrogel for promoting wound healing.When the MN applied to the wound,the increased temperature caused by the inflammation reaction degraded the hydrogel and intelligently released VEGF[188].
4.Conclusions and future outlook
The application field of MN@SRS is broad.In this review article,we surveyed typical articles instead of all the related studies to exhibit extensive therapeutic applications of MN@SRS.Altogether,MN@SRS that combined MNs patch with sustained release drug delivery system ingeniously,were viewed as a successfully platform to deliver drugs and vaccines.MN@SRS can be also used as synergistic therapies,realizing the strategies of “two birds with one stone”.The self-administration potential of MN@SRS increased patient’s access to medications and further enhanced patient compliance.In this regard,women who need contraception and HIV-infected patients benefit greatly.They both require long-acting formulations that are convenient for self-administration to reduce the pill burden.Especially for AIDS patients,they are often unwilling to expose the fact that they are sick to other people.On the other hand,MN@SRS directly deliver vaccines into skin tissue where has rich immune cells,subsequently generating robust immune stimulation due to the durable release of vaccines,which is beneficial for immunotherapy.In addition,MN@SRS can avoid cold chain transportation and address storage difficulties of unstable drugs,such as peptides,proteins and nucleosides,reducing the cost of drugs.
Even though MN@SRS exhibited great potential for improving therapeutic efficacy,practical clinical translation is still in their infancy,and patients do not really get the benefits from this novel platform.In this sense,there are still some issues need to be solved.Firstly,the cooperation between academia,industry and regulatory authorities must be strengthened,not only to develop new products,but also to boost explore of new good manufacturing practices(GMP)and realize industrialization.Furthermore,MN@SRS are a new TDD system instead of substitute of conventional transdermal patches,therefore,their mechanism of action and the safety of materials are crucial.Most of MN@SRS are made of FDAapproved polymers,such as PLA,PLGA,etc.Although the safety of these materials has been established for a long time,they have never been administered intradermally.The routes of metabolism and elimination of these materials and the safety of deposition into the skin for a long-term need to be investigated.Unlike conventional transdermal or topical drug delivery systems,MN@SRS will encounter viable skin cells,whether the MN@SRS keep sterile is another question worth considering.All these factors will delay the commercialization of MN@SRS.Also,due to the complexity of the sustained release compared with bolus release,the release kinetics of MN@SRS need further explore.Nevertheless,with the success of MNs on an industrial scale,for example,Zosano Pharma Corporation(USA)announced the acceptance of their New Drug Application (NDA) by the FDA for their product Qtrypta,a zolmitriptan-loaded MN patch for the treatment of migraine headaches [189].MN@SRS should also be available in the clinic in the future.Qtrypta is an array of close to two thousand drug-coated titanium MNs,which is mounted on the skin-facing surface of a backing that resembles an adhesive bandage.The length of each individual MN is about 3 times the width of a human hair.When the MN patch is applied,the MNs penetrate the outermost layer of the epidermis.The coated zolmitriptan is dissolved by SIF and absorbed rapidly into the bloodstream.The shallow depth of penetration limits the likelihood of stimulating sensory nerve endings and causing pain.To this end,impressive advance in this area has been made by Vaxess Technologies(USA),its proprietary MIMIXTMtherapies based on technology originally developed at Tufts University and MIT.MIMIXTMtherapies are silk fibroin-based MN@SRS for oncology and infectious disease development.This technology is utilizing the natural properties of silk fibroin to obtain the most effective delivery profiles of various drugs and achieve better treatment effect.The silk fibroin-based MN@SRS was fabricated by micromolding method,specifically,silk fibroin was used as needle tip material and PAA played a role as separation layer.After the MN patch is applied on the skin,PAA layer dissolved quickly,silk fibroin-based needle tips were implanted into skin act as drug reservoir to achieve long-term treatment.If these products successful approved by FDA,which will represent a key milestone for the field.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China(32071342 and 31922042),Guangdong Special Support Program (2019TQ05Y209),and the Fundamental Research Funds for the Central Universities(19ykzd31).
REFERENCE
[1] Chen Z,He J,Qi J,Zhu Q,Wu W,Lu Y.Long-acting microneedles:a progress report of the state-of-the-art techniques.Drug Discov Today 2020;25(8):1462-8.
[2] Prausnitz MR,Langer R.Transdermal drug delivery.Nat Biotechnol 2008;26(11):1261-8.
[3] Chen M,Quan G,Sun Y,Yang D,Pan X,Wu C.Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery.J Control Release 2020;325:163-75.
[4] Singh P,Carrier A,Chen Y,Lin S,Wang J,Cui S.Polymeric microneedles for controlled transdermal drug delivery.J Control Release 2019;315:97-113.
[5] Yarmush ML,Golberg A,Sersa G,Kotnik T,Miklavcic D.Electroporation-based technologies for medicine:principles,applications,and challenges.Annu Rev Biomed Eng 2014;16:295-320.
[6] Malinovskaja-Gomez K,Labouta HI,Schneider M,Hirvonen J,Laaksonen T.Transdermal iontophoresis of flufenamic acid loaded PLGA nanoparticles.Eur J Pharm Sci 2016;89:154-62.
[7] Prausnitz MR,Mitragotri S,Langer R.Current status and future potential of transdermal drug delivery.Nat Rev Drug Discov 2004;3(2):115-24.
[8] Alexander A,Dwivedi S,Ajazuddin GTK,Saraf S,Saraf S.Approaches for breaking the barriers of drug permeation through transdermal drug delivery.J Control Release 2012;164(1):26-40.
[9] Wang M,Hu L,Xu C.Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing.Lab Chip 2017;17(8):1373-87.
[10] Ochoa M,Mousoulis C,Ziaie B.Polymeric microdevices for transdermal and subcutaneous drug delivery.Adv Drug Deliv Rev 2012;64(14):1603-16.
[11] Hong X,Wei L,Wu F,Wu Z,Chen L,Liu Z.Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine.Drug Des Devel Ther 2013;7:945-52.
[12] Gupta J,Park SS,Bondy B,Felner EI,Prausnitz MR.Infusion pressure and pain during microneedle injection into skin of human subjects.Biomaterials 2011;32(28):6823-31.
[13] Rzhevskiy AS,Singh TRR,Donnelly RF,Anissimov YG.Microneedles as the technique of drug delivery enhancement in diverse organs and tissues.J Control Release 2018;270:184-202.
[14] Hu X,Yu J,Qian C,Lu Y,Kahkoska AR,Xie Z.H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery.ACS Nano 2017;11(1):613-20.
[15] McCrudden MT,Alkilani AZ,McCrudden CM,McAlister E,McCarthy HO,Woolfson AD.Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose,low molecular weight drugs.J Control Release 2014;180:71-80.
[16] Yan Q,Wang W,Weng J,Zhang Z,Yin L,Yang Q.Dissolving microneedles for transdermal delivery of huperzine A for the treatment of Alzheimer’s disease.Drug Deliv 2020;27(1):1147-55.
[17] Chiu YH,Chen MC,Wan SW.Sodium hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system.Biomacromolecules 2018;19(6):2278-85.
[18] He M,Yang G,Zhao X,Zhang S,Gao Y.Intradermal implantable PLGA microneedles for etonogestrel sustained release.J Pharm Sci 2020;109(6):1958-66.
[19] Donnelly RF,Larraneta E.Microarray patches:potentially useful delivery systems for long-acting nanosuspensions.Drug Discov Today 2018;23(5):1026-33.
[20] Deadman CM,Kellaway IW,Yasin M,Dickinson PA,Murdan S.An investigation into the influence of drug lipophilicity on the in vivo absorption profiles from subcutaneous microspheres and in situ forming depots.J Control Release 2007;122(1):79-85.
[21] Kempe S,Mader K.In situ forming implants-an attractive formulation principle for parenteral depot formulations.J Control Release 2012;161(2):668-79.
[22] Ito Y,Murano H,Hamasaki N,Fukushima K,Takada K.Incidence of low bioavailability of leuprolide acetate after percutaneous administration to rats by dissolving microneedles.Int J Pharm 2011;407(1-2):126-31.
[23] Ito Y,Hagiwara E,Saeki A,Sugioka N,Takada K.Feasibility of microneedles for percutaneous absorption of insulin.Eur J Pharm Sci 2006;29(1):82-8.
[24] Hao Y,Chen Y,He X,Yang F,Han R,Yang C.Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy.Bioact Mater 2020;5(3):542-52.
[25] Monkare J,Reza Nejadnik M,Baccouche K,Romeijn S,Jiskoot W,Bouwstra JA.IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery.J Control Release 2015;218:53-62.
[26] Lee IC,He JS,Tsai MT,Lin KC.Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery.J Mater Chem B 2015;3(2):276-85.
[27] Tekko IA,Permana AD,Vora L,Hatahet T,McCarthy HO,Donnelly RF.Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays:potential for enhanced treatment of psoriasis.Eur J Pharm Sci 2020;152:105469.
[28] Mc Crudden MTC,Larraneta E,Clark A,Jarrahian C,Rein-Weston A,Lachau-Durand S.Design,formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension.J Control Release 2018;292:119-29.
[29] DeMuth PC,Min Y,Huang B,Kramer JA,Miller AD,Barouch DH.Polymer multilayer tattooing for enhanced DNA vaccination.Nat Mater 2013;12(4):367-76.
[30] Terashima S,Tatsukawa C,Suzuki M,Takahashi T,Aoyagi S.Fabrication of microneedle using poly lactic acid sheets by thermal nanoimprint.Precis Eng 2019;59:110-19.
[31] Kim M,Jung B,Park JH.Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin.Biomaterials 2012;33(2):668-78.
[32] Jin J,Reese V,Coler R,Carter D,Rolandi M.Chitin microneedles for an easy-to-use tuberculosis skin test.Adv Healthc Mater 2014;3(3):349-53.
[33] Chen MC,Huang SF,Lai KY,Ling MH.Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination.Biomaterials 2013;34(12):3077-86.
[34] Yavuz B,Chambre L,Kaplan DL.Extended release formulations using silk proteins for controlled delivery of therapeutics.Expert Opin Drug Deliv 2019;16(7):741-56.
[35] Tsioris K,Raja WK,Pritchard EM,Panilaitis B,Kaplan DL,Omenetto FG.Fabrication of silk microneedles for controlled-release drug delivery.Adv Funct Mater 2012;22(2):330-5.
[36] Li W,Terry RN,Tang J,Feng MR,Schwendeman SP,Prausnitz MR.Rapidly separable microneedle patch for the sustained release of a contraceptive.Nat Biomed Eng 2019;3(3):220-9.
[37] Chen MC,Lai KY,Ling MH,Lin CW.Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design.Acta Biomater 2018;65:66-75.
[38] Yavuz B,Chambre L,Harrington K,Kluge J,Valenti L,Kaplan DL.Silk fibroin microneedle patches for the sustained release of levonorgestrel.ACS Appl Bio Mater 2020;3(8):5375-82.
[39] Donnelly RF,McCrudden MT,Zaid Alkilani A,Larraneta E,McAlister E,Courtenay AJ.Hydrogel-forming microneedles prepared from"super swelling"polymers combined with lyophilised wafers for transdermal drug delivery.PLoS One 2014;9(10):e111547.
[40] Zhu M,Liu Y,Jiang F,Cao J,Kundu SC,Lu S.Combined silk fibroin microneedles for insulin delivery.ACS Biomater Sci Eng 2020;6(6):3422-9.
[41] Lee JW,Park JH,Prausnitz MR.Dissolving microneedles for transdermal drug delivery.Biomaterials 2008;29(13):2113-24.
[42] Lee IC,He JS,Tsai MT,Lin KC.Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery.J Mat Chem B 2015;3(2):276-85.
[43] Kim JD,Kim M,Yang H,Lee K,Jung H.Droplet-born air blowing:novel dissolving microneedle fabrication.J Control Release 2013;170(3):430-6.
[44] Lee K,Jung H.Drawing lithography for microneedles:a review of fundamentals and biomedical applications.Biomaterials 2012;33(30):7309-26.
[45] Ruggiero F,Vecchione R,Bhowmick S,Coppola G,Coppola S,Esposito E.Electro-drawn polymer microneedle arrays with controlled shape and dimension.Sens Actuators B 2018;255:1553-60.
[46] Dardano P,Calio A,Di Palma V,Bevilacqua MF,Di Matteo A,De Stefano L.A photolithographic approach to polymeric microneedles array fabrication.Materials 2015;8(12):8661-73.
[47] Johnson AR,Caudill CL,Tumbleston JR,Bloomquist CJ,Moga KA,Ermoshkin A.Single-step fabrication of computationally designed microneedles by continuous liquid interface production.Plos One 2016;11(9):e0162518.
[48] Petruney T,Wilson LC,Stanback J,Cates W.Family planning and the post-2015 development agenda.Bull World Health Organ 2014;92(8)548-A.
[49] Sedgh G,Singh S,Hussain R.Intended and unintended pregnancies worldwide in 2012 and recent trends.Stud Fam Plann 2014;45(3):301-14.
[50] Finer LB,Zolna MR.Declines in unintended pregnancy in the United States.2008-2011.N Engl J Med 2016;374(9):843-52.
[51] Sedgh G,Bearak J,Singh S,Bankole A,Popinchalk A,Ganatra B.Abortion incidence between 1990 and 2014:global,regional,and subregional levels and trends.Lancet 2016;388(10041):258-67.
[52] Sun Y,Wang J,Zhang X,Zhang Z,Zheng Y,Chen D.Synchronic release of two hormonal contraceptives for about one month from the PLGA microspheres:in vitroand in vivo studies.J Control Release 2008;129(3):192-9.
[53] Kaunitz AM.Long-acting injectable contraception with depot medroxyprogesterone acetate.Am J Obstet Gynecol 1994;170(5):1543-9.
[54] Espey E,Ogburn T.Long-acting reversible contraceptives:intrauterine devices and the contraceptive implant.Obstet Gynecol 2011;117(3):705-19.
[55] Hoggart L,Newton VL.Young women’s experiences of side-effects from contraceptive implants:a challenge to bodily control.Reprod Health Matters 2013;21(41):196-204.
[56] Seong KY,Seo MS,Hwang DY,O’Cearbhaill ED,Sreenan S,Karp JM.A self-adherent,bullet-shaped microneedle patch for controlled transdermal delivery of insulin.J Control Release 2017;265:48-56.
[57] Karp JM,Langer R.Materials science dry solution to a sticky problem.Nature 2011;477(7362):42-3.
[58] Li W,Tang J,Terry RN,Li S,Brunie A,Callahan RL.Long-acting reversible contraception by effervescent microneedle patch.Sci Adv 2019;5(11):eaaw8145.
[59] Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.CA Cancer J Clin 2018;68(6):394-424.
[60] Sun T,Zhang YS,Pang B,Hyun DC,Yang M,Xia Y.Engineered nanoparticles for drug delivery in cancer therapy.Angew Chem Int Ed 2014;53(46):12320-64.
[61] Emens LA,Middleton G.The interplay of immunotherapy and chemotherapy:harnessing potential synergies.Cancer Immunol Res 2015;3(5):436-43.
[62] Chen YL,Chang MC,Cheng WF.Metronomic chemotherapy and immunotherapy in cancer treatment.Cancer Lett 2017;400:282-92.
[63] Riley RS,June CH,Langer R,Mitchell MJ.Delivery technologies for cancer immunotherapy.Nat Rev Drug Discov 2019;18(3):175-96.
[64] Hare JI,Lammers T,Ashford MB,Puri S,Storm G,Barry ST.Challenges and strategies in anti-cancer nanomedicine development:an industry perspective.Adv Drug Deliv Rev 2017;108:25-38.
[65] Cheng W,Nie J,Gao N,Liu G,Tao W,Xiao X.A multifunctional nanoplatform against multidrug resistant cancer:merging the best of targeted chemo/gene/photothermal therapy.Adv Funct Mater 2017;27(45):1704135.
[66] Wang W,Tang Z,Zhang Y,Wang Q,Liang Z,Zeng X.Mussel-inspired polydopamine:the bridge for targeting drug delivery system and synergistic cancer treatment.Macromol Biosci 2020;20(10):e2000222.
[67] Perera E,Gnaneswaran N,Jennens R,Sinclair R.Malignant melanoma.Healthcare 2013;2(1):1-19(Basel).
[68] Li Z,Shan X,Chen Z,Gao N,Zeng W,Zeng X.Applications of surface modification technologies in nanomedicine for deep tumor penetration.Adv Sci 2021;8(1):2002589.
[69] Li D,Hu D,Xu H,Patra HK,Liu X,Zhou Z.Progress and perspective of microneedle system for anti-cancer drug delivery.Biomaterials 2021;264:120410.
[70] Zeng X,Luo M,Liu G,Wang X,Tao W,Lin Y.Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments.Adv Sci 2018;5(10):1800510(Weinh).
[71] Cheng W,Zeng X,Chen H,Li Z,Zeng W,Mei L.Versatile polydopamine platforms:synthesis and promising applications for surface modification and advanced nanomedicine.ACS Nano 2019;13(8):8537-65.
[72] Lu Y,Mantha SN,Crowder DC,Chinchilla S,Shah KN,Yun YH.Microstereolithography and characterization of poly(propylene fumarate)-based drug-loaded microneedle arrays.Biofabrication 2015;7(4):045001.
[73] Ahmad Z,Khan MI,Siddique MI,Sarwar HS,Shahnaz G,Hussain SZ.Fabrication and characterization of thiolated chitosan microneedle patch for transdermal delivery of tacrolimus.AAPS PharmSciTech 2020;21(2):68.
[74] Luo Z,Sun W,Fang J,Lee K,Li S,Gu Z.Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery.Adv Healthc Mater 2019;8(3):e1801054.
[75] Kim H,Lee HS,Jeon Y,Park W,Zhang Y,Kim B.Bioresorbable,miniaturized porous silicon needles on a flexible water-soluble backing for unobtrusive,sustained delivery of chemotherapy.ACS Nano 2020;14(6):7227-36.
[76] Mellman I,Coukos G,Dranoff G.Cancer immunotherapy comes of age.Nature 2011;480(7378):480-9.
[77] Milling L,Zhang Y,Irvine DJ.Delivering safer immunotherapies for cancer.Adv Drug Deliv Rev 2017;114:79-101.
[78] CWt S,Wang LL,Evans MA,Mitragotri S.Materials for immunotherapy.Adv Mater 2020;32(13):e1901633.
[79] Fesnak AD,June CH,Levine BL.EngineeredTcells:the promise and challenges of cancer immunotherapy.Nat Rev Cancer 2016;16(9):566-81.
[80] Rosenberg SA,Restifo NP.Adoptive cell transfer as personalized immunotherapy for human cancer.Science 2015;348(6230):62-8.
[81] Zou W,Wolchok JD,Chen L.PD-L1(B7-H1)and PD-1 pathway blockade for cancer therapy:mechanisms,response biomarkers,and combinations.Sci Transl Med 2016;8(328):328rv4.
[82] Sharma P,Allison JP.The future of immune checkpoint therapy.Science 2015;348(6230):56-61.
[83] Valsecchi ME.Combined nivolumab and ipilimumab or monotherapy in untreated melanoma.N Engl J Med 2015;373(13):1270-1.
[84] Boutros C,Tarhini A,Routier E,Lambotte O,Ladurie FL,Carbonnel F.Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination.Nat Rev Clin Oncol 2016;13(8):473-86.
[85] Wang C,Ye YQ,Hochu GM,Sadeghifar H,Gu Z.Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody.Nano Lett 2016;16(4):2334-40.
[86] Larkin J,Chiarion-Sileni V,Gonzalez R,Grob JJ,Cowey CL,Lao CD.Combined nivolumab and ipilimumab or monotherapy in untreated melanoma.N Engl J Med 2015;373(1):23-34.
[87] Zhou Z,Pang J,Wu X,Wu W,Chen X,Kong M.Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle.Nano Res 2020;13(6):1509-18.
[88] Cortez MA,Masrorpour F,Ivan C,Zhang J,Younes AI,Lu Y.Bone morphogenetic protein 7 promotes resistance to immunotherapy.Nat Commun 2020;11(1):4840.
[89] Chen G,Chen Z,Wen D,Wang Z,Li H,Zeng Y.Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy.Proc Natl Acad Sci USA 2020;117(7):3687-92.
[90] Wang W,Kryczek I,Dostal L,Lin H,Tan L,Zhao L.EffectorTcells abrogate stroma-mediated chemoresistance in ovarian cancer.Cell 2016;165(5):1092-105.
[91] Sharma P,Hu-Lieskovan S,Wargo JA,Primary RA.Adaptive,and acquired resistance to cancer immunotherapy.Cell 2017;168(4):707-23.
[92] Hobson J,Gummadidala P,Silverstrim B,Grier D,Bunn J,James T.Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis.Breast Cancer Res Treat 2013;139(2):391-401.
[93] Hansen NM,Ye X,Grube B,Giuliano AE.Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer.Arch Surg 2004;139(6):634-9.
[94] Estourgie SH,Nieweg OE,Kroon BBR.High incidence of in-transit metastases after sentinel node biopsy in patients with melanoma.Br J Surg 2004;91(10):1370-1.
[95] Lu X,Miao L,Gao W,Chen Z,McHugh KJ,Sun Y.Engineered PLGA microparticles for long-term,pulsatile release of STING agonist for cancer immunotherapy.Sci Transl Med 2020;12(556):eaaz6606.
[96] Mellor AL,Munn DH.IDO expression by dendritic cells:tolerance and tryptophan catabolism.Nat Rev Immunol 2004;4(10):762-74.
[97] Moon YW,Hajjar J,Hwu P,Naing A.Targeting the indoleamine 2,3-dioxygenase pathway in cancer.J Immunother Cancer 2015;3:51.
[98] Haibe Y,El Husseini Z,El Sayed R,Shamseddine A.Resisting resistance to immune checkpoint therapy:a systematic review.Int J Mol Sci 2020;21(17):6176.
[99] Ye Y,Wang J,Hu Q,Hochu GM,Xin H,Wang C.Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors.ACS Nano 2016;10(9):8956-63.
[100] Ye Y,Wang C,Zhang X,Hu Q,Zhang Y,Liu Q.A melanin-mediated cancer immunotherapy patch.Sci Immunol 2017;2(17):eaan5692.
[101] Zakrewsky M,Kumar S,Mitragotri S.Nucleic acid delivery into skin for the treatment of skin disease:proofs-of-concept,potential impact,and remaining challenges.J Control Release 2015;219:445-56.
[102] Pecot CV,Calin GA,Coleman RL,Lopez-Berestein G,Sood AK.RNA interference in the clinic:challenges and future directions.Nat Rev Cancer 2011;11(1):59-67.
[103] Sun XY,Zeng LH,Huang YZ.Transcutaneous delivery of DNA/mRNA for cancer therapeutic vaccination.J Gene Med 2019;21(7):e3089.
[104] Darrow JJ.Luxturna:FDA documents reveal the value of a costly gene therapy.Drug Discov Today 2019;24(4):949-54.
[105] Somia N,Verma IM.Gene therapy:trials and tribulations.Nat Rev Genet 2000;1(2):91-9.
[106] Duong HTT,Kim NW,Thambi T,Giang Phan VH,Lee MS,Yin Y.Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses.J Control Release 2018;269:225-34.
[107] Duong HTT,Yin Y,Thambi T,Nguyen TL,Giang Phan VH,Lee MS.Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy.Biomaterials 2018;185:13-24.
[108] Khademhosseini A,Sun W,Gu Z,Zhu S,Dokmeci MR,Ahadian S.Biodegradable microneedle patch for transdermal gene delivery.Nanoscale 2020;12(32):16724-9.
[109] American Diabetes AssociationDiagnosis and classification of diabetes mellitus.Diabetes Care 2013;36:S67-74 Suppl 1.
[110] Jin X,Zhu DD,Chen BZ,Ashfaq M,Guo XD.Insulin delivery systems combined with microneedle technology.Adv Drug Deliv Rev 2018;127:119-37.
[111] Chen X,Wang L,Yu H,Li C,Feng J,Haq F.Preparation,properties and challenges of the microneedles-based insulin delivery system.J Control Release 2018;288:173-88.
[112] Asif M.The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern.J Educ Health Promot 2014;3:1.
[113] Owens DR,Zinman B,Bolli GB.Insulins today and beyond.Lancet 2001;358(9283):739-46.
[114] Pathak V,Pathak NM,O’Neill CL,Guduric-Fuchs J,Medina RJ.Therapies for type 1 diabetes:current scenario and future perspectives.Clin Med Insights Endocrinol Diabetes 2019;12:1179551419844521.
[115] Shah RB,Patel M,Maahs DM,Shah VN.Insulin delivery methods:Past,present and future.Int J Pharm Investig 2016;6(1):1-9.
[116] Wilson LM,Castle JR.Recent advances in insulin therapy.Diabetes Technol Ther 2020;22(12):929-36.
[117] Matteucci E,Giampietro O,Covolan V,Giustarini D,Fanti P,Rossi R.Insulin administration:present strategies and future directions for a noninvasive(possibly more physiological)delivery.Drug Des Devel Ther 2015;9:3109-18.
[118] Xie S,Li ZJ,Yu ZQ.Microneedles for transdermal delivery of insulin.J Drug Deliv Sci Technol 2015;28:11-17.
[119] Chu LY.Controlled release systems for insulin delivery.Expert Opin Ther Patents 2005;15(9):1147-55.
[120] Barnett AH.The role of GLP-1 mimetics and basal insulin analogues in type 2 diabetes mellitus:guidance from studies of liraglutide.Diabetes Obes Metab 2012;14(4):304-14.
[121] Syed YY,McCormack PL.Exenatide extended-release:an updated review of its use in type 2 diabetes mellitus.Drugs 2015;75(10):1141-52.
[122] Qiao Q,Ouwens MJ,Grandy S,Johnsson K,Kostev K.Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany.Diabetes Metab Syndr Obes 2016;9:201-5.
[123] Jin X,Zhu DD,Chen BZ,Ashfaq M,Guo XD.Insulin delivery systems combined with microneedle technology.Adv Drug Deliv Rev 2018;127:119-37.
[124] Zhang YQ,Yu JC,Kahkoska AR,Wang JQ,Buse JB,Gu Z.Advances in transdermal insulin delivery.Adv Drug Deliv Rev 2019;139:51-70.
[125] Chen X,Wang L,Yu HJ,Li CJ,Feng JY,Haq F.Preparation,properties and challenges of the microneedles-based insulin delivery system.J Control Release 2018;288:173-188.
[126] Liu S,Jin MN,Quan YS,Kamiyama F,Katsumi H,Sakane T.The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid,and their application in the transdermal delivery of insulin.J Control Release 2012;161(3):933-41.
[127] Chen MC,Ling MH,Kusuma SJ.Poly-gamma-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin.Acta Biomater 2015;24:106-16.
[128] Yu WJ,Jiang GH,Zhang Y,Liu DP,Xu B,Zhou JY.Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin.Mater Sci Eng C Mater Biol Appl 2017;80:187-96.
[129] Chen CH,Shyu VBH,Chen CT.Dissolving microneedle patches for transdermal insulin delivery in diabetic mice:potential for clinical applications.Materials 2018;11(9):1625.
[130] Ito Y,Hirono M,Fukushima K,Sugioka N,Takada K.Two-layered dissolving microneedles formulated with intermediate-acting insulin.Int J Pharm 2012;436(1-2):387-93.
[131] Zhang Y,Jiang GH,Yu WJ,Liu DP,Xu B.Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats.Mater Sci Eng C Mater Biol Appl 2018;85:18-26.
[132] Yu WJ,Jiang GH,Zhang Y,Liu DP,Xu B,Zhou JY.Near-infrared light triggered and separable microneedles for transdermal delivery of metformin in diabetic rats.J Mat Chem B 2017;5(48):9507-13.
[133] VandenBerg MA,Webber MJ.Biologically inspired and chemically derived methods for glucose-responsive insulin therapy.Adv Healthc Mater 2019;8(12):13.
[134] Yu JC,Wang JQ,Zhang YQ,Chen GJ,Mao WW,Ye YQ.Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs.Nat Biomed Eng 2020;4(5):499-506.
[135] Donnelly RF,Singh TRR,Garland MJ,Migalska K,Majithiya R,McCrudden CM.Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery.Adv Funct Mater 2012;22(23):4879-90.
[136] Chen S,Matsumoto H,Moro-oka Y,Tanaka M,Miyahara Y,Suganami T.Microneedle-array patch fabricated with enzyme-free polymeric components capable of on-demand insulin delivery.Adv Funct Mater 2019;29(7):1807369.
[137] Morens DM,Folkers GK,Fauci AS.The challenge of emerging and re-emerging infectious diseases.Nature 2004;430(6996):242-9.
[138] Rohrl JR,Barrett CB,Civitello DJ,Craft ME,Delius B,DeLeo GA.Emerging human infectious diseases and the links to global food production.Nat Sustain 2019;2(6):445-56.
[139] Morens DM,Fauci AS.Emerging infectious diseases:threats to human health and global stability.PLoS Pathog 2013;9(7):e1003467.
[140] Nicola M,Alsafi Z,Sohrabi C,Kerwan A,Al-Jabir A,Iosifidis C.The socio-economic implications of the coronavirus pandemic(COVID-19):a review.Int J Surg 2020;78:185-93.
[141] Miller IF,Becker AD,Grenfell BT,Metcalf CJE.Disease and healthcare burden of COVID-19 in the United States.Nat Med 2020;26(8):1212-17.
[142] Anderson RM,Heesterbeek H,Klinkenberg D,Hollingsworth TD.How will country-based mitigation measures influence the course of the COVID-19 epidemic?Lancet 2020;395(10228):931-4.
[143] Pati R,Shevtsov M,Sonawane A.Nanoparticle vaccines against infectious diseases.Front Immunol 2018;9:16.
[144] Zhao Z,Ukidve A,Dasgupta A,Mitragotri S.Transdermal immunomodulation:principles,advances and perspectives.Adv Drug Deliv Rev 2018;127:3-19.
[145] Harder J,Schroeder J-M,Glaeser R.The skin surface as antimicrobial barrier:present concepts and future outlooks.Exp Dermatol 2013;22(1):1-5.
[146] Kabashima K,Honda T,Ginhoux F,Egawa G.The immunological anatomy of the skin.Nat Rev Immunol 2019;19(1):19-30.
[147] Palucka K,Banchereau J.Dendritic-cell-based therapeutic cancer vaccines.Immunity 2013;39(1):38-48.
[148] Haniffa M,Gunawan M,Jardine L.Human skin dendritic cells in health and disease.J Dermatol Sci 2015;77(2):85-92.
[149] J.Banchereau,E.Klechevsky,N.Schmitt,R.Morita,K.Palucka,H.Ueno Harnessing human dendritic cell subsets to design novel caccines.In:R.Steinman,J.Banchereau,O.J.Finn,editors.Cancer Vaccines;2009;1174:24-32.
[150] Levin C,Perrin H,Combadiere B.Tailored immunity by skin antigen-presenting cells.Hum Vaccin Immunother 2015;11(1):27-36.
[151] Laurent PE,Bonnet S,Alchas P,Regolini P,Mikszta JA,Pettis R.Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system.Vaccine 2007;25(52):8833-42.
[152] Arnon R,Ben-Yedidia T.Old and new vaccine approaches.Int Immunopharmacol 2003;3(8):1195-204.
[153] Kersten GFA,Crommelin DJA.Liposomes and ISCOMs.Vaccine 2003;21(9-10):915-20.
[154] Hu JK,Crampton JC,Cupo A,Ketas T,van Gils MJ,Sliepen K.Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity.J Virol 2015;89(20):10383-98.
[155] Tam HH,Melo MB,Kang M,Pelet JM,Ruda VM,Foley MH.Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination.Proc Natl Acad Sci USA 2016;113(43):E6639-48.
[156] Schipper P,van der Maaden K,Romeijn S,Oomens C,Kersten G,Jiskoot W.Repeated fractional intradermal dosing of an inactivated polio vaccine by a single hollow microneedle leads to superior immune responses.J Control Release 2016;242:141-7.
[157] Pauthner M,Havenar-Daughton C,Sok D,Nkolola JP,Bastidas R,Boopathy AV.Elicitation of robust Tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches.Immunity 2017;46(6):1073-88.
[158] Boopathy AV,Mandal A,Kulp DW,Menis S,Bennett NR,Watkins HC.Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination.Proc Natl Acad Sci USA 2019;116(33):16473-8.
[159] DeMuth PC,Min Y,Irvine DJ,Hammond PT.Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization.Adv Healthc Mater 2014;3(1):47-58.
[160] Chen YH,Lai KY,Chiu YH,Wu YW,Shiau AL,Chen MC.Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination.Acta Biomater 2019;97:230-8.
[161] Hahnel E,Blume-Peytavi U,Trojahn C,Dobos G,Jahnke I,Kanti V.Prevalence and associated factors of skin diseases in aged nursing home residents:a multicentre prevalence study.Bmj Open 2017;7(9):e018283.
[162] Yang G,Chen Q,Wen D,Chen Z,Wang J,Chen G.A Therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth.ACS Nano 2019;13(4):4354-60.
[163] Kim MS,Lee SM,Sung HS,Won CH,Chang S,Lee MW.Clinical analysis of deep cutaneous mycoses:a 12-year experience at a single institution.Mycoses 2012;55(6):501-506.
[164] Jamaledin R,Yiu CKY,Zare EN,Niu LN,Vecchione R,Chen G.Advances in antimicrobial microneedle patches for combating infections.Adv Mater 2020;32(33):2002129.
[165] Karimkhani C,Dellavalle RP,Coffeng LE,Flohr C,Hay RJ,Langan SM.Global skin disease morbidity and mortality an update from the global burden of disease study 2013.JAMA Dermatol 2017;153(5):406-12.
[166] How KN,Yap WH,Lim CLH,Goh BH,Lai ZW.Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases:a mini review.Front Pharmacol 2020;11:1105.
[167] Zhang Z,Tsai P-C,Ramezanli T,Michniak-Kohn BB.Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases.WIREs Nanomed Nanobiotechnol 2013;5(3):205-18.
[168] Zhang Y,Feng P,Yu J,Yang J,Zhao J,Wang J.ROS-responsive microneedle patch for acne vulgaris treatment.Adv Ther 2018;1(3):1800035.
[169] Tan CWX,Tan WD,Srivastava R,Yow AP,Wong DWK,Tey HL.Dissolving triamcinolone-embedded microneedles for the treatment of keloids:a single-blinded intra-individual controlled clinical trial.Dermatol Ther 2019;9(3):601-11.
[170] Lin S,Quan G,Hou A,Yang P,Peng T,Gu Y.Strategy for hypertrophic scar therapy:improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array.J Control Release 2019;306:69-82.
[171] Zhang T,Sun B,Guo J,Wang M,Cui H,Mao H.Active pharmaceutical ingredient poly(ionic liquid)-based microneedles for the treatment of skin acne infection.Acta Biomater 2020;115:136-47.
[172] Havlickova B,Czaika VA,Friedrich M.Epidemiological trends in skin mycoses worldwide.Mycoses 2008;51:2-15.
[173] Garcia-Solache MA,Casadevall A.Global warming will bring new fungal diseases for mammals.Mbio 2010;1(1):e00061-10.
[174] Huang H,Ostroff GR,Lee CK,Wang JP,Specht CA,Levitz SM.Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists.Infect Immun 2009;77(5):1774-81.
[175] Akhtar N,Verma A,Pathak K.Topical delivery of drugs for the effective treatment of fungal infections of skin.Curr Pharm Des 2015;21(20):2892-913.
[176] Spernovasilis N,Kofteridis DP.Pre-existing liver disease and toxicity of antifungals.J Fungi 2018;4(4):133.
[177] Tuffanelli L,Milburn PB.Treatment of chromoblastomycosis.J Am Acad Dermatol 1990;23:728-32 4 Pt 1.
[178] Wang J,Chou S,Yang Z,Yang Y,Wang Z,Song J.Combating drug-resistant fungi with novel imperfectly amphipathic palindromic peptides.J Med Chem 2019;62(7):3782.
[179] Cowen LE,Sanglard D,Howard SJ,Rogers PD,Perlin DS.Mechanisms of antifungal drug resistance.Cold Spring Harb Perspect Med 2014;5(7):a019752.
[180] Zan P,Than A,Duong PK,Song J,Xu C,Chen P.Antimicrobial microneedle patch for treating deep cutaneous fungal infection.Adv Ther 2019;2(10):1900064.
[181] Barnum L,Samandari M,Schmidt TA,Tamayol A.Microneedle arrays for the treatment of chronic wounds.Expert Opin Drug Deliv 2020;17(12):1767-80.
[182] Dowsett C,Bielby A,Searle R.Reconciling increasing wound care demands with available resources.J Wound Care 2014;23(11):552-62.
[183] Moore K,McCallion R,Searle RJ,Stacey MC,Harding KG.Prediction and monitoring the therapeutic response of chronic dermal wounds.Int Wound J 2006;3(2):89-96.
[184] Saghazadeh S,Rinoldi C,Schot M,Kashaf SS,Sharifi F,Jalilian E.Drug delivery systems and materials for wound healing applications.Adv Drug Deliv Rev 2018;127:138-66.
[185] Stojadinovic A,Carlson JW,Schultz GS,Davis TA,Elster EA.Topical advances in wound care.Gynecol Oncol 2008;111(2):S70-80.
[186] Kiwanuka E,Junker J,Eriksson E.Harnessing growth factors to influence wound healing.Clin Plast Surg 2012;39(3):239.
[187] Hu C,Long L,Cao J,Zhang S,Wang Y.Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release.Chem Eng J 2021;411:128564.
[188] Chi J,Zhang X,Chen C,Shao C,Zhao Y,Wang Y.Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing.Bioact Mater 2020;5(2):253-9.
[189] Paredes AJ,McKenna PE,Ramöller IK,Naser YA,Volpe-Zanutto F,Li M.Microarray patches:poking a hole in the challenges faced when delivering poorly soluble drugs.Adv Funct Mater 2020;31(1):2005792.
 Asian Journal of Pharmacentical Sciences2022年1期
Asian Journal of Pharmacentical Sciences2022年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Calcium ion nanomodulators for mitochondriatargeted multimodal cancer therapy
- Biomaterials reinforced MSCs transplantation for spinal cord injury repair
- Exosomes:Emerging implementation of nanotechnology for detecting and managing novel corona virus-SARS-CoV-2
- Emerging pro-drug and nano-drug strategies for gemcitabine-based cancer therapy
- Plant-derived exosome-like nanoparticles and their therapeutic activities
- Sodium alginate and naloxone loaded macrophagederived nanovesicles for the treatment of spinalcord injury
