Plant-derived exosome-like nanoparticles and their therapeutic activities
aDepartment of Pharmaceutics,School of Pharmacy,Ministry of Education,Fudan University&Key Laboratory of Smart Drug Delivery,Shanghai 201203,China
bInstitute of Integrated Chinese and Western Medicine,Fudan University,Shanghai 200040,China
Keyword:Exosomes Plant-derived exosome-like nanoparticles Drug-delivery systems Nanocarriers
ABSTRACT Nanotechnologies have been successfully applied to the treatment of various diseases.Plant-derived exosome-like nanoparticles (PENs) are expected to become effective therapeutic modalities for treating disease or in drug-delivery.PENs are minimally cytotoxic to healthy tissues,with which they show excellent biocompatibility,and are biased towards tumors by targeting specific tissues through special endocytosis mechanisms.Thus,the use of these PENs may expand the scope of drug therapies while reducing the off-target effects.In this review,we summarize the fundamental features and bioactivities of PENs extracted from the grape,grapefruit,ginger,lemon,and broccoli and discuss the applications of these particles as therapeutics and nanocarriers.
1.Introduction
1.1.Mammalian cell-derived exosomes(MDEs)
Exosomes,which consist of lipid membranes,are spherical nanovesicles with a diameter of 40-150 nm (approximately 100 nm on average) [1-4],and are constitutively generated by the inward budding of the plasma membrane to form early endosomes.The partial early endosomes integrate the surrounding lamina to generate intraluminal vesicles (ILVs),which encapsulate exosomes within large intracellular multivesicular bodies (MVBs).(Fig.1) The subsequent fusion of MVBs with the plasma membrane leads to the secretion of exosomes from most ILVs into the extracellular space [3,5-7].Exosomes are typically defined by their size,composition,and specific exosome marker proteins,such as CD9,CD81,CD63,flotillin,and TSG101 [3,8,9].As exosomes are biogenetically derived,their architecture,components,and molecular processing reflect the processes taking place in their origin cells;therefore,the components of exosomes may contain beneficial agents from the parental cell [10,11].With the same topology as those in the origin cells,exosomes can carry chemical cargo with innocuous traits into the biological environment and perform multiple functions,such as transmitting signals to recipient cells and recognizing antigen-presentation molecules in cell-to-cell communication[12-18].
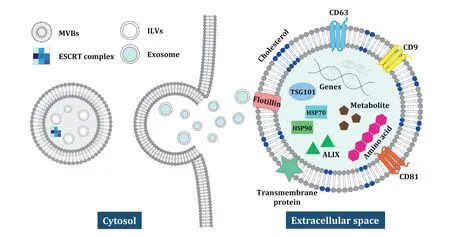
Fig.1-Biogenesis and components of MDEs.MDEs are secreted into the extracellular space through an ESCRT-dependent mechanism.The MDEs consist of lipids surrounding proteins,nucleic acids,metabolites,and amino acids,which are responsible for the therapeutic activities.
1.1.1.Biogenesis and character of MDE
In the biogenesis of MDEs,the endosomal sorting complex required for transport (ESCRT) machinery is responsible for sorting the cargo proteins of ILVs.ESCRT is divided into four complexes,ESCRT-0,-I,-II,-III,which work cooperatively to generate MVBs with associated proteins VPS4,VTA1,ALG-2 interacting protein (ALIX).Multivalent ubiquitin-binding ESCRT-0 consisting of hepatocyte growth factor-regulated tyrosine kinase(HRS)and STAM recruits TSG101 of the ESCRTI complex and isolates the ubiquitinated proteins in the endosomal membrane.The ESCRT-I complex is essential and responsible for cargo sorting in the MVBs,as it induces budding by deforming the plasma membrane.Subsequently,ESCRT-I activates ESCRT-III,which is responsible for the concentration of MVBs’cargo molecules using the ESCRT-II complex or ALIX,and the ESCRT-III/VPS4 complex triggers the constriction and induces the cleavage of the vesicle buds during abscission.Afterwards,MVBs interact with a specific combination of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) within the plasma membrane,and MDEs are secreted to the extracellular milieu of the cell[9,19].
Proteins,such as Tetraspanins CD9,CD63,CD81,CD82 and adhesion molecules CD11b and CD54,in MDEs have been widely used for exosomal markers due to their prevalence in MDEs;however,exosomal proteins could play more important roles determining the functions of MDEs than markers of MDEs.Depending on the particular composition of their exosomal proteins in MDEs,the fate and function of MDEs in biogenesis of MDEs,cargo selection,targeting ability,and endocytosis under both physiological and pathological conditions can vary.Among the protein cargoes of MDEs,those proteins participating in cell adhesion (e.g.,integrin,lactadherin,ICAM),intracellular trafficking(e.g.,RAB GTPases,annexin),signal transduction(e.g.,protein kinases,β-catenin,14-3-3,G proteins),biogenesis factors (e.g.,ALIX,TSG101,syntenin,ubiquitin,clathrin,VPS32,VPS4),and as well as chaperones (e.g.,HSP70,HSP90),have been evaluated [20].MDEs are characterized by the presence of the specific lipids such as phosphatidylserine,cholesterol,sphingomyelins,and ceramides,which are responsible for intercellular signaling as well as being essential in structural stability.In addition,MDEs may also carry genetic materials such as messenger RNA (mRNA),microRNAs (miRNAs),and non-coding RNAs.Overall,MDEs are not only considered to be effective intercellular transporters of proteins,lipids,and nucleic acids,but also novel regulators that can alter the physiological and pathological functions of both recipient and parent cells via their various components of MDEs.
1.1.2.A traditional isolation method of MDEs
Differential ultracentrifugation is a conventional method for the isolation of MDEs.Following a previously optimized protocol,a standard MDEs can be extracted with differential ultracentrifugation (Fig.2).Although the velocity of centrifugation depends on whether the original resources of MDEs is cell culture conditioned medium or body fluids,the conventional isolation method is generally carried out by differential ultracentrifugation.After 48-72 h of cell culture,the conditioned medium is collected and centrifuged at 2000 × g for 10 min to remove live and dead cells.The supernatant is separated and centrifuged at 10 000 × g for 20 min to remove the cell debris.To collect MDEs’fraction,the supernatant is transferred to a fresh tube and subjected to a high speed centrifugation at 100 000 × g for 90 min.The supernatant is retrieved and centrifuged at 100 000-200 000 × g for 90 min to isolate the MDEs.The supernatant is gently removed,and finally,the MDEs’pellet is collected.In addition to the seeding of cells and MDE isolation and purification,sufficient preparation time is needed to extract optimal quantities of MDEs.Thus,including the preparation of cells and isolation of MDEs,the complete process requires at least 3-4 d
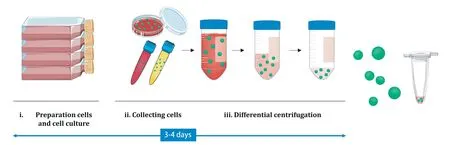
Fig.2-A conventional isolation method of MDEs by differential centrifugation.Depending on the original resources of the MDEs,the velocity of centrifugation used may differ.After cell preparation and cell culture,the conditioned medium is collected and purified by differential centrifugation.MDEs pellets are gently dissolved in PBS.
1.1.3.MDEs’therapy
Exosomes were originally regarded as cellular by-products or waste from cellular processing;however,since the discovery of mammalian cell-derived exosomes (MDEs) in 1983 [21],the characteristics,functions,and regulatory mechanisms of exosomes have been elucidated.For example,Thomou et al.[22]observed the role of miRNAs in serum-derived exosomes against fibroblast growth factor 21(FGF21)within mice with an adipose-tissue-specific miRNA knock-out-processing enzyme Dicer.They found that the exosomes carrying miRNAs,which were delivered to adipose tissue implanted in the liver by intravenous (IV) administration,successfully suppressedFGF213′UTR activity compared to the control,resulting in improved glucose tolerance,decreased insulin levels,and lower expression of FGF21.This study emphasized that MDEs and circulating exosomal miRNAs from different adipose deposits can regulate and reconstitute metabolism in tissues by targeting the predicted regulator.Additionally,because of the molecular composition and naturally harmless traits of MDEs,they represent an outstanding platform for the development of therapeutic vehicles for drug and gene delivery.There are many advantages of using MDEs as drugdelivery systems (DDS),such as their high stability under physiological conditions of pH and temperature,resulting from their native existence in body fluids,and their tolerance to long-term storage [23,24].For example,Gu et al.extractedLactobacillus rhamnosusGG (LGG)-derived exosomes (LDNPs)and applied them in the treatment of alcohol-associated liver disease.When placed in acidic solution at pH 2.2,LDNPs showed no degradation of p75 or p40 proteins,as they have high microenvironmental pH tolerance [25].Furthermore,MDEs are less toxic and immunogenic compared to synthesized nanoparticles,especially when they are extracted from milk or macrophage cells (dendritic cells and monocytes)[26-28].
Therefore,there have been many attempts to demonstrate the encapsulation of hydrophobic drugs and conjugate exosomes with other therapeutic agents [2,29-32].Although,the application of MDEs is promising,several major issues limit the clinical use of these exosomes,including (1) their low production yield,(2) the time-consuming and laborious production processes,and (3) the difficulties involved with achieving high-quality and uniform exosomes[33-37].
1.2.Plant-derived exosome like nanoparticles
Plant-derived exosome-like nanoparticles(PENs)are currently under investigation for their suitability as an alternative to MDEs,enabling researchers to circumvent the technical limitations of mammalian vesicles.In terms of their largescale producibility,PENs have great potential for application in disease therapy as well as in the development of nanocarrier DDS capable of administering various dosages as a result of their physiological,chemical,and biological characteristics[38-41].For example,Wang et al.first developed multifaceted PENs as nanovectors for delivering therapeutic agents to brain tumors [42].They showed that the PENs accumulated at certain tissuein vivoand circulated long-term in the peripheral blood due to the high nanovector stability.Additionally,researchers evaluated the quantity of PENs in plants,and found 1.76 mg/g in grape,2.21 mg/g in grapefruit,and 0.44 mg/g from tomato,suggesting that certain plants could contribute to the large-scale production of PENs.
Even though studies on PENs have only recently begun,there are several,much earlier articles describing the biogenesis and mechanisms of PENs.According to the early evidences,plants are known to produce EVs in response to numerous biotic and abiotic environmental stresses,including pathogen infection and attack [43,44].To provide convincing evidence of the generation and secretion of PENs,Qianli et al.observed the proliferation of intravacuolar MVBs in the cytoplasm and the structural perturbation of the organelle and its membrane by trafficking in barley leaf cells [45].The biotrophic powdery mildew fungus attacks the hypersensitive cells and their intact neighboring cells.Intravacuolar MVBs containing antimicrobial compounds against pathogens were promoted in intact epidermal cells to whether preventing fungal penetration or in response to interactions with the fungal plasma membrane [44-47].Additionally,there have been several reports that enhancement of fungal infection induces the proliferation of MVBs at the site of infection by stimulating plant innate immune responses in plant defense[43,48].
Intravacuolar MVBs contract with cell wall-associated paramural bodies (PMBs) enclosing vesicles,in which membranous or vesicular structures that are placed between the plasma membrane and curved cell wall regions of plant and fungal cells,possibly resulting in the obstruction of growing papillae.Like in the biogenesis of MDEs,ESCRT complexes (ESCRT-0,I,II,and III) are proposed to be involved in the maturation of PENs and cargo-sorting of PENs in plants.However,there is no canonical ESCRT-0 complex in higher plants;instead,TOM1-like(TOL)proteins with conserved VHS(VPS27,HRS,STAM)domains act as substitutions for ESCRT-0 as ubiquitin binding proteins and play a role in the vacuolar sorting of the auxin efflux facilitator PIN-FORMED 2 (PIN2) in early endosome [49].The cargo is subsequently transported to the ESCRT-I and ESCRT-II complexes via the ubiquitinbinding proteins,then the ESCRT-II complex stimulates and recruits ESCRT-III through an interaction between VPS25 and VPS20.The ESCRT-III complex constricts the plasma membrane and cleaves the necks of the buds that from on the cytosolic face to release ILVs containing cargo into endosomal[46,50].
However,some important progresses of the biogenesis of PENs are slightly divergent from those of MDEs,as molecular cargo is transported to the plasma membrane for budding and release,and they may have distinct characteristics and activities based on their different functions.Although there is some evidence,a clear demonstration of the MVB-mediated secretion of exosome-like nanovesicles in plants is needed[51-53].
There remain an urgent need to advance the use of innovative drug development approaches for more effective and efficient to treatment of diseases.Nanotechnology-based drug development has become an attractive strategy;thus,PENs-based therapies could be a potential new approach in the treatment of cancers,inflammations,and immune-related diseases.PENs,which are generally natural nanoparticles secreted by edible plants,such as grape [54],grapefruit[55,56],ginger [57],lemon [58],broccoli [59],carrot [60],coconut [61,62],and apple [63],offer obvious advantageous therapeutic effects stemming from the natural biochemicals in the origin plants.PENs and their plant chemicals have diverse activities on physiological and pathological processes.Ginger-derived exosomes like nanoparticles (GDENs) and their cargo have been proven to suppress tumor cell proliferation and inflammatory bowel disease (IBD),and normalize the microbiota after tissue damage by its targeting and regulating gut bacteria [64-67].Furthermore,there is no evidence of inflammation or toxicity associated with the application of PENs,[67,68]and they are considered safer than artificial nanocarriers such as copolymer-based nanoparticles,metal-based nanoparticles (gold/silver),and carbon-based nanoparticles[69-71].
In this review,we aim to provide a comprehensive overview of PENs,including their functions,therapeutic uses,and applications in drug delivery.
2.Character and function of PENs
PENs have only recently been reported in the therapeutic field,and PEN preparation has been attempted using many common plants,such as grapefruit,grape,lemon,broccoli,carrot,apple,and ginger (Fig.3A).PENs are known to be similar to MDEs in terms of properties such as size distribution,surface electric charge,morphology,density,and certain components[54,58,59].Like MDEs,PENs also comprise biomolecules,such as RNAs,proteins,and lipids,that regulate physiological processes [67] (Fig.3B).While PENs themselves can be used as transportation vesicles,the structural and functional biomolecules they contain can also have useful clinical applications[42,68].
One of the benefits of PENs is that they can be derived from numerous edible plants,which allows their effective and abundant production.Because of these advantages,PENs also hold promise as candidate endogenous carriers for drug delivery.Depending on the PENs’origin cell,each PEN has different characteristics and components,and thus PENs and the intrinsic molecules show distinct patterns of regulation of signaling pathways by various mechanisms and cellular uptake (Fig.3C and 3D).For example,Zhuang et al.confirmed that a 6-shogaol rich in GDENs activates Nrf2 by regulating TLR4/TRIF pathway,protecting against alcohol-induced liver damage through the anti-inflammatory actions of this pathway [72].Additionally,Ju et al.reported that grape-exosome like nanoparticles induced the recovery of intestinal stem cell through the Wnt/β-catenin signaling pathway,which regulates genes including AXIN-2,Cycline D1,c-MYC,and EGF (Fig.3D) [54].In terms of the cellular uptake of grape-derived exosome-like nanoparticles (GELNs),GELNs showed a high selectivity to intestinal stem cells and was significantly inhibited by a cytochalasin-D inhibitor,known as micropinocytosis inhibitor.In contrast,a clathrinmediated endocytosis inhibitor did not affect their uptake[54].It is assumed that there are specific ligands and receptor routes between PENs and intestinal stem cells;however,it has been challenging to ascertain the functions of the specific molecules and ligands in PENs because the mechanisms underlying the delivery and internalization of PENs recipient cells are still somewhat elusive.
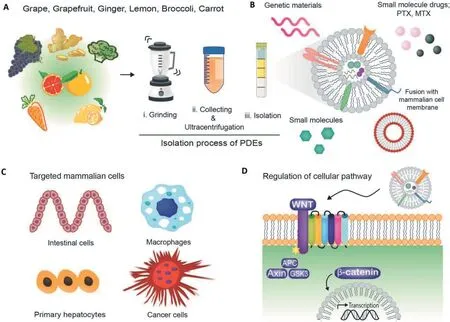
Fig.3-A schematic illustration of edible-plant-derived exosome-like nanoparticles(PENs).(A)Brief description of the procedure for the isolation of PENs via density-gradient separation.(B)Application of PENs carrying genes,pharmaceuticals,or small molecules.(C)Targeting of mammalian cells by various PENs.(D)Mechanism of PEN-mediated regulation of cellular pathways.
Despite many similarities between PENs and MDEs,the two groups of vesicles show some differences in many aspects.The lipid bilayer of MDEs are mainly composed of cholesterol,glycoshingolipids,ceramides and phosphatidylserine,which provide stability and a unique rigidity [73-76].In contrast,the exosomal membranes of PENs are enriched with phosphatidic acid(PA),phosphatidylcholines(PC),digalactosyldiacylglycerol(DGDG),and monogalactosyldiacylglycerol (MGDG) [67],and these distinct lipid characteristics provide inherent mammaliancell-regulating activities.Among phospholipids,especially PA is notable among phospholipids for its ability to target and stimulate the mammalian target of rapamcin (mTOR),and is commonly found in PENs.The mTOR pathway is responsible for cell growth,proliferation,recovery,and operates in a wide variety of human health and disease process.PC is a source of choline in the body and may protect a cell wall in large intestine by building a cellular block in the cell membrane.Teng et al.isolated GDENs and analyzed their genes,proteins and lipid profile.They found that phospholipid-enriched membranes of GDENs were responsible for preferential uptake of microbiota and could regulate the gut bacteria milieu.Additionally,the presence of specific proteins and genes indicated that GDENs could adjust the intestinal microenvironment.Together,the unique features of PENs may contribute to interspecies communication[67].
As a consequence of these valuable properties,PENs are considered to provide outstanding therapeutic advantages compared with MDEs or artificial nanoparticles.These advantages include facile large-scale production [66],low toxicity,reduced immunogenicity [59],efficient cellular uptake [42] and high biocompatibility and stability [77].Although there have been multitude of studies related to the characteristics and treatment efficacy of PENs,unfortunately,many aspects of these vesicles are not fully understood yet.Thus,additional studies are needed to enhance our understanding of the bioactivities and applications of PENs.Here,we present a universal methodology for the isolation of PENs and provide details about their roles in health and disease,therapeutic potential,and application as nanocarriers in DDS.
3.Manufacturing and characterization of PENs
3.1.General methodology for isolation of PENs
Density gradient separation by differential ultracentrifugation is the gold-standard approach for the isolation of PENs[61,66,78,79].Fresh plant tissue is homogenized with a highspeed grinder (Fig.4),and the collected fluid is filtered to remove large solid impurities and residues.Subsequently,the crude solution is centrifuged at a comparatively low speed to remove rough debris.The supernatant is then centrifuged,and the resulting supernatant is transferred to a fresh sterile tube and centrifuged at a speed of 100 000-120 000×g for 90 min in a high-speed refrigerated centrifuge.Afterwards,the nanoparticles aggregated at the bottom of the tube and are resuspended in phosphate-buffered saline (PBS) for collection.Layer-wise sucrose gradient separation (8%,30%,45% and 60% sucrose in PBS) is carefully performed (without disturbing the sucrose layers)to extract the specific exosomelike nanoparticles at their characteristic density zone (1.13-1.19 g/ml).All processes are performed at 4°C[63].

Fig.4-A schematic illustration of a general method for isolating PENs.Various plants are collected and homogenized.The juice is centrifuged twice at low speed,and the supernatant is sequentially centrifuged at high speeds to isolate nanovesicles containing exosome-like nanoparticles.The pellet and cushion layer are obtained and applied to sucrose gradient fractionation to purify exosome-like nanoparticles via their different buoyant densities.
This traditional technique for isolating exosomes is considered to be effective;however,it may result in the destruction of a large number of extracellular vesicles and the aggregation of heterogeneous heavy particles during purification with repeated high-force centrifugation.To preserve the structures of different-sized nanoparticles,including the traditional cup-shape,a thin layer of sucrose cushion can be applied followed by a simple pelleting step as an alternative to ultracentrifugation isolation.When applying sucrose cushion,the certain density of sucrose(1.12 to 1.18 g/ml) is parallel to that of exosomes (1.15 to 1.19 g/ml) to induce a cushioning effect collecting pure exosomes by preventing mixing protein aggregates of the high density (1.22 g/ml).As a result,during repeated high force ultracentrifugation,EVs were protected by sucrose cushion layers,the PENs will not aggregate or degrade.Moreover,it shows higher yield,increased purity with minimal protein contamination,and the maintenance of vesicle integrity are achieved [67,80-82].As a brief introduction to the methodology,a layer of highly concentrated sucrose is added to the bottom of the tube to serve as a cushion.Then,a layer of lower-concentration sucrose is added over the previous layer,while causing minimum disturbance to the layer below,and the supernatant is laid on top.After highspeed ultracentrifugation,specific vesicles gather between the sucrose layers and maintain their representative structures and shapes[33,66,78].
3.2.Characterization of PENs
To elucidate and support quantitative and qualitative aspects of isolated PENs,PENs are verified in the size,zeta potential,their shape of structure,and the concentration of the chemical composition.Determination of PENs’size and surface charge are considered to be an indispensable factor for accurate characterization of PENs since it confirms the exosomal integrity.After isolating PENs via a standard extraction method,their size and surface charge are determined via dynamic light scattering (DLS).PENs have a diameter of approximately 50 to 500 nm and display negative charge between-25 and-15 mV.To morphological analysis of PENs,transmission electron microscopy(TEM)or scanning electron microscopy (SEM) revealed that PENs substantially exhibit a uniform structure and cup-shaped morphology.
To evaluate quantitative assessments of PENs,through the nanoparticle-tracking analysis(NTA),particle size distribution and particle concentration of a sample can be determined.Li et al.[66] determined that the nanoparticle concentration of GDENs was counted at approximately 3.5×1010/ml,and the total protein concentration was estimated at 5.76 μg/ml of GDENs.It is assumed that if the preparation of isolation is pure,the relatively high ratio of the particle counts to the protein concentration can be achieved by minimizing the contaminating protein to the samples [83].To evaluate the purity of PENs,Teng et al.isolated PENs using a high-purity preparation procedure and calculated the ratio of the counts of PENs to the total protein concentration.The purity of PENs was evaluated as approximately 1.3×1011/mg protein of PENs[67].
3.3.Extraction of lipids from PENs and fabrication
Our knowledge of the biological roles of PEN lipids is increasing due to the several advantages of PENs,such as the stability of their membranes under both physiological and pathological conditions and the increased delivery efficiency of certain lipids that can bind to specific receptors in the target tissue.To perform reproducible uniform-sized nanovectors and stably incorporate of hydrophobic drugs into PEN lipids,the lipids are extracted by the Bligh and Dyer method.The hydrophilic components of PENs are separated by an appropriate volume of MeOH/EtOH (2:1,v/v).Chloroform and ddH2O (1:1,v/v) are sequentially added and centrifuged at 2000×g for 10 min at room temperature to separate the aqueous and organic phases.The organic phase is separated,sequentially washed with 1 M of KCl and ddH2O,and dried by heating (60 °C) under nitrogen.To create PEN-derived nanovectors,residual organic compounds are removed under a vacuum pump,and the PEN-derived nanovectors are suspended in PBS.Afterwards,the solution is passed through a membrane filter using a liposomes extruder to collect homogeneously sized the liposome-like nanovesicles.Therefore,lipids are readily available nanoparticle for the delivery of cargoes and could represent a promising resource in the development of drug therapies[64,65].
4.Applications of PENs and their therapeutic effects
Fresh plant materials,such as herbs,seeds,vegetables,fruits,and their extracts,are a cornucopia of vitamins,minerals,fiber,proteins,and other nutrients,and have versatile and nutritious components that are beneficial for human health.Moreover,they also reduce the risk of cancer,chronic disease,and inflammation.Therefore,biomolecules of plants have attracted much attention for their potential to improve health and provide protection against various ailments.The Food and Agriculture Organization stated that there are over 50,000 therapeutic plants in the world [84,85],and medicinal plants provide promising natural resources for modern drug discovery [86-91].A number of natural bioactive compounds from medicinal plants have demonstrated great significance in the clinical treatment of various diseases,and many chemical drugs have been sourced from natural compounds.However,the development of drugs from botanicals is associated with multiple challenges attributable to their complicated compositions and the lack of clinically validated quality standards.The therapeutic efficacy of herbs is known to depend on the many ingredients of the plant,but the active components of most herbal plants have not been identified and evaluated [92].Thus,it is important to ensure plantderived products contain all possible active components to improve the efficacy of these products and ensure low dosages and consistent quality.
PENs are naturally generated and carry innocuous components from their parent cells,some of which have been proven to be therapeutic.Additionally,PENs can intrinsically localize at target tissues -one of the most important traits of a targeted delivery system.However,PENs are a new concept in nanomedicine,and not all of their aspects have been fully identified and described.The therapeutic potential of edible PENs has been recently demonstrated in several disease models.Various plant-derived exosomes have been extensively applied in the development of novel drugs to treat specific diseases or maintain healthy body functions.A diversity of edible plants has been used for the isolation of therapeutically effective exosomes with various functionalities.Table 1 lists several important plants that have been used to extract PENs and their therapeutic applications.
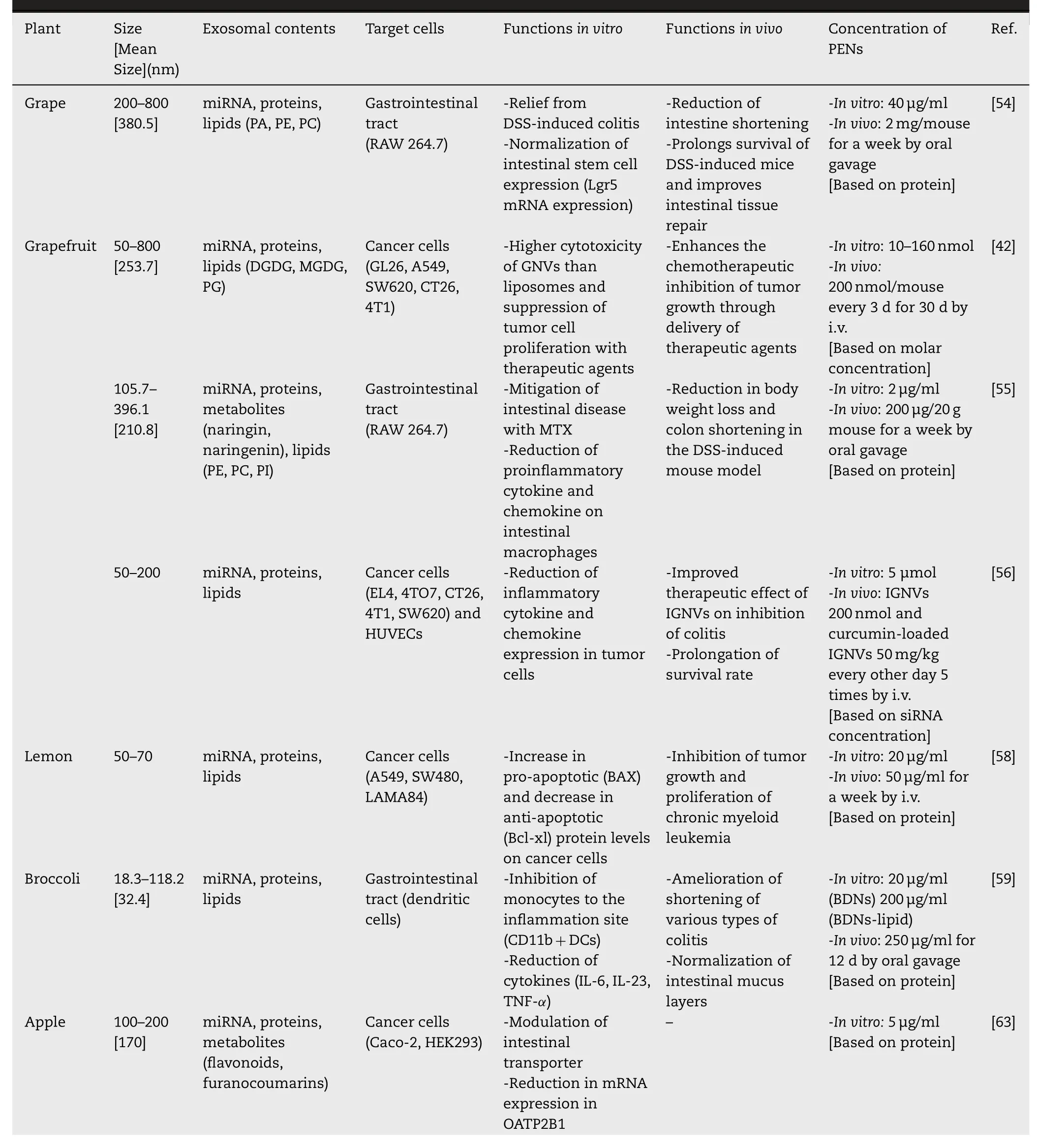
Table 1-Identification,characterization,and therapeutic potentials of PENs.
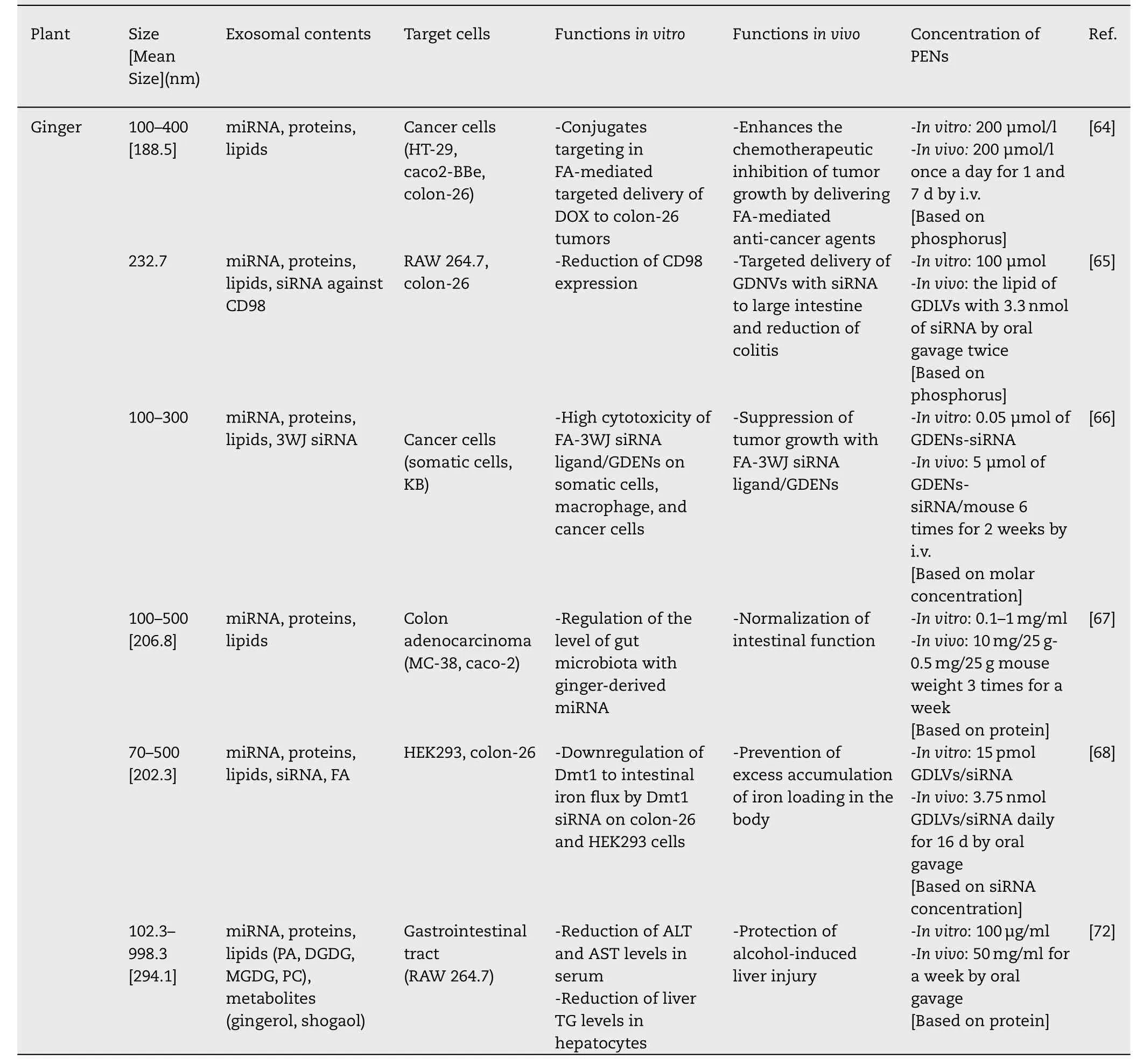
Table 1 (continued)
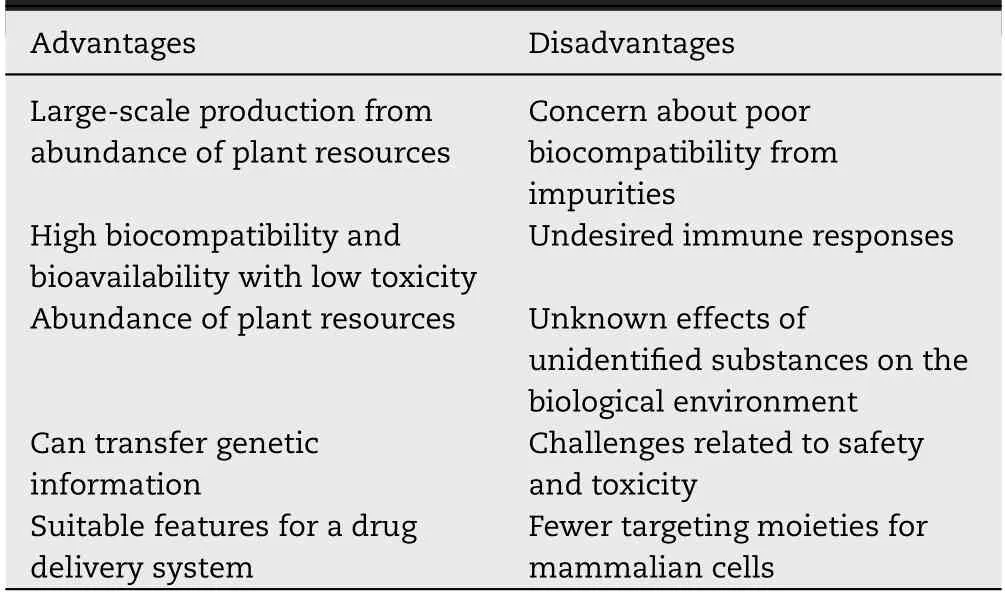
Table 2-Advantages and disadvantages of PENs.
4.1.PENs as therapeutic agents
Although PENs are a relatively recent discovery,they have already been utilized for the treatment of a variety of diseases because of their beneficial therapeutic effects and tissuespecific targeting [55,67].In particular,published reports describe their use in the treatment of intestinal bowel disease.Despite the limited amount of research,a handful of studies have revealed some of the specific biomolecules in PENs,such as proteins and lipids,that bind to receptors and act as recognition elements for distant sites[54,67].Grape exosomelike nanoparticles (GELNs) have been used to relieve dextran sulfate sodium (DSS)-induced colitis in mice [54].Under physiological conditions,GELNs have been shown to increase the proliferation of intestinal stem cells,which are reported to be the only stem cells to yield long-lived intestinal organoid structuresin vitro.In the DSS-induced mouse colitis model,GELNs dramatically promoted the proliferation of intestinal stem cells,accelerated mucosal epithelium regeneration,and helped restore the intestinal architecture throughout the entire length of the intestine.The group further studied broccoli-derived nanoparticles(BDNs)[59]and found that they showed preventive and therapeutic effects on three types of colitis.In anin vivoassay,BDNs showed potent preventive effects on acute and chronic colitis with elevated levels of anti-inflammatory cytokines,as evidenced by a reduction in colon shortening,weight loss,and inflammatory infiltrate in the mucosa,and an increased histoscore in comparison with the control group.Furthermore,BDNs preserved the intestinal immune environment with minimal adverse reactions by regulating AMP-activated protein kinase (AMPK) activation[59].
Exosomes or exosome-like nanoparticles derived from plants have also been reported to show anti-cancer activity.Raimondo et al.[58] investigated the application ofCitrus limon(lemon)-juice-derived nanovesicles for the treatment of chronic myeloid leukemia (CML).The lemon-derived nanovesicles greatly suppressed the growth of tumors in a CML model with the intended targeting effect and inhibited the secretion of multiple cytokines related to angiogenesis with TNF-alpha-related-apoptosis-inducingligand (TRAIL)-mediated cell death.In vivobiodistribution studies showed that the nanovesicles targeted the tumorous tissue within 15 min,and their effects lasted for over 24 h.The nanovesicles mediated cross-kingdom communication and helped eradicate cancer cells through TRAIL signaling,which supports the viability of their application as chemotherapeutic agents.
Another interesting therapeutic potential of PENs is the application of GDENs in protection against alcohol-induced liver damage[72].Ginger has been used in traditional medical practice for thousands of years,and its intrinsic chemical compounds,such as shogaol and gingerol,offer many health benefits [57,93-95].GDENs-treated model mice with alcoholinduced liver damage showed increased expression of a group of detoxifying/antioxidant genes,including HO-1,NQO1,GCLM,and GCLC.Furthermore,histological analysis revealed that,mice treated with GDENs showed a remarkable reduction in lipid droplets in the liver.Additionally,GDENs significantly decreased liver triglyceride levels and liver weight.The mice that were administered GDENs also showed histopathological changes in the liver,demonstrating that GDENs protect the liver from alcohol-induced damage.
At present,many studies have been devoted to developing DDS that can target specific cells,tissues,or organs.PENs show a natural ability to target desired tissues,which makes them very attractive to drug researchers.In one study aiming to develop methods of protecting liver cells against alcohol-induced damage,GDENs showed unique transportation properties.In anin vivostudy,administrated DiR-labeled GDENs were found to primarily accumulate in the liver.Confocal microscopic imaging of immunostaining further confirmed that GDENs could access and move withinthe hepatic vascular system.Apple-derived exosomes were also found to be internalized by human epithelial colorectal adenocarcinoma(Caco-2)cellsin vivoafter co-incubation[63].Thus,the natural targeting abilities of PENs are likely to facilitate their utilization as natural DDS.
4.2.PENs as nanocarriers for therapeutic agents
Conventional DDS,including artificially synthesized nanoparticles and liposomes,show a number of limitations that are currently unresolved,such as their low biocompatibility,toxicity,poor targeting efficiency,and short retention time in the circulatory system [96-98].In contrast,PENs,which are produced naturally by plants,provide stability,rigidity,and a suitable morphology,can incorporate drugs within their lipid bilayer,and target the desired tissues [42,56,67].PENs are membranous vesicles consisting of various lipids with unique benefits,such as strength and site-specific targeting [66,99].Furthermore,the PENs’specificity,which is conferred by a particular orientation of proteins and lipids,and their ability to manipulate genes for therapy,transfer hydrophobic drugs,and evade immune attack makes them very suitable as DDS for future medical applications.
PENs,as nanovehicles,can safely deliver drugs and have long-term blood circulating properties after systemic administration,and are,therefore,considered promising targeted delivery vectors for tumorigenic disorders and chronic illnesses [40,67,68].To evaluate the toxicity of PENs,Zhang et al.used GDNVs capable of targeting tumors and examined whether noticeable tissue damage in organs occurred.[64]Due to the targeting ability of GDNVs to tumors,the accumulation of GDNVs in spleen and liver was reduced,which could elicit reduced systemic toxicity of drugs to healthy tissues while prolonging the blood circulation of administered drugs.Histological analysis of the heart,liver,spleen,lung or kidney revealed no significant damaging effect compared with the control group,indicating that PENs can be applied as a drug delivery nano-platform,increasing drug efficacy and reducing potential drug toxicity.
In terms of a drug loading capacity of PENs,Zhang et al.[64] demonstrated ginger-derived nanovectors (GDNVs)loaded Doxorubicin(Dox)and evaluated their properties such as drug loading efficiency and drug release of GDNVs using a pre-loading strategy.Under the five different pH values (pH 5.5,6.0,6.5,7.0 and 7.5),the drug release kinetics of GDNVs loaded with Dox and DC-Chol/DOPE liposomes loaded with Dox were evaluated for 48 h.GDNVs loaded with Dox released the cargo in an acidic tumor-like microenvironment,and more intriguingly GDNVs loaded with Dox diffused it more rapidly than the commercially available liposomes.
PENs that can ensure sufficient biodistribution with high biocompatibility have potential as nano-transporters to deliver chemical drugs,genes,and small molecules.Thus,they can deliver drugs with precise and specific targeting of tissues rather than producing systemic effects,leading to improved therapeutic effects and fewer adverse effects.The following paragraphs summarize the use of PENs as drug carriers.
Wang et al.evaluated the potential of nanovesicles released from grapefruit (GNVs) to provide targeted drug delivery to intestinal macrophages [42].Evidence suggested that the majority of orally administrated GNVs were taken up in the lamina propria of both the small and large intestines by intestinal macrophages,which are the major immune cells of the intestine.In contrast,commercially available liposomes were much less efficient at targeting intestinal macrophages.In further research,GNVs were conjugated with methotrexate(MTX),an immunosuppressant and anti-inflammatory agent.In a DSS-induced colitis model,GNVs conjugated to MTX(GMTX) significantly reduced body weight loss and colon shortening in mice.These results were further supported by the less severe colon tissue damage and inflammatory cell infiltration in mice treated with GMTX.As a proof of concept,GNVs were demonstrated to be suitable for targeting intestinal macrophages in the treatment of intestinal inflammatoryrelated diseases.
DDS carrying genetic material,such as siRNA and miRNA,have been thoroughly studied.However,because of their low loading efficiencies,their therapeutic effects are less than ideal,and adverse effects seem unavoidable [100,101].To address the problems associated with the use of GDENs,Zhang et al.extracted lipids from GDENs and loaded them with siRNA against CD98 to treat ulcerative colitis [65],and they found that GDENs showed high biocompatibility with less toxicity and apoptosis of macrophage and colon-26 cellsin vitroin comparison with a commercially available preparation of DC-Chol/DOPE liposomes.GDENs were then transfected with siRNA against CD98 using sonication and applied to evaluate the mRNA expression of CD98 in anin vivostudy.The siRNA-CD98/GDENs complexes were effectively retained in the gastrointestinal tract after oral administration and significantly reduced the expression of CD98 in the intestine in comparison with scrambled siRNA/GDENs.
On the basis of their low toxicity and high biocompatibility compared with synthetic liposomes,ginger-derived lipid vectors (GDLVs) were found to be suitable for carrying divalent metal transporter 1(Dmt1)-siRNA blunts to intestinal epithelial cells to mitigate iron loading in hereditary hemochromatosis [68].Furthermore,to improve GDLVs’ability to target the duodenum,they were infused with folic acid (FA),which could then be integrated into the duodenum and jejunum by the proton-coupled folate transporter.After siRNA-FA-GDLVs were administered to mice,iron loading was mitigated through a reduction of Dmt1-mRNA expression,leading to lower levels of ferritin,TSAT,and non-heme Fe in various organs,including the liver,kidney,pancreas,and heart.
Recently,several methods of loading different cargoes into PENs have been developed.Passive loading techniques such as co-incubating of exosomes and drugs allow PENs to be loaded with desired cargo molecules [102].However,passive loading techniques may fail to achieve a high yield of encapsulation rate.Therefore,sonication,freeze-thaw cycling and other mechanical interventions can temporarily disrupt the integrity of PEN membranes and enhance cargo loading efficiency.To avoid the possibility of toxicityin vivothat may result from impurity and to obtain nanocarriers of a uniform size,nanovectors can also be fabricated using lipids from PENs,and cargo can be mixed during the preparation of lipid thin film [66].The approach can be used to load a wide range of cargoes such as lipophilic drug doxorubicin[64],biomacromolecule such as siRNA,antibodies and DNA expression vector [42].Such methods have yielded high encapsulation rates (95.9% ± 0.26%) of doxorubicin [64],however,the encapsulation efficiency of other cargoes was not explored.
4.3.Engineered PENs for targeted drug delivery
Previous studies have shown that PENs naturally possess specific-cell-targeting ability.However,information regarding the underlying mechanisms of the natural targeting potential of PENs is limited because PENs have been studied less extensively than MDEs.Nevertheless,based on studies of the origin plants,the preferential uptake of PENs by certain cells has been suggested to be due to specific genes (encoding miRNAs and siRNAs) and small molecules,which become extracellular ligands for the targeting moieties on the cells[42,67].In comparison with the artificial nanoparticles,PENs possess high intrinsic targeting capability,low off-targeting effects and no detectable toxicity.This eliminates the need for additional modifications to improve their biocompatibility,in vivostability,and pharmacokinetic properties that would otherwise be required to enhance their therapeutic potency when they are functionalized with certain ligands.PENs guarantee high biocompatibility and stability under a variety of physiological conditions (e.g.blood stream and various pH levels).PENs can also be functionalized as vectors to encapsulate siRNAs/miRNAs and chemotherapeutic drugs,especially,hydrophobic compounds.
Based on the results of a previous study on the drugloading capability of exosome nanocarriers extracted from ginger and grapefruit,PENs appear to be outstanding candidates for drug delivery.Furthermore,PENs are considered ideal nanocarriers because they contain common proteins that participate in transferring and trafficking[54,55].For the efficient intracellular delivery of hydrophobic drugs and therapeutic agents with enhanced accumulation,a novel therapeutic approach is desired.Despite PENs’intrinsic targeting features [67],there have been many attempts to further fine-tune their targeting effects and enhance the efficiency of their intracellular transfer.Through modifications using targeting ligands,such as small molecules and genetic material on the surface of the exosomal membrane,and cell membrane fusion,PEN’s nanovectors can be developed to target organs or cells with more accurate and consistent localization [42].Additionally,the biomolecular engineering of PEN nanocarriers has improved the stability and consistency of the nanostructures[66].
PENs membrane can be modified in multiple ways to incorporate ligands or cell membrane.For example,small molecules such as FA are added to the extracted lipids from PENs to form a lipid thin film and consequently extruded to fabricate nanovesicles.The ligands are displayed on the surface of the membrane to carry out a targeting effect,which are proved byin vitrocell uptake andin vivobiodistribution studies [42,46].In another article,GNVs were prepared to generate a customized delivery vector by fusing with leukocyte-derived cell membrane by the extrusion process.Through this procedure,the modified GNVs can have a targeting ability to the inflammatory sites in disease and reduce off-targeting effects delivering the therapeutic agents[56].
Li et al.reported GDENs fused with siRNA as targeting ligands [66].The three-way junction RNA (3WJ) architecture was manipulated to produce different physical configurations and angles,known as arrow-tail and arrow-head.When their uptake by cancer was analyzed,the findings showed different intensities of cellular internalization depending on the 3WJ nanostructure orientation.To preferentially target folate receptor-overexpressing cancer,FA was used as a targeting ligand conjugated with arrow-tail siRNA on the outer surface of the GDENs.The therapeutic potential of FA-3WJ-GDENs was confirmed by the reduced survivin mRNA expression levels.Thein vivosuppression of tumor growth administered FA-3WJ-GDENs was significantly reduced than that in the control group.Additionally,treatment significantly down-regulated survivin protein levels in comparison with both a scrambled-RNA control group and a group treated without FA.
In another study aiming at improving the targeting performance of PENs,FA was also used to modify GNVs[42] and the modified vectors resulted in a much better distribution to tumorous tissues in comparison with unmodified GNVs.When loaded with the chemotherapy drug,paclitaxel (PTX),GNV-FA accumulated primarily in tumors,while free-PTX or GNVs mainly targeted the spleen and liver.It is hoped that the use of this novel DDS can avoid the adverse effects of chemotherapy by delivering more of the drug to tumors instead of healthy organs.Another advancement in the treatment of colon cancer was reported by Zhang et al.,who used FA-modified GDLVs carrying the therapeutic agent doxorubicin(DOX)[64].Briefly,the methods involved the extraction of total lipids from GDLVs,which were mixed with DOX and/or FA in dimethyl sulfoxide (DMSO) in chloroform and dried under nitrogen to obtain a thin lipidcomplex film.A standard method based on the hydration of lipid films,similar to that used for the production of liposomes,was employed to fabricate DOX-FA-GDLVs or FA-GDLVs.In comparison with unmodified GDLVs,FA-GDLVs showed an improved capacity to target colon-26 tumors,probably through active FA-FRs interactions.However,the FA-GDLVs were found to accumulate significantly less in the spleen and liver than GDLVs,indicating that employing FA-GDLVs as carriers could decrease the systemic toxicity of drugs to normal tissues.Anin vivostudy showed that,after IV injection,FA-GDLVs were still detectable after 48 h of circulation,giving them a greater opportunity to penetrate tumors.Therefore,FA-GDLVs could be an ideal drug-delivery platform to exert anti-tumor effects while avoiding the adverse effects of free-circulating drugs.
Wang et al.developed a smart nanocarrier using GNVs coated with an activated leukocyte-derived plasma membrane to enhance their therapeutic potential and targeting specificity [56].Through the modification of the membrane-coated GNVs,inflammatory-related receptorenriched plasma membrane-coated GNVs (IGNVs) with specific cellular targeting were derived.A human umbilical vein endothelial cell (HUVEC) monolayer was cultured in a transwell plate to establish anin vitroblood-brain barrier(BBB) model,and IGNVs or GNVs were added to the upper layer to assess their ability to inflammation sites.The experiment resulted in much higher numbers of IGNVs transmigrating through HUVECs over 48 h than GNVs,and the migration efficiency was further enhanced with the addition of chemokines.The effect of the peripheral circulation of IGNVs was further confirmed in inflammatory models and chronic inflammatory cancer models.The IGNVs showed significantly increased accumulation at the inflammatory sites in comparison with GNVs,and IGNVs and DOX were consistently used to treat cancers in CT26 and 4T1 cellsin vivo.IGNVs were shown to enhance tumor permeability and tumor volume reduction in comparison with free-DOX and GNV-DOX.Because inflammation is a common process in many diseased tissues,IGNVs’abilities to target inflammation sites make them excellent targeted nanocarriers.
4.4.Non-invasive administration of PENs
In the advancement of nanotechnology for pharmaceutical applications,it is very important to improve medication efficacy.As previous studies of nanomedicines show,IV injection facilitates efficient pharmaceutical drug effects.IV injection is commonly used for treatment because it has high bioavailability,a rapid effect,and avoids first-pass effect and gastric manipulation [103,104].However,in the administration of any drugs,safety and the number of side effects have become the main concerns,especially within the body [105-109].Therefore,medication strategies should be less invasive and aim to minimize concerns,such as a severe and immediate allergic reaction,while considering drug absorption.
Oral administration is the preferred and most convenient route for pharmaceuticals since it provides a low risk of infection (unlike direct injection),improves the permeability of the whole gastrointestinal tract (GI) track tract,and avoids blood clearance [103,110,111].This non-invasive method has been previously applied using MDEs.For example,Lin et al.reported the use of bovine and porcine milk exosomes for altering miRNA profiles.The bovine and porcine milk-derived exosome systemically contained various biochemical compounds,relevant to immune responses.They considered the exosomes to be promising agent for pharmacological application.Bovine and porcine milkderived exosome containing certain miRNAs were orally administered and finally detected in intestinal cells [112].However,MDEs from only a few sources have been developed for oral administration[26,113-117],and most MDEs were not employed for oral delivery due to their low stability at various pH and temperatures,rapid degradation of biomolecules in the digestive tract,and the limitations of industrial scale production for oral dosing.[118,119]
The oral administration of PENs has been recently developed.PENs can be administered through versatile methods due to their tolerability,biocompatibility,and ability to target certain tissues.Therefore,administering PEN via the oral route also leads to prompt effects in pharmacotherapy[65,66,68].For example,GNVs were found to carry MTX to intestinal macrophages after oral administration [55],it is important to note that these GNVs had better targeting efficiency than commercially available liposomes.
To study the stability of PENsin vitro,researchers suspended them in solutions of different pH:water,0.5 N HCl,and 0.5 N NaOH.Their stability was assessed on the basis of their size and changes in the zeta potential.Most PENs were found to have reduced size heterogeneity when suspended in acidic solutions [55,120],whereas alkaline environments had no such effect.Additionally,the stability of PENs was tested after serial digestion in gastric and intestinal enzymatic solutions.GNVs were highly resistant to digestion by gastric pepsin,intestinal pancreatin,and bile-extract solutions [55].An intestinal-like solution had no effect on PENs derived from grape,grapefruit,and carrots;however,GDENs showed a reduction in negative charge [120].In contrast,most PENs in acidic solution show a significant reduction in surface charge [120].Interestingly,the surface charge of GDENs changed from negative to positive in stomach-like solution,then reverted back to negative in intestine-like solution[72].
PENs can be specifically taken up by target cells or tissues after oral administration.For example,GDENs were concentrated primarily in the liver after oral administration,and few or none were detected in the lung,spleen,or other organs [72].This intriguing trait of PENs may minimize the adverse effects caused by the non-specific distribution of therapeutics such as anti-cancer drugs.Additionally,GDENs were specifically taken up by albumin+hepatocytes,while grapefruit-derived exosome like nanovesicles co-localized with F4/80+liver Kupffer cells,indicating that PENs from different plants have distinct targeting abilities.The findings also substantiated the hypothesis that GDENs migrate to the liver from the gut primarily through vascular vessels[72].
PENs can carry hydrophobic anti-cancer drugs and genes to target cells or tissues through oral gavage.The anti-inflammatory drug MTX was incorporated into grapefruit-derived exosome like nanovesicles,which were found to target F4/80+macrophages in the lamina propria and maintained the therapeutic effects of MTX,providing evidence for the drug-preserving effect of these PENs.Additionally,compared with commercially available liposomes,PENs were much more efficient at transfecting intestinal macrophages after oral delivery.More recently,GDENs were used to deliver RNA that preferentially targeted bacterial genes in LGG[67].GDENs with the potential to target genes with gma-miR396e were selectively taken up by gut bacteria,which led to the regulation of LGG mRNA expression through IL-22 mechanisms.Orally administered GDENs stably delivered genes to the intestine in mice,as determined by qPCR analysis of GDENs miRNA in the gut and feces after treatment.Ultimately,GDENs ameliorated mouse colitis by influencing the gut microbiota composition.Hence,PENs are considered compelling candidates for oral-delivery products.
Many of the PENs mentioned above have been orally administered,while most MDEs or liposomes are,to our knowledge,usually administered by IV injection.Only a few MDEs are applied by oral administration for different disease models.Thus,it is conceivable that orally administered PENs not only guarantee the therapeutic effect with improved targeting ability,but also a reduced risk of infection,hence the therapeutic approach of using PENs by oral administration could be also a good strategy to greatly improve patient compliance in clinical trials.
Another possible approach in the administration of PENs,intranasal administration,has been demonstrated.Intranasal administration has been commonly used for the treatment of pulmonary disease,and it provides advantages such as allowing local delivery;the delivery of a concentrated dose to the target organ;and direct delivery to the respiratory system,the upper nose,and brain [121-126].Zhuang et al.reported the use of intranasal route-based GNV and miRNA targeting brain tumor [127].GNVs carrying miRNA were used for therapeutic treatment,after post-intranasal administration,GNVs showed an enhanced ability to deliver to a glioma,in olfactory bulb,compared with the standard liposomes.Basically,GNV phospholipids and cholesterol provide high stability to the cargo genes and are also effective bilayer anchors for targeting ligands in PENs.Therefore,the GNVs showed an improved capacity to contain and deliver RNA to the target tissue.The main obstacles for the clinical use of MDEs and chemically synthesized nanocarriers,i.e.,their biosafety and toxicity,could be overcome with GNVs.GNVs with miRNA were effectively delivered to the brain with high specificity,thereby slowing the growth of tumors and prolonging the survival rate.
4.5.PEN therapy in clinical trials
Because of the advantages mentioned above,i.e.,PENs can act as vesicles for transporting cell-created contents and are suitable for large-scale production by standardized manufacturing processes,their application of PENs has been tested in preclinical and clinical trials.In contrast to MDEs,PENs are in the early stages of development,and,certain sources,e.g.,grape (NCT01668849),ginger and aloe (NCT03493984),have just been selected and have been registered for clinical trials.To manage the oral mucositis pain induced by radiation and chemotherapy,Ju et al.developed GELNs that can be applied to recover tissue after mucosal damage [54].During the subsequent clinical trial,the GELNs should have been administered for 60 patients diagnosed with head and neck cancer during 35 d of chemoradiation therapy,but these patients could not be recruited.Bohler et al.set out to demonstrate that GDENs and aloe exosome-like nanoparticles can be used to treat diseases and improve the health of patients diagnosed with polycystic ovary syndrome;however,these GDENs and aloe exosome-like nanoparticles have not been approved for clinical trials until now (NCT03493984).There are also plans to use plant derived exosomes as nanocarriers to deliver hydrophobic drugs.Miller et al.conjugated curcumin with plant exosomes and tried to apply participants diagnosed with colon cancer(NCT01294072);however this has not yet reached the recruitment phase.
Even with the enormous advantages of PENs,their production from cultivation,isolation,and characterization to clinical trials and manufacture has not yet been fully realized.Therefore,to overcome the obstacles in the clinical applications of PENs,the remaining issues in their good manufacturing practice (GMP) production should be considered,and GMP-grade PENs should be investigated.
5.Summary and future perspective
According to previous reports,PENs are known to have similar properties to MDEs[39],and they have also been used for therapeutic purposes for various diseases [128,129].In comparison with MDEs,PENs offer several advantages.First,they can be obtained via large-scale manufacturing processes from a number of beneficial renewable sources [130],thereby meeting the requirements for the urgent production of high-grade exosomes.Second,the constituents of PENs,which naturally evolved in plant cells,promise enhanced biocompatibility and safety and minimal cytotoxicity or any other negative side effect.Third,various sources of PENs are available,which may allow researchers to choose from a diversity of nanovesicles depending on their applicability to a disease.Furthermore,PENs generally contain similar intrinsic therapeutic materials as MDEs,which can be transferred to the targeted cells.Additionally,PENs also have the potential for use as DDS nanocarriers because of their lipid membrane stability,and they can be easily modified to target specific ligands.Additionally,PENs can be tested in a comparably short time via eco-friendly protocols[64,131].Precisely characterizing PEN varieties will enable toestablish a standardized procedure for their production of the corresponding variety.These combined features highlight the important contributions PENs can make in the development of therapeutics.
Despite the therapeutic benefits of PENs,there are still some downsides.The main concerns regarding the use of PENs are related to their heterogeneous size and appearance,which might lead to them being considered as impurities by the body,leading to undesirable immune responses and regulation activities by underlying mechanisms that have not been studied yet during treatment [66].Furthermore,the roles and functions of PENs are still somewhat unclear;therefore,they could have unpredictable effects as a result of unidentified biological substances.In the application of PENs,some challenges related to biosafety and toxicity may also be faced because of the unknown bioactive constituents of plants.In addition,PENs may have poorer targeting capabilities in the certain targeting tissue than MDEs because PENs are not derived from biological fluids,tissues,or cells [56,132].To overcome these drawbacks of using PENs,methods for their isolation should be optimized to achieve the uniformed nanovesicles,and their morphological features,the quantitative aspects,and chemical components should be evaluated in detail to obtain information on their functional roles.All the advantages and disadvantages of PENs in the context of treatment development are summarized in Table 2.
In this article,we reviewed some of the recent researches on the uses and functions of PENs.As part of the current developments in medicine and DDS nanotechnology,PENs may open new avenues for drug discovery.PENs provide compelling features:they are derived from biological sources and can promote the immune system to combat inflammatory diseases.PENs exhibit less toxicity than artificial vesicles,such as synthetic nanoparticles and liposomes.Furthermore,the composition of the lipid bilayer of PENs is distinct from that of MDEs.PENs can act as satisfactory DDS for targeting the diseased tissue due to their high stability and promising activity based on their high phospholipid composition and lack of cholesterol.In combination with proteins,miRNAs,and bioactive phytochemicals,PENs may serve as a vital tool for drug discovery.However,PENs are not derived from human sources,which could be a major concern in terms of their biocompatibility in the body.Additionally,the detailed mechanisms involved in the functions and roles of PENs in pathogenic processes have not yet been fully clarified;thus,the risks of associated with their clinical use are elusive.Overall,PENs demonstrate a broad therapeutic potential that may benefit patients,and they may constitute the next generation therapeutics in the near future.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81773911,81690263 and 81573616)and the Development Project of Shanghai Peak Disciplines-Integrated Medicine (No.20180101).Jisu Kim and Shiyi Li contributed equally to this work.
 Asian Journal of Pharmacentical Sciences2022年1期
Asian Journal of Pharmacentical Sciences2022年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Calcium ion nanomodulators for mitochondriatargeted multimodal cancer therapy
- Biomaterials reinforced MSCs transplantation for spinal cord injury repair
- Exosomes:Emerging implementation of nanotechnology for detecting and managing novel corona virus-SARS-CoV-2
- Emerging pro-drug and nano-drug strategies for gemcitabine-based cancer therapy
- Polymeric microneedle-mediated sustained release systems:Design strategies and promisingapplications for drug delivery
- Sodium alginate and naloxone loaded macrophagederived nanovesicles for the treatment of spinalcord injury
