A Spectroscopic Investigation of pH Sensitivities of Resorufin and Resazurin
SHEN Yujie,ZOU Jinhui,ZHAO Bolin,TAN Qinqin,ZHANG Yuwei
(Guangzhou Key Laboratory of Sensing Materials and Devices,Centre for Advanced Analytical Science,c/o School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China)
Abstract:Resazurin is a commonly used dye for single molecule fluorescence microscopy.However,its fluorescence properties change obviously with the change of environment,which leads to the instability of single molecule fluorescence signal. Herein,based on the influence of pH on luminescence properties of fluorescent probe,the fluorescence properties of resorufin and resazurin in phosphate buffer solution were reported and the fluorescence optimization conditions were obtained. In addition,a mathematical model was further built and the dependence between fluorescence intensity and hydrogen ion was obtained combining with theoretical calculation,which provided a theoretical explanation for pH response performance. The results showed that based on the increase of pH, the fluorescence intensity increased first and then decreased. Theoretical studies showed that the fluorescence quenching correlates with the protonation of the resazurin and resorufin.
Key words:fluorescence;dye;resazurin;resorufin;pH
Dyes are used quite frequently as probes to analyze the behavior of various types of systems.Resazurin (RZ), a heterocyclic N-oxide dye, is often used to study biological material[1-2]. The‘resazurin reduction test’has been used for about 50 years to monitor bacterial and yeast contamination of milk,and also to assess semen quality. Resazurin is blue and scarcely fluorescent. In biological tests it is reduced to highly red fluorescent resorufin (RF).Resorufin is also an important medium in singlemolecule biological and chemical processes[3], which is further reduced to dihydroresorufin (uncolored and nonfluorescent) as shown in Fig. 1. Resazurin and resorufin have shown an interesting chemical and photochemical property in protic solvent which strongly depends on temperature, viscosity and the unique structure of the solvent[4-6]. In contrast, few studies on the fluorescence intensity of the dyes in PBS solution with different pH have been carried out. Since many biological applications involved resazurin and resorufin, knowledge of the spectroscopy of resazurin and resorufin is very important.
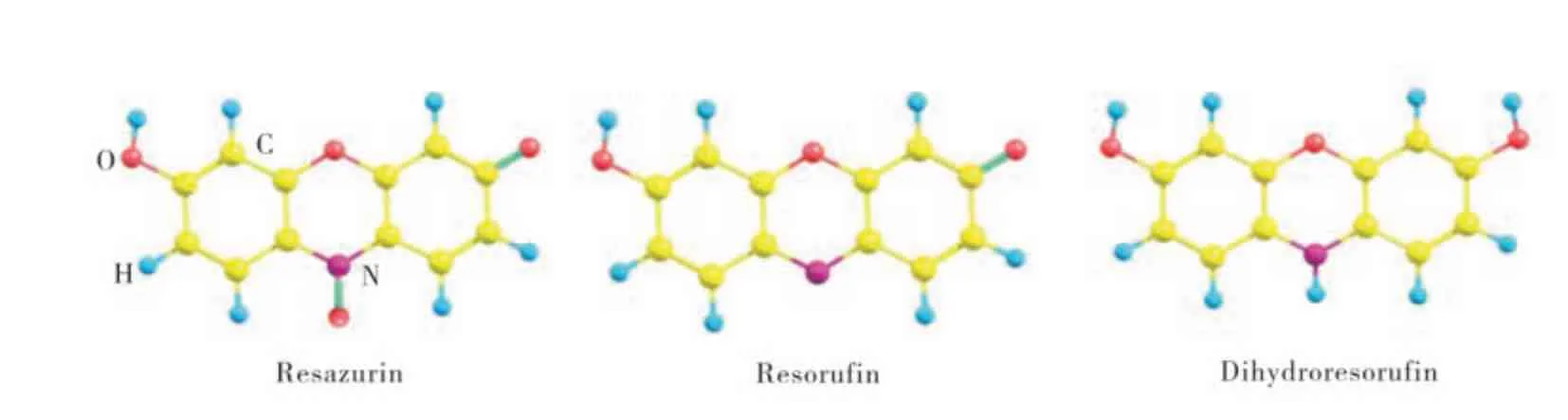
Fig. 1 Structure of resazurin,resorufin and dihydroresorufin
In order to provide new insights into the nature of the interactions between dyes and solvent environment, we present a study on the effect upon the fluorescence strength of resazurin and resorufin in PBS with different pH. The fluorescence intensity of resorufin increased with the pH of PBS. Resazurin presents a clear dependence in the absorption with the pH of PBS.
1 Materials and methods
Resazurin and resorufin were purchased from Aldrich and used as supplied. 10 μM resazurin and resorufin in PBS solution were used in fluorescence and absorption spectra measurement. Dye solutions were purged with argon for 30 min before their use.
The UV-Vis spectra were acquired using a UVVis spectrophotometer Shimadzu model 1650, for solutions prepared in PBS with distinct pH. Static fluorescence determinations were carried out with a Perkin-Elmer LS-55. All optical experiments were carried out at room temperature.
DFT method at M062X/6-31+G* level has been carried out adopting Gaussian 09 program[7].The solvent effect in water was employed in the SCRF calculations by using the conductor-like screening model (COSMO) method[8]. The total energy has been corrected by high basis set of 6-311++G** with solvation effect. All reactants,intermediates and products have been confirmed to be the minimum with no imaginary frequencies. Each transition state has only one imaginary frequency.
2 Results and discussion
2.1 UV-visible absorption of resazurin and resorufin in different pH
The UV-Vis absorption spectra of resazurin in different pH PBS solution are shown in Fig. 2. As shown in Fig. 2, the spectra basically comprised two peaks, 600 cm−1and 570 cm−1. The intense absorption band at 602 nm are assigned to the π-π*transition of the phenoxazin-3-one[9]. In PBS solution with different pH, the absorption strength shows differences. At pH = 5.00, the strength is the lowest. As the pH increased, the absorption intensity increased and reached the highest at pH 8.00. Then the intensity of adsorption decreased at higher pH. In the case of the resorufin shown in Fig. 3, the absorption intensity showed the similar tendency as the resazurin, which showed the absorption increased as the pH increased first and then decreased as the pH increased, and the turning point is about pH 7.41.
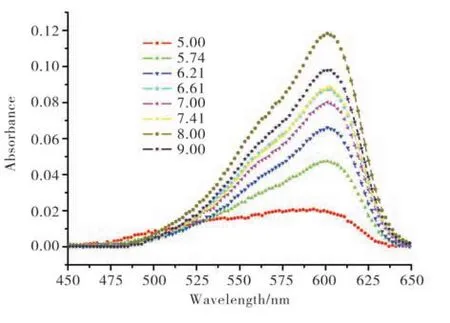
Fig. 2 Absorption spectra of resazurin in different pH PBS(pH 5.00-pH 9.00)
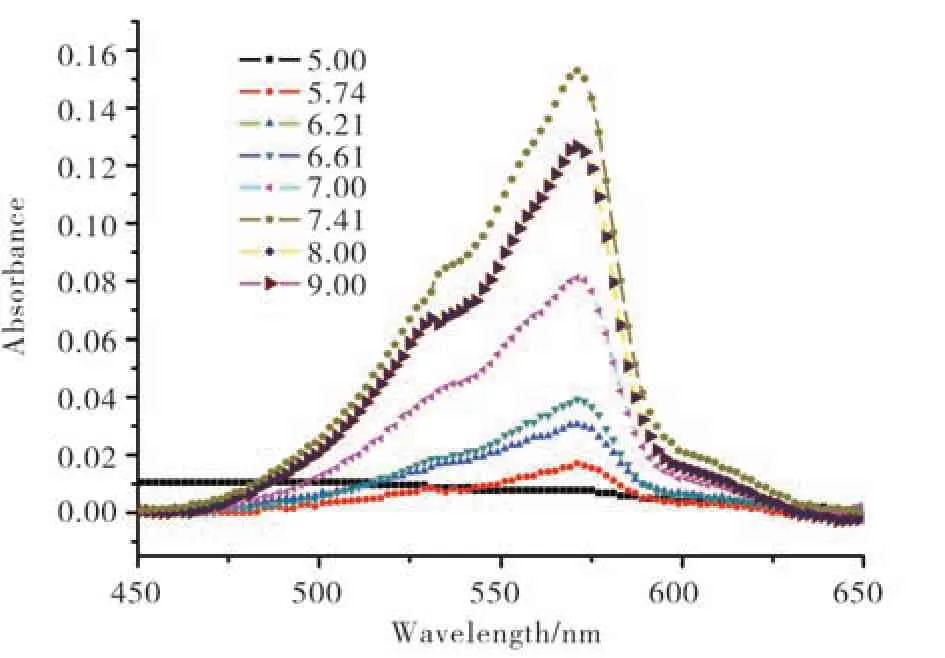
Fig. 3 Absorption spectra of resorufin in different pH PBS(pH 5.00-pH 9.00)
2.2 Fluorescence strength of resorufin in PBS with distinctive pH
As shown in Fig. 4, resorufin excited at 532 nm and exhibited a strong fluorescence emission with a broad band centered at 592 nm. A progressive decrease in intensity without change in the band shape was observed from pH 7.41 to 5.00. For pH 5.00, low fluorescence was detected, indicating merely complete protonation of resorufin.

Fig. 4 Fluorescence spectra of resorufin in different pH PBS
The weak fluorescence of resorufin in acid solution could be illustrated by the DFT and TDDFT. Planar rigid molecules usually show stronger fluorescence due to better electron delocalization over the whole molecule[10-11]. For resorufin in the reduction process, the oxygen- and nitrogen-bridge connect the two sides of the sixmembered ring as a planar geometry in ground states(S0), which is similar with that in corresponding singlet excited states (S1). And the large oscillator strengths (f=1.0356 andf=0.7820, respectively)for S0→S1indicate the HOMO→LUMO transition is allowed. Moreover, the electron transition for HOMO→LUMO is assigned to the local excited(LE) state (π →π*) over the planar moiety, so the emission process from LUMO→HOMO is confirmed as the strong turn-on fluorescence for reactant resorufin. By contrast, the attaching of H to N atom in the final reduction product dihydroresorufin is sp3hybridization, and the triangular pyramid steric configuration induces the whole molecules distorting dramatically to be nonplanar Λ -type geometry. Moreover, the forbidden transitions for S0→S1withf=0.0457 andf=0.0395 respectively show S1state is nearly the dark one. Herein, the electrons in LUMO and LUMO+3 in product dihydroresorufin are localized on two sides of the molecule respectively. The emission from the first allowed S5state (f=0.3600)in product dihydroresorufin needs to pass S1→S0emission via LUMO+3 and LUMO. Therefore, the large intermolecular charge transition (ICT) will result in a non-radiative pathway from six-membered ring at two sides to the product dihydroresorufin, and then reduce the fluorescence intensity (Fig. 5)[12-16].In addition, the quasi-planar geometry of S1state for the product dihydroresorufin is quite different from S0state, thereby, the obvious non-radiative pathways(great geometry relaxation in excited state as well as the ICT process) are predicted to be the turn-off fluorescence for product dihydroresorufin. In other words, the sp3hybridization in N atom from protonation increases the non-radiative transition pathway due to the ICT process and great geometry relaxation for S1state, which plays an important role in turning off the fluorescence in redox process for resorufin.
The fluorescence of resorufin in PBS was efficiently quenched by the H+in, which coincide with the theory calculation result.Bimolecular quenching rate constants (Ksv) were determined from the Stern-Volmer (SV) plots ofI0/Ivs. proton concentration [H+] (Fig. 6), whereI0andIstand for the fluorescence intensity in the absence and in the presence of[H+],

Fig. 5 Fluorescence quenching process of resorufin by protonation

Fig. 6 Relationship of fluorescence intensity and[H+],the red line means the data after fitting

The Stern-Volmer plots determined from the fluorescence intensity showed a linear relationship.From the slope of the plot, a value of 1.036 × 10−7forKsvcould be obtained, supporting that the quenching was induced by the proton.
3 Conclusion
In summary, fluorescence spectroscopic properties of resazurin and resorufin have been quantitatively investigated in PBS with different pH.Based on the measurements of absorption and fluorescence spectra, both dyes have strong spectral properties only in their anionic forms at pH above 7.0. Resorufin presents high fluorescence in neutral and basic media. The fluorescence intensity has been illustrated by DFT and TDDFT methods. All evidence allows us to suppose that the quenching proceeds through a protonation mechanism. And the kinetics parameter for the triplet quenching is 1.036 × 10−7.

