Systematic review of Shufeng Jiedu capsules for acute attack of chronic bronchitis
DanDou, Yuan-Ping Jia, Chuan-Hui Yao, Yu-Juan Li, Shu-Ying Lv, Hong-Chun Zhang✉
1. Beijing University of Chinese Medicine,Beijing 100029, China
2. Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing 100029, China
ABSTRACT Objective: To systematically evaluate the efficacy and safety of Shufeng Jiedu Capsules in the treatment of acute attacks of chronic bronchitis. Methods: CNKI, VIP, CBM,WanFang, PubMed, EMbase, Cochrane Library and ChiCTR were searched by computer,and randomized controlled trials of Shufeng Jiedu capsules in the treatment of AECB were integrated.The retrieval time was from the date of establishing the database to December 31,2020. Meta-analysis of outcome indicators [including effective rate, C-reactive protein (CRP),lung function [FEV1% and/or FEV1/FVC], interleukin-8 (IL-8), tumor necrosis factor-α(TNF-α)] were carried out by RevMan 5.3 software, and the effective rate was tested by sequential analysis (TSA), and the quality of evidence was evaluated according to GRADE standards. Results: A total of 680 cases in 8 articles were included. Shufeng Jiedu Capsules improved the effective rate of clinicaltreatment [RR=1.20, 95%CI (1.13, 1.28)], improved lung function [FEV1:MD=0.33, 95% CI (0.22, 0.45), FEV1/FVC:MD=10.17,95%CI(8.15,12.19)],reduced CRP[MD=-7.32, 95%CI(-8.42,-6.22)], IL-8[MD=-63.39, 95%CI(-73.49,- 53.29)],TNF-α[MD=-7.44, 95%CI (-8.35, -6.53)] levels. The above differences were statistically significant (P<0.05), and no serious adverse reactions were reported in all studies.The results of TSA analysis showed that the experimental group had definite evidence for improving the efficiency. According to the GRADE evaluation system, the efficiency, TNF-αwere mediumquality evidences, FEV1 was low-quality evidence, and FEV1/FVC, CRP, and IL-8 were extremely low-quality evidence. Conclusions: The curative effect of Shufeng Jiedu capsule combined with western medicine in the treatment of AECB was better than that of single western medicine. Nevertheless, considering the limited sample size and the quality of includedarticles,higher quality RCTs are still needed to further confirm its effectiveness and safety.
Keywords:Shufeng Jiedu capsule Acute attack of chronic bronchitis Meta analysis Sequential analysis
1. Introduction
Chronicbronchitis (CB) is a common respiratory disease in clinic, which is a chronic nonspecific inflammation[1] caused by many reasons,such as hyperplasia of trachea and bronchus glands and increased mucussecretion.The main symptoms are cough,expectoration and wheezing, which are easy to recur and difficult to heal, and seriously affect the quality of life of patients.Respiratory tract infection can induce acute attack of chronic bronchitis (AECB)and accelerate the deterioration of lungfunction, making chronic obstructive pulmonary disease aggravate repeatedly, which increase the mortality rate[2].AECB mainly adopts symptomatic treatment such as controlling infection,expelling phlegm and relievingcough,relieving spasm and asthma, but with the repeated attack of symptoms and the continuous development of the disease,the number of drug-resistant strains increases gradually,and the therapeutic effect is not
Satisfactory. AECB is mainly composed of elderly people, with poor airway immune function, although simple routine western medicine treatment can alleviate the symptoms of patients, the disease control is poor,and there are many allergic reactions and adverse reactions.The combination of traditional Chinese and western medicine can reduce the adversereactions and improve the curative effect.
TCM classifies CB as "cough", "asthma syndrome", "phlegm drink" and other categories[3]. The elderly are physically weak,immune decline, low resistance, induced by external factors, easy to induce AECB. AECB is characterized by the syndrome of windheat attacking the lung and phlegm-heat accumulating in the lung,which should be treated by clearing heat and purging the lung,resolving phlegm and relieving cough, and relieving the acute symptoms of the patients[4]. Shufeng Jiedu Capsule is a proprietary Chinese medicine composed of Forsythia suspensa, Polygonum cuspidatum, Radix Isatidis, Bupleurum, Patrinia officinalis, Verbena,Reed Root and Glycyrrhiza uralensis,which is derived from Xiangxi ancestral Miao medicine "Qudu San", which has the effects of dispelling wind, clearing heat, detoxifying andrelieving sore throat.Modern pharmacological studies[5-6] have shown thatthe drug has immunomodulatory and anti-inflammatory effects, and is widely used in the adjuvant treatment of upper respiratory tract infectionand COVID-19. In recent years, a number of studies have shown that Shufeng Jiedu Capsule can inhibit inflammation and improve immunity in the treatment of AECB, with good safety.Therefore, the purpose of this study is to systematically evaluate and sequentially analyze the clinical efficacy of Shufeng jiedu capsule in the treatment of AECB, and evaluatethe quality of GRADE evidence, so as to provide evidence-based basis for clinical diagnosis and treatment.
2. Materials and methods
2.1 Literature inclusion criteria
(1) Study type: Randomized controlled trial (RCT); (2) Subjects:Definitely diagnosed as AECB, regardless of gender, age, race or region;(3) Intervention measures: The control group was given routine treatment such as anti-infection, expectorant and antiasthmatic, while the experimental group was given Shufeng Jiedu Capsule on the basis of the control group; (4) Outcome indicators:The main indicators are effective rate, while the secondary indicators are CRP, lung function (including forced expiratory volume in the first second (FEV1), forced expiratory volume in the first second as a percentage of forced vital capacity (FEV1/FVC)), TNF-α and IL-8.The safety index is the occurrence of adverse reactions.
2.2 Literature exclusion criteria
(1) Semi-randomized trials with other diseases; 2) The control group was treated with other TCMs. 3) Take only one document with repeated reports or the same data; 4) Research that can't get complete data.
2.3 Literature retrieval strategy
Chinese databases searched by computer include CNKI, VIP,CBM,WanFang and CHICTR.English databases include PubMed,EMbase and Cochrane Library, and the retrieval time is from the date of establishment to December 31st, 2020. Chinese search words were Shufeng Jiedu,chronic bronchitis, chronic bronchitis acute attack, chronic bronchitis acute exacerbation, chronic bronchitis acute aggravation, English key words were Shufeng Jiedu, chronic bronchitis, acute attack of chronic bronchitis, acute exacerbation of chronic bronchitis.The retrieval was carried out by the combination of subject words and free words, and there were no restrictions on the type and language of literature publication.
2.4 Literature screening and data extraction
Two researchers independently searched the literature according to the inclusion and exclusion criteria, and cross-checked. If there were differences, the two researchers discussed or discussed with the third researcher, and the selected documents were introduced into the NoteExpress 3.2. After that, the data were extracted and stored in EXCEL. The included materials mainly included: basic information of the literature, methodological elements, characteristics of the research object, course of disease, course of treatment, intervention and control measures, outcome indicators and adverse events and so on.
2.5 Literature quality evaluation
Cochrance collaborative network risk bias assessment tool[7] was used to evaluate the methodological quality of the included literature.The contents of evaluation mainly included: random allocation method, hidden allocation scheme, blind method for research objects and testers, blind method for outcome assessors, completeness of result data, selective reporting of research results and other biases.Finally, the bias risk of each item above was judged as "low", "high"and "uncertain".
2.6 Statistical analysis
The assessment of bias risk was completed by RevMan 5.3 software provided by Cochrane collaboration network.The relative risk (RR) was used to calculate the effect in the second classification variable, andthe mean difference (MD) was used as the curative effect statistic in the continuous variable. The results of both were shown by 95% confidence interval (CI). In addition, the heterogeneous state was evaluated according to the heterogeneous I2index. The larger I2was, the greater heterogeneity was. If P≥0.1 and I2≤50%, the homogeneity was good, sothe fixed effect model is chosen. If P<0.1 or I2>50%, it indicated thatheterogeneity is large, so sensitivity analysis was selected, such as eliminating heterogeneity by excluding low-quality research and changing the standard of nano-exclusion. If the source of heterogeneity cannot be found, the random effect model was used. In addition, the effective rate of the included literature was analyzed in sequence (TSAv0.9,www.ctu.dk/tsa). TSA can not only reduce the false positive results caused byrandom errors, but also estimate the sample size required for Meta-analysis, overcoming the drawbacks of traditional system evaluation.
2.7 GRADE evidence quality rating
The grading method recommended by GRADE system[8] was adopted to evaluate the quality of evidence. The evidence quality was dividedinto four grades, namely, high, medium, low and very low, through thegrading of research limitations, inconsistency of results, indirection of evidence, inaccuracy of results and publication bias.Gradepro system (https://gradepro.org/)[9] was used to evaluate the quality of evidence for all outcome indicators.
3. Results
3.1 Literature retrieval results
A total of 41 relevant literatures were initially retrieved, 30 duplicated literatures were excluded, 1 irrelevant literature was excluded afterreading the title and abstract of the literature, the remaining 10 literatures were downloaded and read the full text, and 2 literatures were further excluded according to the inclusion and exclusion criteria, and finally 8 literatures were included[10-17], all of which were Chinese literatures.
3.2 Basic characteristics of the included literature
A total of 680 patients were included in 8 literatures[10-17],including 340 patients in the control group and 340 patients in the experimental group, with the maximum sample size of 120 cases and the minimum sample size of 36 cases, and the course of treatment varied from 7 to 14 days. The intervention measures of the experimental group were taking Shufeng Jiedu Capsule orally on the basis of routine western medicine treatment in the control group. The dosage is 4 capsules/time,3 times a day.The routine western medicine treatment in the control group included antibiotics, glucocorticoids,expectorants, theophyllines and anticholinergic drugs. Antibiotics included piperacillin sodium tazobactam sodium, cefuroxime and levofloxacin. The expectorant comprises ambroxol hydrochloride solution, ambroxol tablets and acetylcysteine solution for inhalation.Glucocorticoid includes budesonide suspension for inhalation. The ophylline drugs include aminophylline injection and aminophylline tablets. The anticholinergics include compound ipratropium bromide atomized solution. The course of treatment is 7~14 days. The basic information included in the study are shown in Table 1.
3.3 Quality evaluation of included literature
Among the 8 studies, 2 studies[11,16] only mentioned the word"random", indicating uncertain risk; 5 studies[10,12,13,15,17] used the random number table method, indicating low risk. One study[14]randomly assignedpatients according to the time of seeing a doctor,which belonged to alternating distribution and is of high risk. None of the studies reported the allocation of hidden and blind methods for risk uncertainty. No shedding cases were reported in all the studies,and all the observation indexes mentioned in the research scheme were reported in the result part. The result data was complete and there was no selective report, which was classified as low risk. Due to insufficient literature information,other bias risks were classified as risk uncertainty. The bias risk assessment included in the study is shown in figure 1 and Table 2.
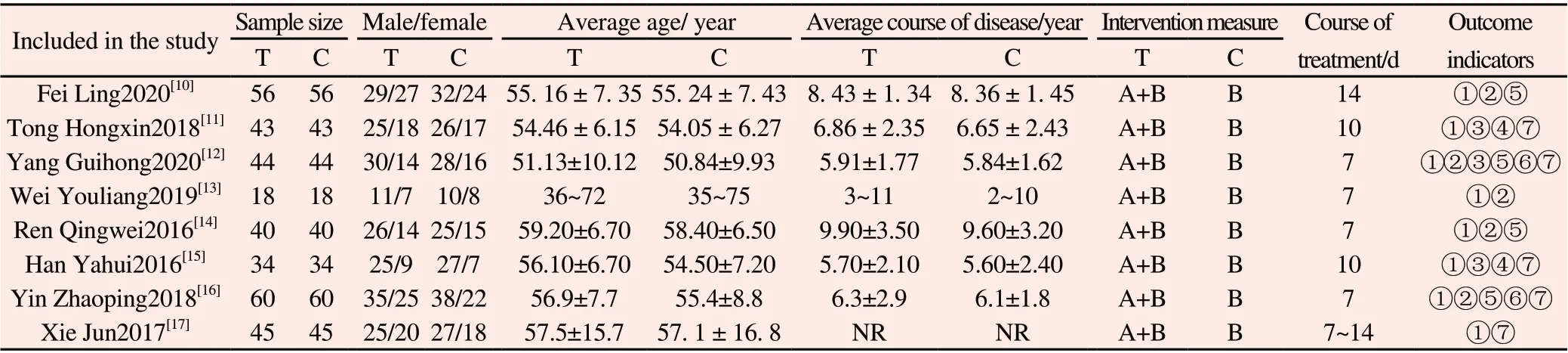
Table 1 Basic information included in the study

Table 2 Quality evaluation of included literature
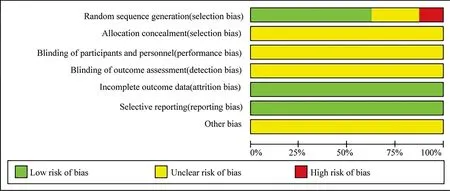
Figure1 Percentage of projects included in the study that produced a risk of bias.
3.4 Meta-analysis results
3.4.1 Efficiency
Eight literatures[10-17] reported the effective rate of clinical treatment, totaling 680 patients. Heterogeneity test P = 0.94, I2= 0, there was no obvious heterogeneity among the studies,so the fixed effect model was used for Meta-analysis. The results showed that RR=1.20, 95%CI[1.13, 1.28], P<0.00001, and the difference was statistically significant.The cl inical effective rate of the experimental group was 1.2 times that of t he control group. Shufeng Jiedu Capsule can obviously improve the ef fective rate of AECB treatment, as shown in Figure 2.
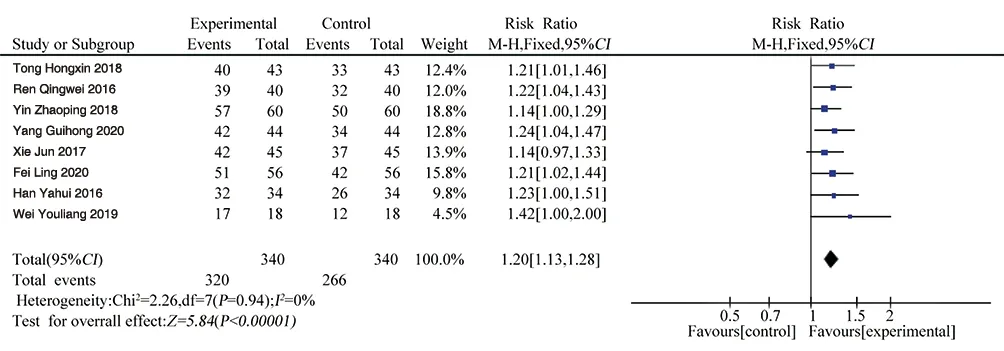
Figure 2 Forest plot of effective rate
3.4.2 C-reactive protein
CRP was reported in 5 literatures[10,12-14,16], with a total of 436 patients. Heterogeneity test (P < 0.00001, I2= 94%)showed obvious heterogeneity among the studies, so sensitivity analysis was carried out to find the reasons for heterogeneity. It was observed that the CRP recorded[13] in was lower than the other four literatures as a whole, and the heterogeneity disappeared after removing [13], which may be caused by the mild illness of the cases included in [13].After eliminating, the results showed that P = 0.75, I2= 0, and the fixed effect model was used for Meta-analysis. The results showed that MD=-7.32, 95%CI[-8.42, -6.22], P<0.00001, the difference was statistically significant, the mean difference of CRP between the experimental group and the control group was -7.32, and the CRP of the experimental group was lower than that of the control group, as shown in Figure 3.3.4.3 Lung function

Figure 3 Forest plot of CRP
(1) FEV1: FEV1 was reported in 3 literatures[11, 12, 15], with a totalof 242 patients. Heterogeneity test P = 0.90, I2= 0,and there was no obvious heterogeneity among the studies, so the fixed effect model wasused for Meta-analysis. The result showed that MD=0.33,95%CI[0.22, 0.45], P<0.00001, the difference was statistically significant. The FEV1difference between the experimental group and the control group was 0.33, and the FEV1 of the experimental group was higher than that of the control group, seeing Figure 4 for details.(2) FEV1/FVC (%): FEV1/FVC was reported in 2 literatures[11,15], with 154patients in total. Heterogeneity test P = 0.98, I2= 0,there was no obvious heterogeneity among the studies, so the fixed effect model was used for Meta-analysis. The result showed that MD=10.17, 95%CI[8.15, 12.19], P<0.00001, the difference was statistically significant. The mean difference of FEV1/FVC between the experimental group and the control group was 10.17, and FEV1/FVC of the experimental group was higher than that of the control group, as shown in Figure 5.3.4.4 Interleukin 8

Figure 4 Forest plot of FEV1
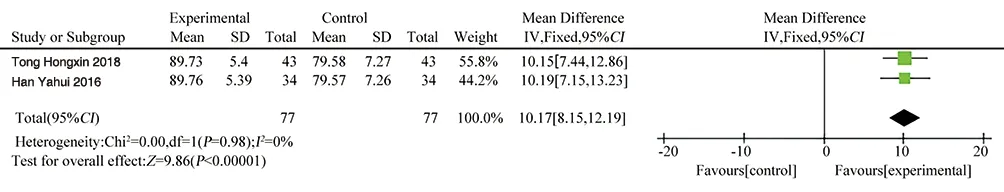
Figure 5 Forest plot of FEV1/FVC
IL-8 was reported in two literatures[12, 16], involving a total of 208patients. Heterogeneity test P =0.0005, I2= 92%. There was significantheterogeneity among various studies, so random effects model was usedfor Meta-analysis. The results showed as follows:MD=-63.39, 95%CI[-73.49, -53.29], P<0.00001, and the difference was statistically significant.The mean difference of IL-8 between the test group and the control group was -63.39, and IL-8 in the test group was significantly lower than that in the control group, as shown in Figure 6.
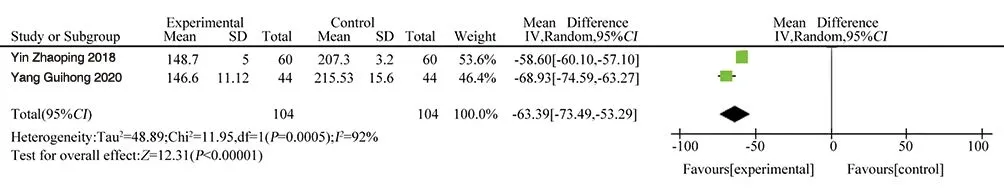
Figure 6 Forest plot of IL-8
3.4.5 Tumor necrosis factor α
Four literatures[10, 12, 14, 16] reported TNF-α, with a total of 400 patients. Heterogeneity test P = 0.27, I2= 23%,there was no obvious heterogeneity among the studies, so the fixed effect model was used for Meta-analysis. The results showed that MD=-7.44, 95%CI[-8.35,-6.53], P<0.00001, the difference was statistically significant. The mean difference of TNF-α between the experimental group and the control group was -7.44, and the TNF-α in the experimental group was significantly lower than that in the control group, as shown in Figure 7.
3.5 Adverse reactions
Five literatures[11,12,15-17] reported adverse reactions, all of which were described in detail, but there were no serious adverse reactions.Eleven cases of adverse reactions occurred in the experimental group, mainly including nausea, abdominal distension and diarrhea,with an incidence of 3.2%(11/340), while 16 cases of adverse reactions occurred in the control group, mainly including nausea,vomiting and diarrhea, withan incidence of 4.7%(16/340). Adverse reactions werenot mentioned in the other three literatures.
3.6 GRADE evidence quality rating
GRADE evaluation of each outcome indicators showed that middlegrade evidence support that Shufeng Jiedu Capsule combined with western medicine routine treatment could improve the effective rate and reduce TNF-α level compared with western medicine routine treatment alone. Low-grade evidence has supported that Shufeng Jiedu Capsule combined with Western medicine routine treatment can increase FEV1 compared with Western medicine routine treatment alone, and extremely low-grade evidence has supported that Shufeng Jiedu Capsule combined with Western medicine routine treatment can reduce CRP, IL-8 and increase FEV1/FVC compared with Western medicine routine treatment alone,as shown in Table 3.
3.7 Sequential analysis of tests
The effectiveness of 8 included studies was sequentially analyzed by using TSAv0.9 software.The type I error rate α=0.05, the type II error rate β=0.2, the cutoff Z=1.96, the statistical efficiency was 80%, therelative risk reduction (RRR) was 15%, and the relative event rate of the control group was 70% (refer to the results of this study and related literature Meta analysis[18]). Sample size expected information value (RIS) was set. Results showed that the cumulative Z values (Z-curve) after 4 studies[11] at the same time through the traditional values and the TSA boundary value curve, not through the RIS (RIS=650), that the cumulative amount of information which didn’t meet expectations, but don't need more studies have demonstrated that certain conclusion aheadof schedule, proved the breeze detoxification capsules with conventionalwestern medicine therapy AECB is superior to the conventional westernmedicine therapy alone, in line with the Meta analysis conclusion of this study,as shown in figure 8.
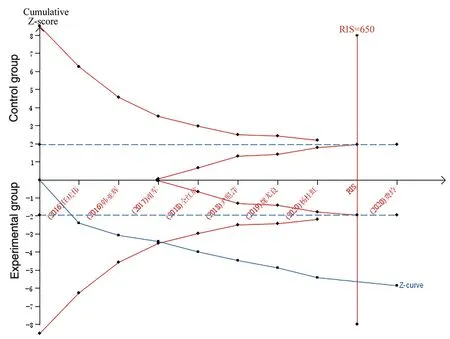
Figure 8 rial sequential analysis of the incidence of effective rate
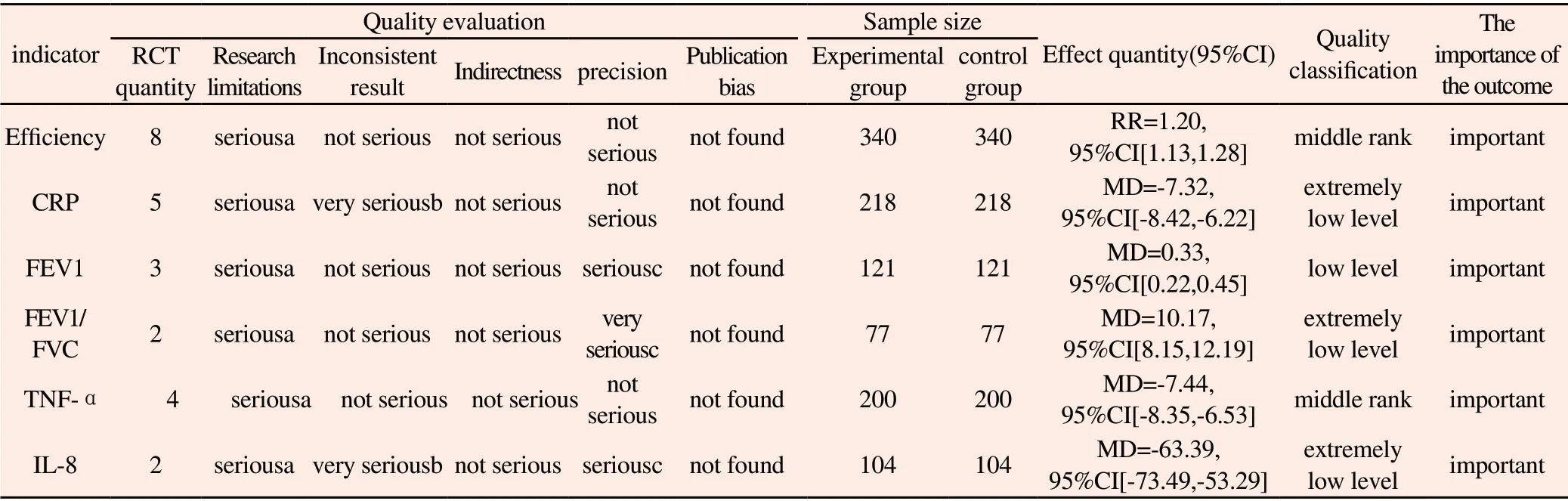
Table 3 Summary of main findings of GRADEpro
4. Discussion
Shufeng Jiedu Capsule is composed of forsythia, Polygonum cuspidatum, Radix Isatidis, Bupleurum, Patrinia officinalis, Verbena,Reed rootand licorice, which has the effect of soothing wind and clearing heat,detoxifying and promoting pharynx. Pharmacological studies[19-21] showed that Shufeng Jiedu Capsule can control bacterial infection, antivirus andenhance immunity, and is widely used in upper respiratory tract infection, community-acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, influenza and other diseases.
CB is one of the common diseases and frequently-occurring diseases in respiratory department, which has a long illness and is prone to recurrent attacks. In the acute attack period, the symptoms of cough, expectoration and wheezing continue to worsen, expectoration increases, and some patients also have fever symptoms. AECB recurs and eventually develops into chronic obstructive pulmonary disease, cor pulmonale and other diseases.AECB can aggravate bronchial mucus secretion, gland hyperplasia,collapse lumen, unsmooth airflow in respiratory tract, increase airway resistance, and lead to the decline of lung function, so lung function is a common index to evaluate AECB.AECB is characterized by airway inflammation, and CRP, TNF-α and IL-8 play an important role in airway allergy and inflammation. Studies[22]have shown thatinflammatory cytokines such as CRP, TNF-α and IL-8 can aggravate the acute attack of CB. CRP can reflect the inflammatory reaction of respiratory system, and is positively correlated with cytokines IL-8 and TNF-α, while serum IL-8 and TNF-α levels are closely related to the progress of acute and chronic airway inflammation[23]. IL-8 is an important chemotactic factor of neutrophils[24], which can activate neutrophils, and then induce inflammatory reaction, which rises sharply at the onsetof AECB. Zhang Tiejun[25] found that Shufeng Jiedu Capsule can not only significantly inhibit the production of thermogenic cytokine TNF-α, and then reduce the production of thermogenic factor cAMP and play an antipyretic role, but also reduce the activation of CRP and IL-8 caused by upper respiratory tract infection by inhibiting the expression of related inflammatory pathways, and play an antiinflammatory role through multiple channels and multiple targets,thus effectively reducing the onset of AECB.
4.1 Effectiveness and Safety Analysis
The results of Meta analysis in this study have shown that in the process of clinical treatment of AECB, compared with routine western medicine treatment, Shufeng jiedu capsule can significantly improve theeffective rate, improve pulmonary function, and has obvious advantages in reducing CRP, TNF-αand IL-8 proinflammatory cytokines. The above differences are statistically significant. In terms of adverse reactions, the incidence of adverse reactions was 3.2% in the test group (11/340) and 4.7% in the control group (16/340). The gastrointestinal symptoms such as nausea and diarrhea were the main symptoms in both groups, and the safety was higher. According to the sequential analysis of8 effective rates included in the study, it is proved that the effective rate of Shufeng Jiedu Capsule combined with western medicine routine treatment for AECB is better than that of western medicine routine treatment alone, and the curative effect is exact. According to the GRADEevaluation system, the outcome index is effective,TNF-α is medium quality evidence, FEV1 is low quality evidence,FEV1/FVC, CRP and IL-8 are extremely low quality evidence.Although the evidence of the effective rate of Shufeng Jiedu Capsule in treating AECB is accurate, theevidence quality level is not high on the whole, and further research isneeded to prove the credibility of the results in the future.
4.2 Shortcomings and Prospects
To sum up, Shufeng Jiedu Capsule combined with Western medicine conventional treatment has certain advantages in improving clinical effective rate, improving lung function and regulating inflammatory factors, and it is safe. However, this study still has the following limitations:① Only Chinese literature was included,and the number of literature was small, and English literature can not be retrieved; ② Distribution concealment, blind method and other bias were not mentioned in all literatures, and the quality of research included in the literature and the overall quality of GRADE evaluation were low; ③ TCM syndrome types included in each research institute were different, such as wind-heatattacking lung syndrome and phlegm-dampness accumulating lung syndrome,which may have an impact on the results; ④ Because less than 10 papers were included in this study and funnel chart was not drawn,it was impossible to judge whether there was publication bias. ⑤The treatment schemes of different control groups were classified as routine Western medicine treatment, and there were some heterogeneity in intervention measures in each study. Therefore,further multi-center, large sample and high-quality clinical trials are needed to verify the effectiveness and safety of Shufeng Jiedu Capsule in treating AECB in the future.
 Journal of Hainan Medical College2022年6期
Journal of Hainan Medical College2022年6期
- Journal of Hainan Medical College的其它文章
- Study on the mechanism of Shenling Baizhu Powder in the treatment of diarrhea-type irritable bowel syndrome based on network pharmacology and molecular docking
- Research on the possible molecular mechanism of Fructus cnidii to improve sleep based on network pharmacology
- Network pharmacological study and molecular docking verification of Capparis spinosa in the treatment of systemic sclerosis
- Effect of pestle intervention in type 2 diabetic peripheral neuropathy on Keap1 / Nrf2 / ARE pathway and the relationship with oxidative stress
- Experimental study on the effect of cryoablation on lung cancer mice based on MAPK/ERK signaling pathway
- Effects of Qishen Yiqi dripping pills-containing serum on KATP channel opening and PI3K/AKT signaling pathway in hypoxic/reoxygenated H9C2 cardiocytes
