桃糖转运蛋白基因PpTST2的功能初探
王宁,孟祥光,文滨滨,和华杰,陈修德*,李玲*
桃糖转运蛋白基因的功能初探
王宁1,2,3,孟祥光1,2,3,文滨滨1,2,3,和华杰1,2,3,陈修德1,2,3*,李玲1,2,3*
1. 山东农业大学园艺科学与工程学院, 山东 泰安 271018 2. 作物生物学国家重点实验室, 山东 泰安 271018 3. 山东省果蔬优质高效协同创新中心, 山东 泰安 271018
桃糖转运蛋白基因在液泡膜糖转运过程中具有重要作用。本文以桃品种 ‘2014-9-34’ 为材料,克隆,并对其进行生物信息学分析及转基因苹果愈伤功能鉴定。结果显示,的cDNA序列总长为2 220 bp,编码740个氨基酸,预测其编码蛋白质分子量为78.91 kDa,等电点为4.8,启动子序列分析发现其具有干旱胁迫、激素响应、光反应和糖响应等多种顺式作用元件,基因的表达明显受ABA的诱导,在‘王林’愈伤组织中过表达可以提高可溶性糖含量。酵母单杂实验表明转录因子PpABRE1能够激活的表达。
桃; 转运蛋白; 基因表达
糖在植物生长发育过程中起着重要作用。风味是影响果实品质的关键因素之一,其主要取决于可溶性糖和有机酸含量[1,2]。糖还参与调节多种信号途径转导和基因表达等[3,4]。
液泡糖转运蛋白(TSTs)最初叫液泡单糖转运蛋白(TMTs),AtTMT1、AtTMT2是拟南芥中最早被发现,具有葡萄糖、果糖转运能力的转运蛋白,且基因在盐、干旱和寒冷等环境胁迫下显著上调[5]。在甜菜中第一次发现可以将蔗糖转运到液泡内[6]。因此将TMTs更名为TSTs[6]。
TSTs在多种植物的糖积累中都有研究。在拟南芥中单糖转运蛋白AtTST1活性的增加改变了拟南芥中细胞糖的分配、糖信号转导和种子产量[7]。梨转入酵母EBY.VW4 000后,可以在低浓度的果糖、蔗糖、山梨醇培养基中生长,在转基因番茄中果糖和葡萄糖含量显著提高,蔗糖含量无明显变化,使转基因番茄比野生型提早开花、成熟[8]。、可以转运果糖和蔗糖[9]。沉默后葡萄糖的含量显著降低,在ABA处理下,抵消了ABA诱导的葡萄糖含量增加[10]。介导了突变体酵母细胞在葡萄糖和果糖以及半乳糖和木糖培养基中的生长[11,12]。黄瓜在酵母中的功能分析表明,它能够转运半乳糖、甘露糖和蔗糖[13]。在水稻液泡葡萄糖储存中发挥了作用,但和在寒冷、盐或干旱情况下表达无变化[14]。在草莓和黄瓜中过表达增加了葡萄糖、果糖、蔗糖的含量,且在高糖甜瓜品种果实中的表达量显著高于低糖品种[15]。在调控西瓜葡萄糖、果糖、蔗糖含量发挥重要作用[16]。在甜高粱中,和的表达量显著高于谷物高粱[17]。在柑橘中在果实成熟期显著上调[18]。
最近在桃中发现了一个基因家族中和糖含量相关的基因,位于控制水果蔗糖含量的QTL区域,且位于果实酸度定位区间[19-21]。基因的第三个外显子存在一个非同义的G/T单核苷酸多态性(SNP),的G和T等位基因分别命名为和[19]。我们将基因转入苹果愈伤中,对基因的功能进一步挖掘。
1 材料和方法
1.1 实验材料
试验材料为山桃()苹果‘王林’的愈伤组织。桃幼苗由山桃种子成熟以后,于30株生长良好的桃树中随机采集400个果实,去除果肉,收集种子,最后用尼龙袋与沙子混合,层积于山东农业大学科技创新园。来年春天播种在穴盘中,放置于光照培养箱,23 ℃,16 h/8 h光暗周期下培养1周,选取长势一致的幼苗使用。苹果‘王林’愈伤组织平均两周继代1次,平铺于加入1.6 mg/L 2,4-D和0.4 mg/L 6-BA的MS固态培养基中,放置在25 ℃的恒温培养箱中暗培养。
1.2 ‘王林’苹果愈伤组织的转化
以‘2014-9-34’油桃cDNA为材料扩增,构建过表达载体pRI101- PpTST2。将构建的过表达载体导入GV3101农杆菌感受态,得到重组农杆菌侵染‘王林’苹果愈伤。
转基因苹果愈伤鉴定:
(1)PCR扩增鉴定转基因植株以待验证植株的DNA为模板,使用Taq酶进行PCR扩增实验,1%琼脂糖凝胶电泳实验检测扩增片段与目的基因大小是否一致。
(2)qRT-PCR鉴定转基因植株
提取待检测植株的RNA,反转录为cDNA,qRT-PCR检测目的基因在各株系与野生型中的表达量。
1.3 总RNA的提取与荧光定量
用RNA提取试剂盒(天根,DP432)提取RNA后,然后用反转录试剂盒(KR106)得cDNA,按照SYBR®Green PCR Master Mix说明书配制荧光定量PCR反应体系,采用2–ΔΔCT方法对数据分析。
1.4 生物信息学分析
ExPASy网站ProtParam在线软件(http://web.expasy.org/protparam/)预测PpTST2蛋白大小,利用(http://bioinformatics.psb.ugent.be/webtooLs/pLantcare/htmL/)网址分析启动子的顺式作用元件。
1.5 苹果愈伤糖含量的测定
苹果愈伤还原性糖和可溶性总糖测定方法,参考李合生[22]实验植物生理生化实验原理和技术。

表1 本实验所用引物序列
1.6 酵母单杂实验
将目的基因启动子片段连接pAbAi表达载体,构建pBait-AbAi质粒,使用1191限制性内切酶线性化pBait-AbAi,将线性化的质粒转入Y1H Gold酵母感受态细胞,涂布SD/-Ura培养基。选取阳性克隆的菌株,用无菌的ddH2O将酵母浓度调整为OD600约为0.002,将稀释后的菌液涂布于含有不同AbA浓度的SD/-Ura固体培养基,确定诱饵菌株的AbA最小抑制浓度。构建PpABREs-pGADT7载体质粒,和已构建pBait-AbAi质粒共同转入Y1H Gold酵母感受态,涂布SD/-Leu-Ura培养基和含有最小抑制浓度的SD/-Leu-Ura培养基,30 ℃倒置培养3~5 d。
1.7 双荧光素酶成像试验
将下游基因的启动子序列连接到pGreenⅡ 0800-LUC载体,将的编码序列连接到pGreenⅡ0029 62-SK载体,将重组质粒分别转入GV3101(pSoup-p19)农杆菌感受态细胞。将农杆菌活化混合后注射烟草叶片,注射完的烟草暗培养1 d后转入光照培养,2~3 d后,在烟草叶片背面涂抹D-虫荧光素钠盐溶液,使用活体成像系统(美国,精诺真)观察拍照。
1.8 数据分析
荧光定量数据用Excel 2019软件数据处理,用GraphPad 6软件进行作图;用SPSS 26软件进行显著性相关分析。
2 实验结果
2.1 PpTST2_G基因的克隆
如图1所示获得了一条2 000 bp长度的条带,基因测序结果显示:基因编码区长度为2 220 bp。蛋白预测大小为78.91 kDa左右,氨基酸等电点pI:4.8。
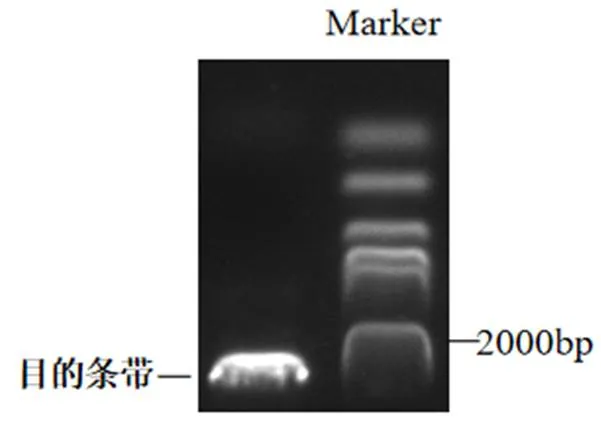
图 1 PpTST2_G基因扩增结果
2.2 PpTST2_G的进化树分析及蛋白序列比对
图2显示和在同一个分支上,但图3氨基酸相似度分析,和的相似度为70.39%,大于和的相似度63.70%,因此()在拟南芥中的同源基因是。

图2 PpTST2_G基因进化树分析
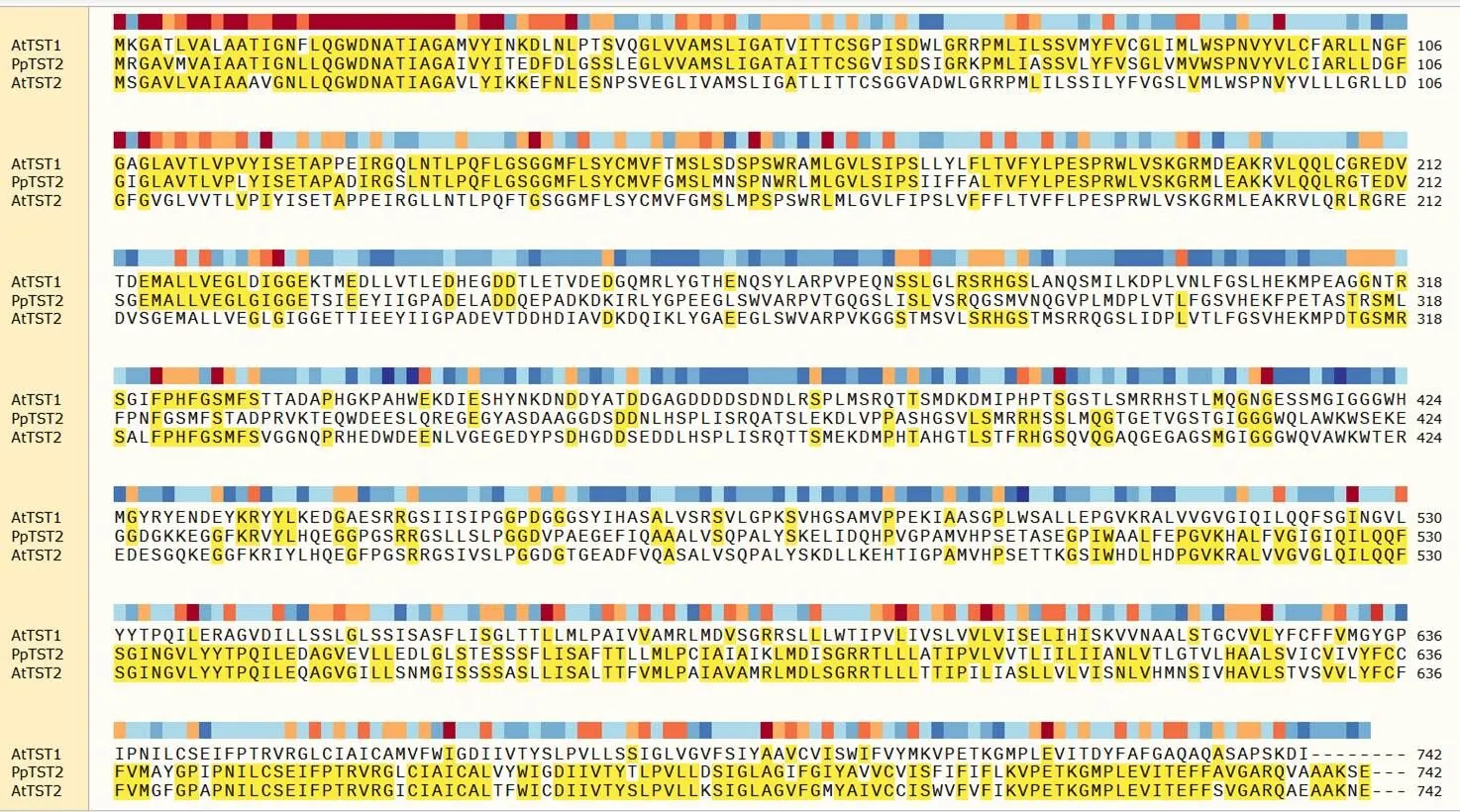
图 3 PpTST2_G与AtTST1、AtTST2氨基酸序列比对
2.3 启动子序列分析及非生物胁迫分析
用PlantCARE在线软件进行分析发现了一些顺式作用元件[23]:CPBCSPOR是细胞分裂素响应元件[24];SREATMSD是与糖信号相关的元件[25]。
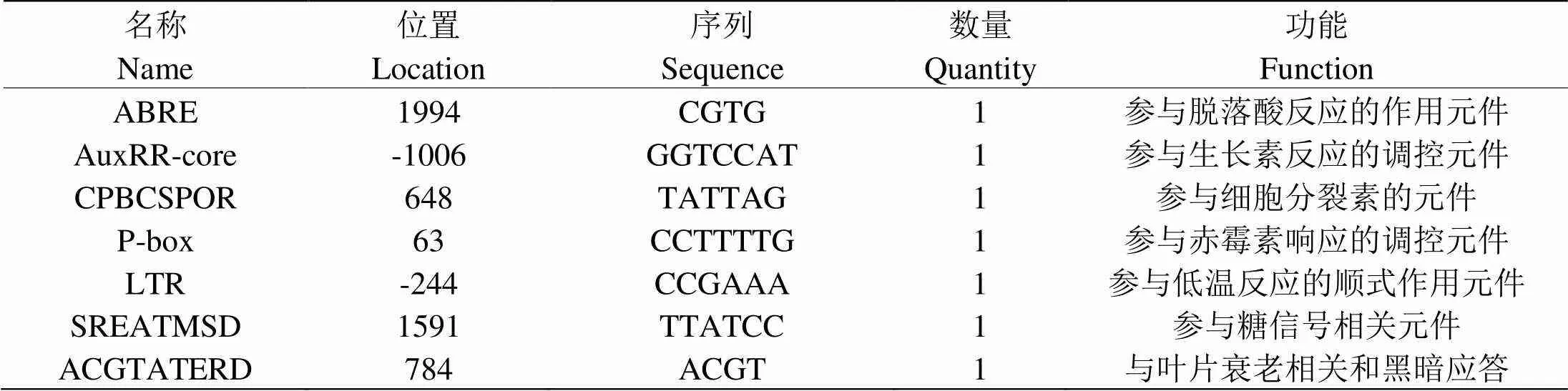
表 2 PpTST2基因启动子序列顺式作用元件分析
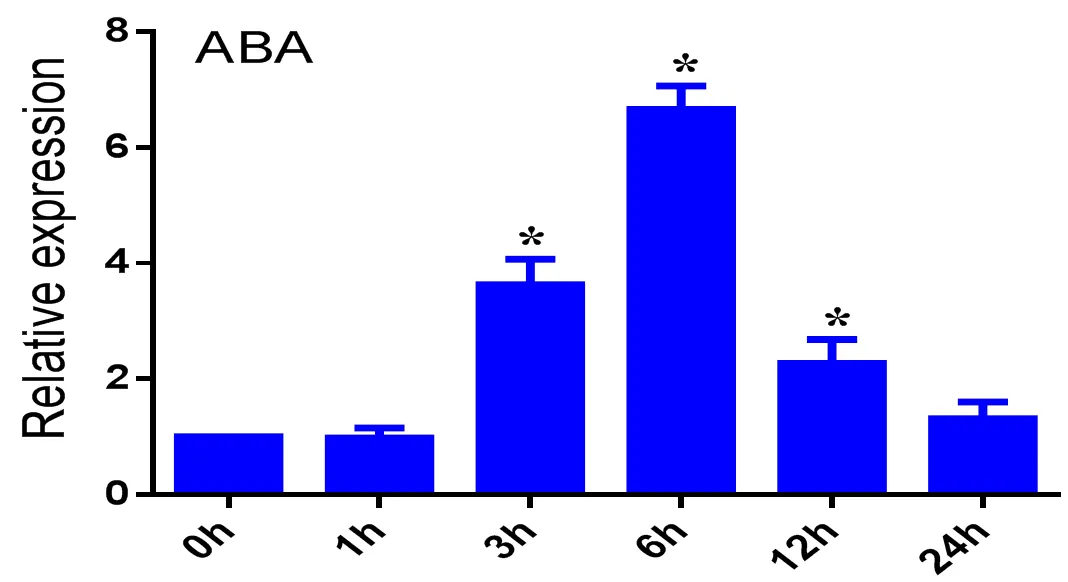
图 4 PpTST2响应ABA信号
注:误差条代表三个生物学重复的标准误差(SE),*:< 0.05,下同。
Note: The error bar represents the standard error (SE) of the three biological replications, *:< 0.05, similarly below
2.4 PpTST2_G在‘王林’愈伤组织中过表达分析
我们扩增了基因,并将其转化进苹果愈伤,还原性糖和和可溶性糖含量都显著提高。还原性糖含量由8.24 mg·g-1上升为12.79 mg·g-1、14.45 mg·g-1,可溶性糖由17.42 mg·g-1上升为20.06 mg·g-1,23.45 mg·g-1。
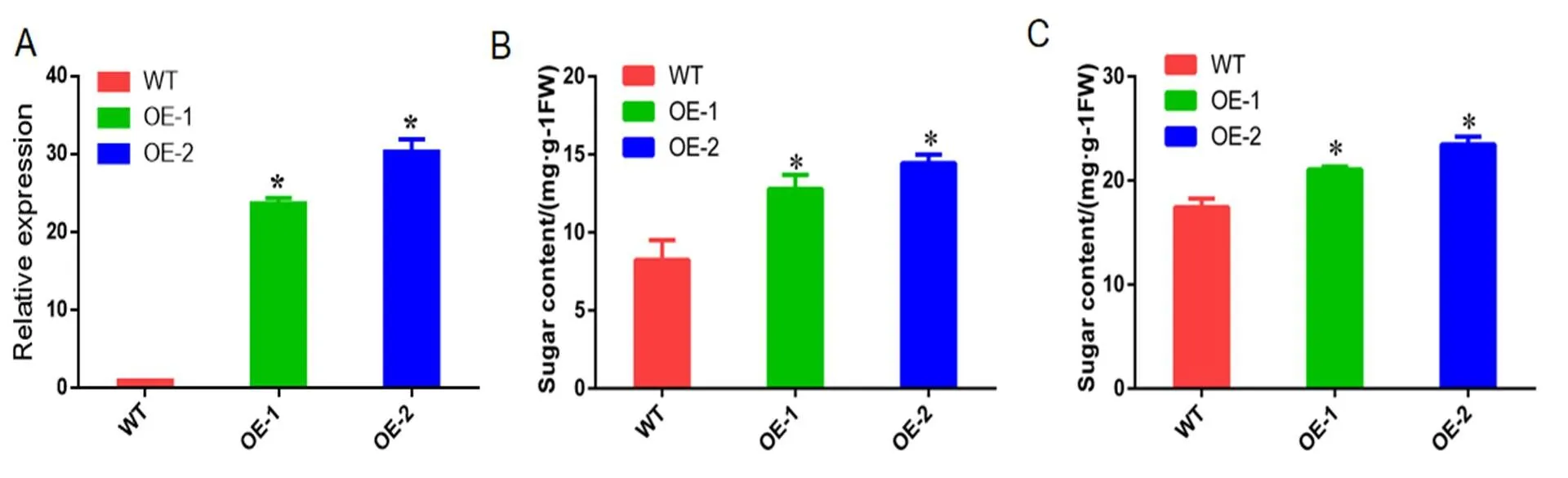
图 5 转基因苹果愈伤和野生型糖含量比较
(A)转基因苹果愈伤表达量分析(B)还原性糖含量 (C)可溶性总糖含量
(A) Analysis ofexpression in transgenic apple callus (B) Reducing sugar content. (C) Total soluble sugar content
2.5 PpABRE1激活基因PpTST2的表达
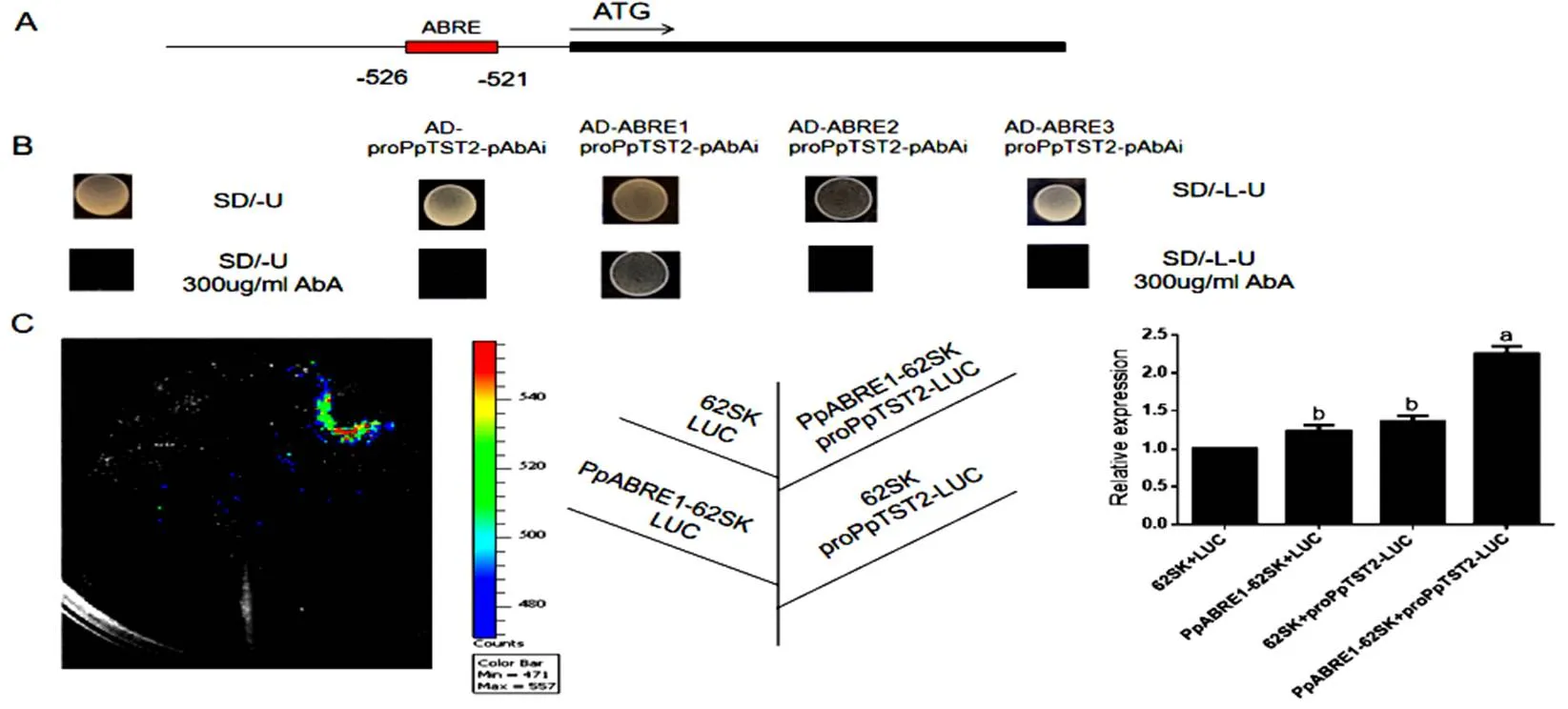
图 6 PpABRE1激活PpTST2的表达
(A) CGTG元件在基因序列中的位置(B)酵母单杂验证PpABRE1可以结合的启动子(C)PpABRE1激活的表达
(A) The position of the CGTG element in thegene sequence; (B) Yeast single hybrid verification that PpABRE1 can bind to the promoter of; (C) PpABRE1 activates the expression of
图4表达分析表明受ABA诱导,此外通过启动子分析,含有ABA响应元件ABRE。因此我们扩增了包含CTGCAC总长为50 bp的启动子序列,随后又扩增了序列号为、、三个基因家族的序列,分别命名为、、,只有AD-- proPpTST2-pAbAi在-L-U+300AbA的培养基上生长,而AD-- proPpTST2-pAbAi、AD-- proPpTST2-pAbAi在SD/L-U+300 AbA的培养基上被抑制不能生长(图6 B)。双荧光素实验证明PpABRE1能够激活的表达(图6 C)。
3 讨 论
通常情况下,液泡积累大量的还原糖,大约占到植物游离己糖的90%,仅有少量的己糖存在于细胞质中,植物液泡占据了细胞体积的90%,并通过膜屏障与周围的细胞成分分离。液泡中的组分通常包括各种糖、多醇、有机酸、氨基酸以及离子[7,26-28]。糖类作为能量物质和信号分子对植物的生长发育和非生物胁迫都有影响[29-32],糖还具有提高植物的抗病能力[33,34]。
和是第一批在拟南芥中发现的具有葡萄糖转运能力的基因[35],这两个基因都属于协助扩散超家族(MFS),所有的TST蛋白都具有一个独特的大约320个氨基酸的中心结构域[5,6,36]。在拟南芥中,和均以三种亚型存在,基因功能的缺失会导致开花延迟;和在大多数植物组织中都有发现,而基因很少表达,可能不参与AtTSTs介导的液泡糖转运,AtTSTs活性缺失影响植物在低温下葡萄糖和果糖的积累[5,36]。活性决定着拟南芥种子的产量,在tDNA插入突变体中,种子脂质和蛋白质含量比野生型少10%左右,过表达种子脂质含量稍高[7]。活性与种子产量呈正相关很可能是由于过表达植株中细胞糖信号响应的改变[37]。由于过表达株系在细胞质中表现出更少的葡萄糖,导致光合作用基因的表达量更高[38]。此外,除了二氧化碳固定活性的增加外,过表达株系表现出呼吸速率降低和蔗糖转运基因表达上调。这些变化使过表达株系能够从叶片中输出更多的光合产物,从而提高种子产量[38]。本实验中我们得到的转基因苹果愈伤与前人研究结果相似,提高了转基因植株的可溶性糖含量。
在互作因子激活AtTST1实验中,前人发现蛋白激酶AtVIK1与AtTST1与互作,并磷酸化AtTST1,促进葡萄糖向液泡中转运[38]。此外,温度改变促进和的磷酸化[28]。前人通过酵母双杂交实验发现膜蛋白MdVGT1与MdTMT1互作[9],下一步可以使用膜系统双杂技术手段进行筛库,寻找PpTST2互作蛋白。
开花是植物从营养生长到生殖生长转换的重要现象,在这一转换期间, 蔗糖含量往往出现短期增加[39]。蔗糖是人们研究最多的与成花过程有关的可溶性糖,葡萄糖对植物生长的影响一般不同于蔗糖,葡萄糖主要影响幼苗的生长、光合作用、淀粉降解和衰老,而蔗糖与成熟的关系更为密切,如开花过程和贮藏器官的发育[40]。种子的发育也由蔗糖控制[8]。提高内源性蔗糖水平也能提高番茄的开花效果[41]。梨的在番茄中过表达后,转基因番茄比WT番茄提早开花2-3周[8,42]。
前人研究发现和的启动子序列中都含有CACGTC核心序列,实验证明MdABRE2特异性结合和的启动子,但MdABRE1不特异性结合和[10],本实验中基因结构与相似,也包含4个外显子和3个内含子。此外,位于果实酸度定位区间[19-21],我们推测此基因和果实酸度有关。
4 结 论
(1)本实验证明了促进苹果愈伤中糖的积累;
(2)转录因子PpABRE1可以激活的表达。
[1] Predieri S, Ragazzini P, Rondelli R. Sensory evaluation and peach fruit quality [J]. Acta Horticulture, 2006,713:429-434
[2] Stadler R, Truernit E, Gahrtz M,. The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis [J]. The Plant Journal, 1999,19(3):269-278
[3] Koch KE Carbohydrate-modulated gene expression in plants [J]. Annu Rev Plant Physiol Plant Mol Biol, 1996,47(1):509-540
[4] Smeekens S, Rook SF. Sugar sensing and sugar-mediated signal transduction in plants [J]. Plant Physiology and Biochemistry, 1997,115(1):7-13
[5] Wormit A, Trentmann O, Feifer I,. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport [J]. Plant Cell, 2006,18(12):3476-3490
[6] Jung B, Ludewig F, Schulz A,. Identification of the transporter responsible for sucrose accumulation in sugar beet taproots [J]. Nat Plants, 2015,1(1):14001
[7] Wingenter K, Schulz A, Wormit A,. Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis [J]. Plant Physiol, 2010,154(2):665-677
[8] Cheng R, Cheng Y, Lu J,. The gene PbTMT4 from pear () mediates vacuolar sugar transport and strongly affects sugar accumulation in fruit [J]. Physiol Plant, 2018,164(3):307-319
[9] Ma XL, Qin Y, Wei XY,. Sequence and expression analysis of apple tonoplast monosaccharide transporter TMT genes and their relationship with sugar accumulation in fruit [J]. Acta Horticulturae Sinica, 2014,41(7):1317-1325
[10] Ma QJ, Sun MH, Lu J,Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes [J]. Plant Physiol, 2017,174(4):2348-2362
[11] Zeng L, Wang Z, Vainstein A,. Cloning, localization, and expression analysis of a new tonoplast monosaccharide transporter fromL. [J]. Journal of Plant Growth Regulation, 2010,30(2):199-212
[12] Afoufa-Bastien D, Medici A, Jeauffre J,. The Vitis vinifera sugar transporter gene family: phylogenetic overview and macroarray expression profiling [J]. BMC Plant Biol, 2010,10(1):245
[13] Huang W, Hu B, Liu J,. Identification and characterization of tonoplast sugar transporter (tst) gene family in cucumber [J]. Horticultural Plant Journal, 2020,6(3):145-157
[14] Cho JI, Burla B, Lee DW,. Expression analysis and functional characterization of the monosaccharide transporters, OsTMTs, involving vacuolar sugar transport in rice () [J]. New Phytol, 2010,186(3):657-68
[15] Cheng J, Wen S, Xiao S,. Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation [J]. J Exp Bot, 2018,69(3):511-523
[16] Ren Y, Guo S, Zhang J,. A tonoplast sugar transporter underlies a sugar accumulation QTL in Watermelon [J]. Plant Physiol, 2018,176(1):836-850
[17] Bihmidine S, Julius BT, Dweikat I,. Tonoplast Sugar Transporters (SbTSTs) putatively control sucrose accumulation in sweet sorghum stems [J]. Plant Signal Behav, 2016,11(1):e1117721
[18] Zheng QM, Tang Z, Xu Q,. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange () [J]. Plant Cell, Tissue and Organ Culture, 2014,119(3):609-624
[19] Peng Q, Wang L, Ogutu C,. Functional analysis reveals the regulatory role of PpTST1 encoding tonoplast sugar transporter in sugar accumulation of peach fruit [J]. Int J Mol Sci, 2020,21(3):1112
[20] Dirlewanger E, Moing A, Rothan C,. Mapping QTLs controlling fruit quality in peach ((L.) Batsch) [J]. Theoretical and Applied Genetics, 1999,98(1):18-31
[21] Etienne C, Rothan C, Moing A,. Candidate genes and QTLs for sugar and organic acid content in peach [(L.) [J]. Batsch], Theor Appl Genet, 2002,105(1):145-159
[22] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000
[23] Lescot M, Déhais P, Thijs G,. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences [J]. Nucleic Acids Research, 2002,30(1):325-327
[24] Fusada N, Masuda T, Kuroda H,. Identification of a novel cis-element exhibiting cytokinin-dependent protein binding in vitro in the 5'-region of NADPH-protochlorophyllide oxidoreductase gene in cucumber [J]. Plant Mol Biol, 2005,59(4):631-645
[25] Tatematsu K, Ward S, Leyser O,. Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis [J]. Plant Physiol, 2005,138(2):757-766
[26] Whiteman SA, Serazetdinova L, Jones AM,. Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana [J]. Proteomics, 2008,8(17):3536-3547
[27] Carter C, Pan S, Zouhar J,. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins [J]. Plant Cell, 2004,16(12):3285-3303
[28] Schulze WX, Schneider T, Starck S,. Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters [J]. Plant Journal, 2012,69(3):529-541
[29] Ohto MA, Onai K, Furukawa Y,. Effects of sugar on vegetative development and floral transition in Arabidopsis [J]. Plant Physiology, 2001,127(1):252-261
[30] Eveland AL, Jackson DP. Sugars, signalling, and plant development [J]. J Exp Bot, 2012,63(9):3367-3377
[31] Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development [J]. J Exp Bot, 2014,65(3):799-807
[32] Sheen J. Master regulators in plant glucose signaling networks [J]. J Plant Biol, 2014,57(2):67-79
[33] Otazu V. Soft rot susceptibility of potatoes with high seducing Sugar Content [J]. Phytopathology, 1981,71(3):290-295
[34] Conrath U, Linke C, Jeblick W,. Enhanced resistance to Phytophthora infestans and Alternaria solani in leaves and tubers, respectively, of potato plants with decreased activity of the plastidic ATP/ADP transporter [J]. Planta, 2003,217(1):75-83
[35] Aluri S, Büttner M. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering [J]. Plant J, 2007,104(7):2537-2542
[36] Hedrich R, Sauer N, Neuhaus HE. Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins [J]. Curr Opin Plant Biol, 2015,25(1):63-70
[37] Wingenter K, Trentmann O, Winschuh I,A member of the mitogen-activated protein 3-kinase family is involved in the regulation of plant vacuolar glucose uptake [J]. Plant J, 2011,68(5):890-900
[38] Cho LH, Pasriga R, Yoon J,. Roles of Sugars in Controlling Flowering Time [J]. Journal of Plant Biology, 2018,61(3):121-130
[39] Tognetti JA, Pontis HG. Martinez-Noel GM Sucrose signaling in plants: a world yet to be explored [J]. Plant Signal Behav, 2013,8(3):e23316
[40] Micallef BJ, Haskins KA, Vanderveer PJ,. Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an increased capacity for sucrose synthesis [J]. Planta, 1995,196(2):327-334
[41] Cheng R, Zhang H, Cheng Y,. Bioinformatics analysis of tonoplast monosaccharide transporter (TMT) gene family in fruit trees of rosaceae [J]. Journal of Nanjing Agricultural University, 2017(4):601-610
Preliminary Study on the Function of Peach Sugar Transporter Gene
WANG Ning1,2,3, MENG Xiang-guang1,2,3, WEN Bin-bin1,2,3, HE Hua-jie1,2,3, CHEN Xiu-de1,2,3*, LI Ling1,2,3*
1.271018,2.271018,3.271018,
The sugar transporter gene() played an important role in tonoplast sugar transport in peach (). We clonedgene from the peach variety ‘2014-9-34’ and performed the bio-information analyses of the, and verified the functions ofby transforming 'Orin' calli (). The results showed that the full length of the cDNA sequence ofwas 2 220 bp, encoding 740 amino acids. The predicted molecular weight and isoelectric point of thewas 78.91 kDa and 4.8, respectively. Analysis of thepromoter sequence showed that it contained drought stress, hormone, light, and sugar response-elements. Additionally, experiment results showed that hormone ABA could significantly induce expression of thegene. Overexpression ofin 'Orin' calli increased the content of soluble sugar. Yeast single-hybrid experiments showed that the transcription factor PpABRE1 could activate the expression of.
peach; transporter; gene expression
S662.1
A
1000-2324(2022)02-0180-08
10.3969/j.issn.1000-2324.2022.02.002
2021-11-14
2022-01-28
国家重点研发计划(2018YFD1000104);国家自然基金面上项目(31872041);山东省农业良种工程项目(2020LZGC007);山东省果品产业技术体系-栽培与土肥岗(SDAIT-06-04)
王宁(1997-),男,硕士在读,主要从事果树分子生物学及生理研究. E-mail:tonyparkerw@163.com
Author for correspondence. E-mail:chenxiude@163.com; lilingsdau@163.com

