The WHO 2021 thymoma classification: a work in progress
Saul Suster
Department of Pathology, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Abstract The updated, recently published 2021 WHO classification of thymic tumors incorporates the most recent advances in the field of thymic pathology, with particular emphasis on primary thymic epithelial neoplasms.This new edition retains the basic format of previous editions for the classification of primary thymic epithelial neoplasms reaffirming the schema originally proposed in the first edition, which utilized a combination of letters and numbers for the designation of these tumors.Only minimal changes have been incorporated in the new edition compared with the previous ones.A new helpful feature is a summary in each chapter of recommendations for “essential” and“desirable” criteria to facilitate the diagnosis.A few issues in this classification, however, still require clarification.In this review, the changes and advances in the classification of thymoma presented in the latest WHO book on Thoracic Tumors will be reviewed along with some of the areas that may still benefit from the additional investigation.
Keywords: Thymoma, WHO classification, thymic epithelial neoplasms, atypical thymoma
INTRODUCTION
The classification of thymoma has experienced numerous changes over the years, reflecting the difficulties in the histopathologic categorization of these tumors.It was noted for many years that pathologic classification of thymoma, regardless of the system proposed, failed to reliably predict the clinical behaviorof the lesions in a consistent manner, and that exceptions to every rule existed, with often unpredictable behavior being exhibited by some of these tumors.One of the problems involved in the morphologic classification of thymoma is the great complexity and heterogeneity they can exhibit[1,2].The variety of morphologic appearances that they can display has given rise to numerous histologic types and variants and led to numerous proposals for their terminology.Despite the great variety of histologic appearances, it has slowly become apparent that from the cytological point of view, thymomas can be divided into major 2 groups: those composed of small oval or spindle cells, and those composed of larger, round, epithelioid cells[Figure 1 A and B].This dichotomy has been tacitly acknowledged by every classification proposal to date,regardless of the terms utilized.Despite the novel introduction of a system of letters and numbers for the classification of thymoma by Dr.Rosai[3]in the first edition of the WHO schema, the system employed today by the WHO classification still relies essentially on tumor cytomorphology for classification and the different terms currently employed simply reflect the composition of the tumor as being either of a spindle,round, or putative “mixed” cell type.
As with all other tumor systems, our understanding of the behavior of thymoma has increased with every generation, and our criteria and progressive understanding of the biology of these tumors has contributed to shaping our approach to their classification.Herein we will explore the status of thymoma classification as currently defined by the WHO and discuss some of the areas in which additional insights would be helpful for a better understanding of these tumors.
WHO 2021 classification of thymoma
The latest issue of the WHO classification of thymic tumors preserves the original framework proposed by Dr.Rosai in the first edition, using a combination of letters and numbers to designate the various categories of thymoma[4].It is stated by the editors that the histologic classification, nomenclature, defining criteria,and reporting strategies for thymoma and thymic carcinoma are largely the same as in the 4th edition,except for two entities that were eliminated, microscopic and sclerosing thymoma[5].
Thymoma type A
Type A thymoma is defined by the WHO as “a thymic epithelial neoplasm with variable growth patterns,composed of usually bland spindle/oval tumor cells with few or no admixed immature lymphocytes”[4].Despite the fact that the tumor is primarily composed of a population of spindle cells, the WHO specifically recommends not using the term “spindle cell thymoma”; no rationale or explanation is offered for that recommendation.The pathogenesis of these tumors is still in dispute.Genetic studies have demonstrated that these tumors harbor few genetic alterations, most often involving chromosome 6q25.2[6].Almost all cases show a missense mutation ofGTF2I, a gene involved in cell regulation, receptor tyrosine kinase signaling, and negative regulation of apoptosis, cell cycle, and DNA damage control[7,8].The tumors are characteristically grossly surrounded by a well-developed fibrous capsule and display a lobular cut surface.The histology is characterized by a fascicular, storiform, or hemangiopericytic growth pattern [Figure 2 A and B].Cystic changes are commonly encountered, and a microcystic or pseudoglandular pattern of growth are other commonly seen features.The tumor cells are characterized by bland-appearing oval to spindleshaped nuclei with finely dispersed chromatin and inconspicuous or absent nucleoli, and a scant rim of amphophilic cytoplasm.Mitoses are rare to non-existent and nuclear pleomorphism or cytologic atypia is absent.Type A thymoma shares with other types of thymomas positivity for cytokeratins (AE1/AE3) and nuclear positivity for p63 and p40.Some cases may show aberrant expression of CD20.The tumor cells are also positive for bcl-2 in about 90% of cases; a feature that can be of help for separating them from type B thymomas, which are uniformly negative[9].The tumor cells are also characterized by an extremely low proliferation rate (average 1% nuclear positivity).
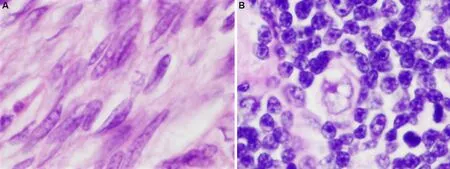
Figure 1.Two basic types of cells in thymoma: (A) spindle or oval cells devoid of nucleoli and with dispersed chromatin pattern and scant cytoplasm characterize type A thymoma (60×); (B) large round to polygonal cells with vesicular nuclei, scant nuclear chromatin and prominent eosinophilic nucleoli surrounded by an ample but indistinct rim of cytoplasm characterize type B thymomas (60×).
The diagnosis of type A thymoma is generally straightforward and does not pose a major problem for diagnosis.In small biopsy samples, the differential diagnosis may include low-grade spindle cell neoplasms such as mediastinal solitary fibrous tumor, a differential that is easily resolved using a basic panel of immunohistochemical stains.Most of these tumors are identified in early stages and are associated with a favorable prognosis; some cases, however, are resected in more advanced stages and can follow a more aggressive behavior with recurrences, distant metastases, and fatal outcomes[10-17].
Atypical type A thymoma
In the 4th edition of the WHO classification of tumors of the thymus, this new category was introduced and stated to represent a subtype of type A thymoma[5].Although a formal definition of atypical type A thymoma is not provided in the current WHO classification, it is indicated that rarely, type A thymomas can display some degree of atypia, such as hypercellularity, increased mitotic counts, and particularly foci of necrosis.The single illustration of atypical type A thymoma provided in the WHO monograph shows atumor with increased cellularity, nuclear crowding, prominent nucleoli, necrosis, and increased mitotic activity.The authors of the WHO state that the literature indicates such tumors are not associated with higher chromosomal alterations or a more aggressive behavior than type A thymomas and stress the fact that metastases can also occur in type A thymomas without such atypical histologic features[4].Very few studies are available on atypical type A thymoma and its full spectrum of histologic features, and biologic behavior still remains poorly understood.As such, this remains a “provisional” category in the current WHO schema.
Thymoma type AB
This is defined by the WHO as “a thymic epithelial neoplasm composed of variable proportions of lymphocyte-poor spindle cell (type A) component and a lymphocyte-rich (type B-like) component with a significant population of immature T cells”[4].Type AB thymoma is a novel type of thymic epithelial neoplasm that was originally introduced in the “histogenetic” classification proposed by Marino and Müller-Hermelink[18]in 1985 under the designation of “mixed thymoma”.It was initially postulated that such tumors showed mixed differentiation by reproducing both the cortical and medullary compartments of the normal thymus.This interpretation was accepted by Dr.Rosai in the first edition of the WHO book on the histologic classification of thymic tumors and was maintained in the subsequent editions of the WHO books[4,5,19].The current WHO classification states that the pathogenesis of these tumors is “a putative thymic epithelial cell precursor with the potential for bilineage, corticomedullary differentiation, and restricted terminal medullary maturation”[4], a statement that is based on a single previously published immunohistochemical study[4,20].The WHO also states that type A and type AB thymomas frequently share genetic alterations and that both harbor a frequent mutation in theGTF2Igene as well as overexpression of a microRNA cluster on chromosome 19q13.42[7,8,21].In their histologic description of type AB thymoma,they state that the tumors are composed of a highly variable admixture of a lymphocyte-poor type A component and a type B-like lymphocyte-rich component, which can either form discrete, separate lobules or be intricately intermingled [Figure 3].However, they also state that the type B-like areas are different from type B1, B2, and B3 thymoma, in that the tumor cells are small and oval, plump and spindly, or polygonal in shape, with round to oval pale-staining nuclei showing dispersed chromatin and inconspicuous nucleoli.It is further stated that the large vesicular nuclei with distinct nucleoli that are characteristic of the neoplastic cells in type B2 thymoma are only rarely seen.In other words, the current WHO tacitly admits that the proliferating epithelial cells in the “type B-like” areas are essentially spindle cells, not the round or polygonal cells of type B thymoma.Yet, the WHO persists in clinging to the notion that these tumors represent biphenotypic proliferations that harbor both type A and type B components, as evidenced by their favored histogenetic theory and their choice of designation for this tumor.We have recently argued that the bulk of the evidence, both from morphology, ultrastructure,immunohistochemistry, and genetics supports that type AB thymoma represents a lymphocyte-rich spindle cell thymoma (in other words, a lymphocyte-rich type A thymoma), in which the proliferating epithelial cells in both the type A component and the “type B-like” component are one and the same, i.e., spindle thymic epithelial cells[22].Moreover, in some cases, the entire tumor is composed of an overwhelmingly lymphocyte-rich component that can closely mimic a type B-1 thymoma, except the neoplastic epithelial cell population is composed of short spindle cells rather than the round epithelioid cells typical of B-1 thymoma [Figure 4 A and B].Not only does the histology (with the exception of the abundance of lymphocytes) and genetic profile of these tumors mirror each other, but their clinical behavior appears to be essentially the same.This begs the question of why we need a separate category for a tumor that simply represents a variant of spindle cell thymoma which simply happens to contain areas with an abundance of immature T-lymphocytes.Additional studies are needed to demonstrate more convincingly and conclusively that type A and type AB thymoma represent two distinct and separate categories of tumors rather than variations of the same neoplasm.
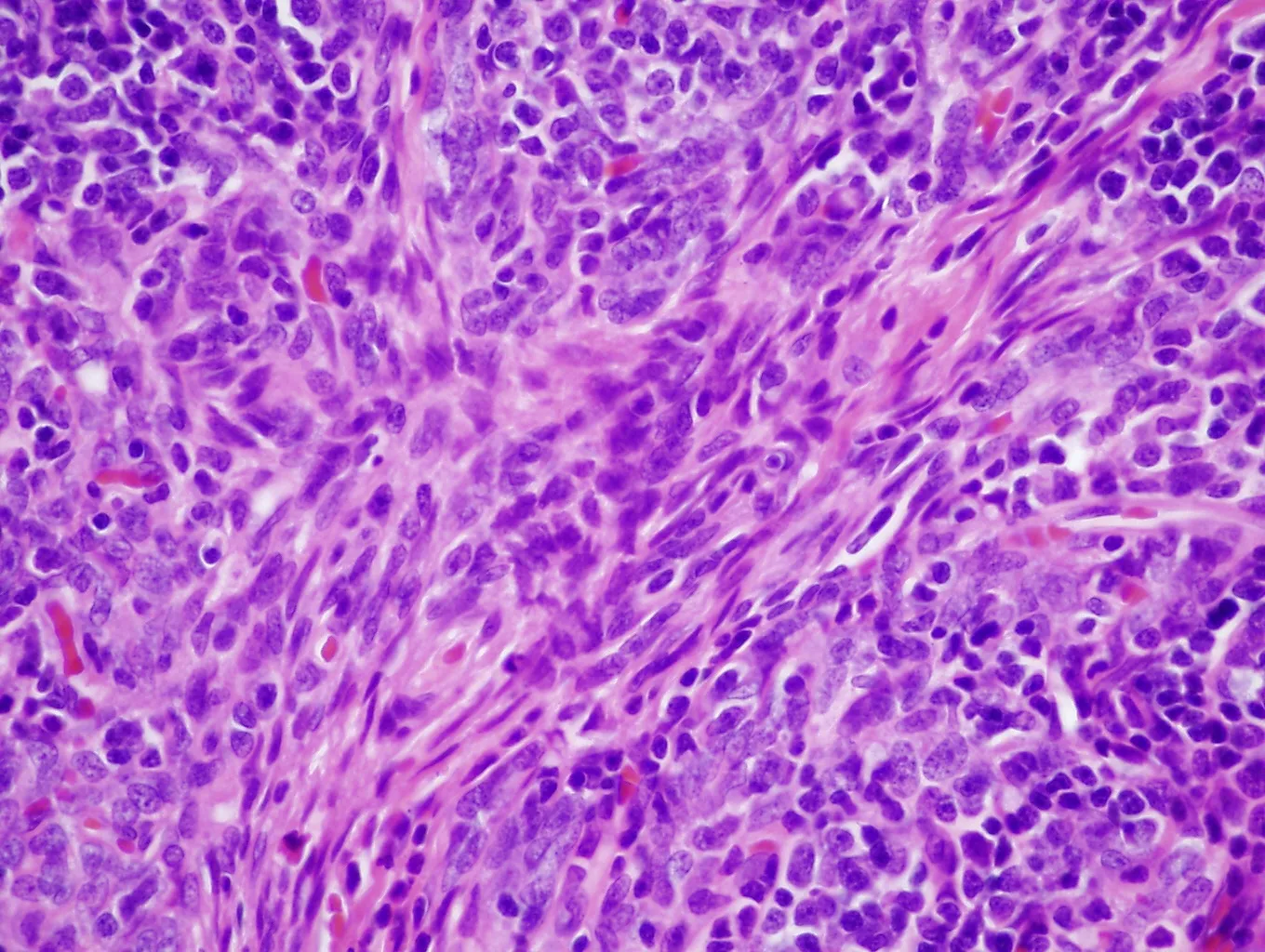
Figure 3.Type AB thymoma is defined as a tumor that contains an admixture of spindle cell areas (center) with paucity of lymphocytes and lymphocyte-rich (“B-like component”) areas, both in the same tumor (40×).
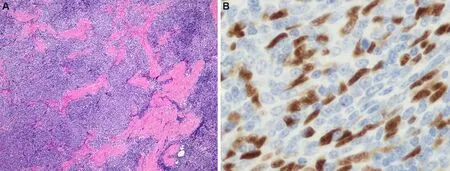
Figure 4.Predominantly lymphocyte-rich AB thymoma: (A) the tumors are composed of a predominantly lymphocyte-rich population of cells harboring scattered small spindle cells in the background; these tumors can resemble on cursory examination a type B1 thymoma(2×); (B) an immunoperoxidase stain for p63 shows on higher magnification the scattered proliferating thymic epithelial cells (dark brown nuclei) surrounded by a large number of small lymphocytes.Notice the spindle shape of the nuclei, unlike the round shape of the nuclei characteristic seen in type B thymoma (60×).
Type B-1 thymoma
This is defined by the WHO as “a thymic epithelial tumor that recapitulates the cytoarchitectural features of the non-involuted normal thymic cortex, accompanied by regions of medullary differentiation”[4].The tumors are characterized histologically by a lobular architecture separated by fibrous bands, and on scanning magnification, they show a deep blue color due to the crowding of small lymphocytes [Figure 5A].On higher magnification, singly scattered larger cells corresponding to the proliferating thymic epithelial cells are identified admixed with the lymphocytes.The tumor cells have large, round to oval nuclei with dispersed chromatin pattern and with easily visualized eosinophilic nucleoli, and they are surrounded by an ample rim of amphophilic cytoplasm.Sharply delineated pale areas containing a lower density of lymphocytes are frequently identified on scanning magnification (so-called areas of medullary differentiation) [Figure 5B], as well as occasional dilated perivascular spaces, which are helpful for separating these tumors from mediastinal lymphomas which they can closely resemble.Immunohistochemical stains for cytokeratin and p63/p40 are helpful for highlighting the proliferating thymic epithelial cells interspersed with the lymphocytes and represent a simple way of distinguishing them from mediastinal lymphomas.
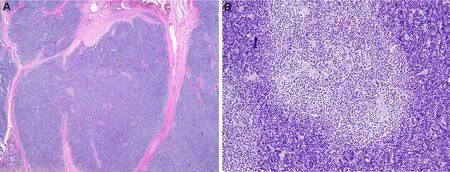
Figure 5.Type B1 thymoma: (A) scanning magnification shows the appearance of a “blue cell tumor” due to the massive amounts of small lymphocytes, and the characteristic lobulation separated by broad fibrous bands (2×); (B) area of “medullary” differentiation is seen in a B1 thymoma, characterized by a rounded zone that looks paler than the surrounding tissue owing to a decrease in the number of small lymphocytes (20×).
Type B-2 thymoma
Is defined by the WHO as a “lymphocyte-rich thymic epithelial tumor composed of polygonal tumor cells accompanied by numerous immature T-cells.The tumor cells occur at a density higher than that of the normal thymus and of type B-1 thymoma”[4].Type B-2 thymoma represents the progression of the neoplastic process in these tumors to the next level, with a noticeable increase in the number of proliferating thymic epithelial cells over that of B-1 thymoma.The tumors can be easily distinguished on scanning magnification from B-1 thymoma because instead of the uniform and compact “blue” appearance resembling a lymphoma, the tumors in B-2 thymoma show a mottled appearance and are less homogeneous due to the increase in the number of proliferating epithelial cells [Figure 6].The tumors can display both areas of “medullary” differentiation and prominent dilated perivascular spaces.On higher magnification,the proliferating thymic epithelial cells are easily visualized and more abundant than in type B-1 thymoma.The tumor cells closely resemble those seen in type B-1 thymoma and contain large, round to oval nuclei with dispersed chromatin and conspicuous eosinophilic nucleoli surrounded by abundant amphophilic to lightly eosinophilic cytoplasm.Because of the abundance of the proliferating cells, clustering of epithelial cells can be seen forming small aggregates of 3-5 contiguous cells, but prominent sheeting of cells or syncytia are not present.Mitotic activity is absent in the epithelial cells.The immunohistochemical and genetic features of these tumors are similar to those of B-1 thymoma.
Type B-3 thymoma
Is defined by the WHO as “a thymic epithelial tumor predominantly composed of mildly or moderately atypical polygonal tumor cells accompanied by small numbers of non-neoplastic immature T-cells”[4].Type B3 thymoma represents a more advanced stage in the progression of malignancy in thymic epithelial neoplasms, whereby the normal architecture of the mature thymus characterized by a densely populated cortical zone in which small lymphocytes overshadow the epithelial cells is replaced by an overwhelmingpopulation of neoplastic thymic epithelial cells with few residual lymphocytes.In other words, there is a loss of functional maturation and an increase in the proliferative activity of the tumor cells in the process.Because of the obvious increase in cytologic atypia of the proliferating epithelial cells, we have proposed the terminology of “atypical thymoma” for these tumors to draw a distinction between them and their better differentiated counterparts (WHO B-1 and B-2)[23].The tumors show a similar growth pattern as that of B-1 and B-2 thymoma, with lobules of tumor cells separated by fibrous bands.They are easily recognized on scanning magnification because of their light pink color, in contrast to the dark blue appearance of B-1 and B-2 thymomas; this is due to the abundance of eosinophilic cytoplasm in the tumor cells and the paucity of small lymphocytes [Figure 7].The most distinguishing feature of type B-3 thymomas is their cohesive,sheet-like growth pattern.Unlike types B-1 and B-2, in which the proliferating thymic epithelial cells are separated by large amounts of small lymphocytes, in B-3 thymoma, the cells form confluent and cohesive sheets of cells that are attached to one another resembling immature squamous epithelium [Figure 8A].In fact, one of the distinguishing features of B-3 thymoma is the pavement-like appearance caused by sheets of cells displaying sharp cell membranes that resemble the immature or “intermediate” cells in mucoepidermoid carcinoma or the squamoid cells of various skin adnexal neoplasms, such as poroma and hidradenoma [Figure 8A].The cytologic features of these tumors can be quite variable, and they can display a spectrum of cell shapes and sizes.Most of the tumor cells are medium to large size, but smaller, less mature cells can also be seen.The cells are characterized by large round to oval nuclei with a generally denser chromatin pattern than that seen in B-1 or B-2 thymomas often containing prominent eosinophilic nucleoli.The cells display abundant densely eosinophilic cytoplasm and, as mentioned above, tend to display sharply outlined cell borders, although lacking intercellular bridges [Figure 8B].Unlike B-1 and B-2 thymoma, they can display variable mitotic activity, which is generally low (1-2 mitoses per 10 high power fields).The mitoses are usually of conventional type, but abnormal mitoses, including tripolar mitoses, can occasionally be seen.The tumors also tend to show prominent dilated perivascular spaces; palisading of epithelial cells along the edges of the perivascular spaces is a common feature.The nuclei of the tumor cells often adopt a “raisinoid” or crinkled appearance and can sometimes display longitudinal nuclear grooves.In some cases, the cytoplasm of the tumor cells can be water-clear or can show a striking clear perinuclear halo.Larger pleomorphic and sometimes multilobated or multinucleated tumor cells with dense chromatin patterns and prominent nucleoli can be occasionally found and do not impart a more ominous prognosis tothese tumors.Foci of abrupt squamous differentiation can be seen in about 15% of cases [Figure 9].These foci can resemble the so-called “squamous eddies” seen in some skin adnexal tumors, or they may become more confluent and prominent, leading to difficulties for distinguishing them from well-differentiated squamous cell carcinoma.Admixtures and transitions of B3 with type B1 and B2 thymoma are frequently present in varying proportions.Development of thymic carcinoma arising from the background of B3 thymoma has also been documented[24,25].The current WHO book also states that “…spindle cells can occur focally in an otherwise typical B3 thymoma.Whether a pure spindle cell subtype of type B3 thymoma exists is unclear, and distinction from atypical type A thymoma is difficult” [Figure 10].Actually, a spindle cell variant of this tumor has been acknowledged in the literature for quite some time[26,27].The acknowledgment that there is the possibility of the existence of a spindle cell type of B3 thymoma by the WHO and thatdistinction from atypical type A thymoma is difficult to make indicates that a clear definition of atypical type A thymoma does not yet exist and that further study and debate needs to take place to clarify this issue.
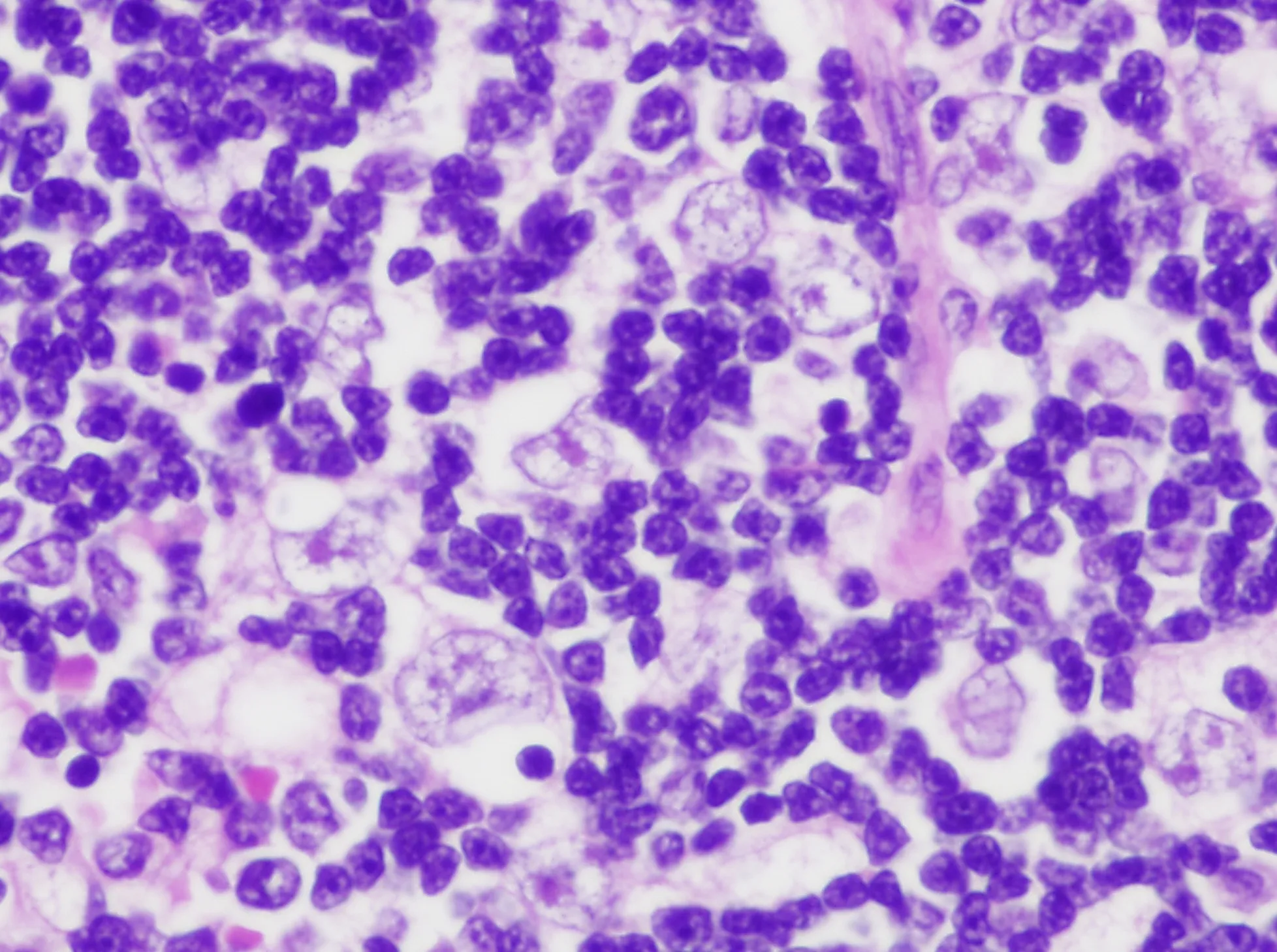
Figure 6.Type B2 thymoma is characterized by the increase in the number of proliferating thymic epithelial cells vis-à-vis the number of lymphocytes.Notice, however, that the appearance of the proliferating thymic epithelial cells is identical to that seen in B1 thymoma,the only difference is the increased number of epithelial cells (40×).
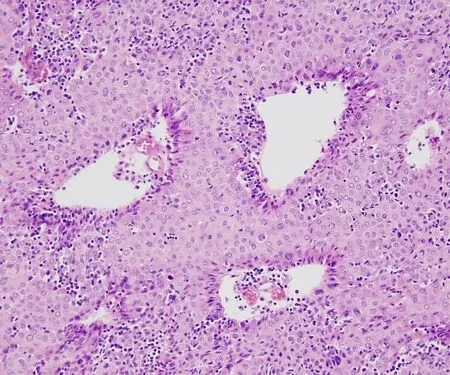
Figure 7.B3 thymoma is characterized by a cohesive sheet-like growth pattern composed of thymic epithelial cells with abundant cytoplasm and frequent dilated perivascular spaces.Notice the paucity of stromal lymphocytes compared with B1 and B2 thymoma(20×).
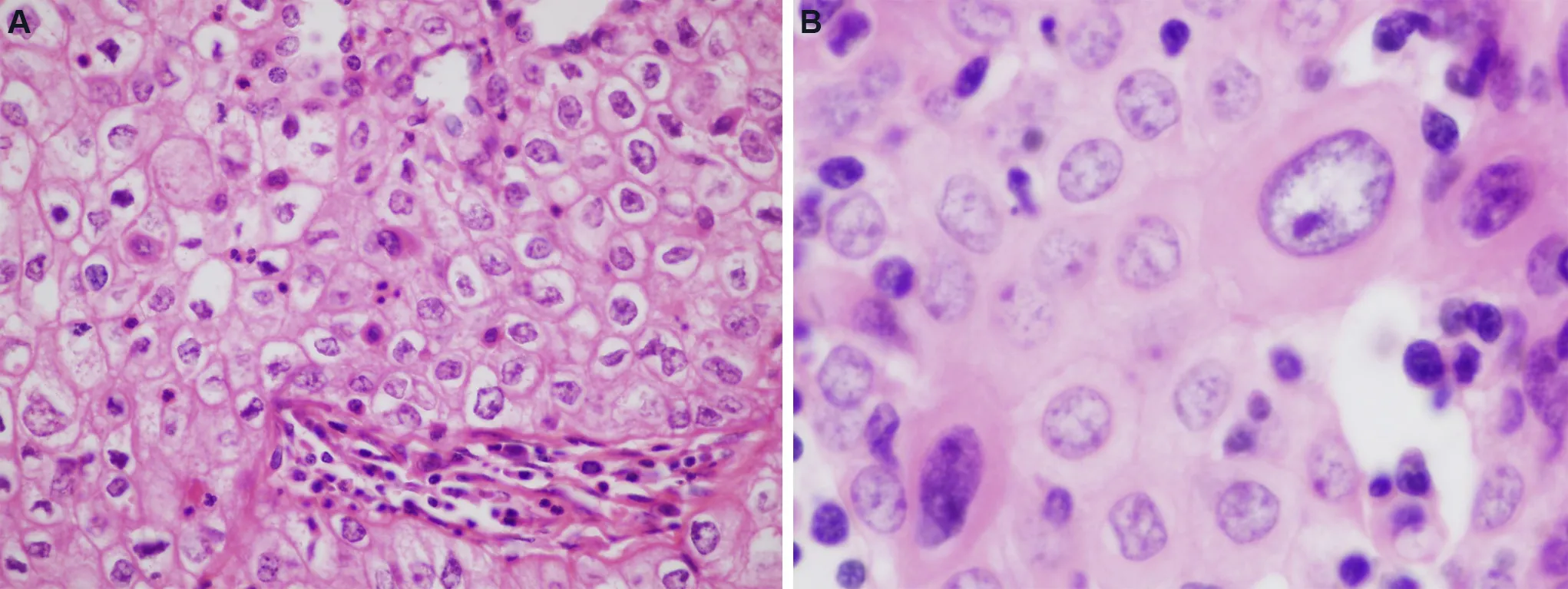
Figure 8.Type B3 thymoma: (A) higher magnification of type B-3 thymoma shows the pavement-like architecture resembling immature squamous epithelium due to the sharply apposed cell membranes (40×); (B) cytologic features of B3 thymoma include enlargement of nuclei, increase in chromatin pattern, and occasional prominent nucleoli and larger, more atypical cells (60×).Low mitotic activity is generally present in these tumors, averaging 1-2 per 10 high power fields.
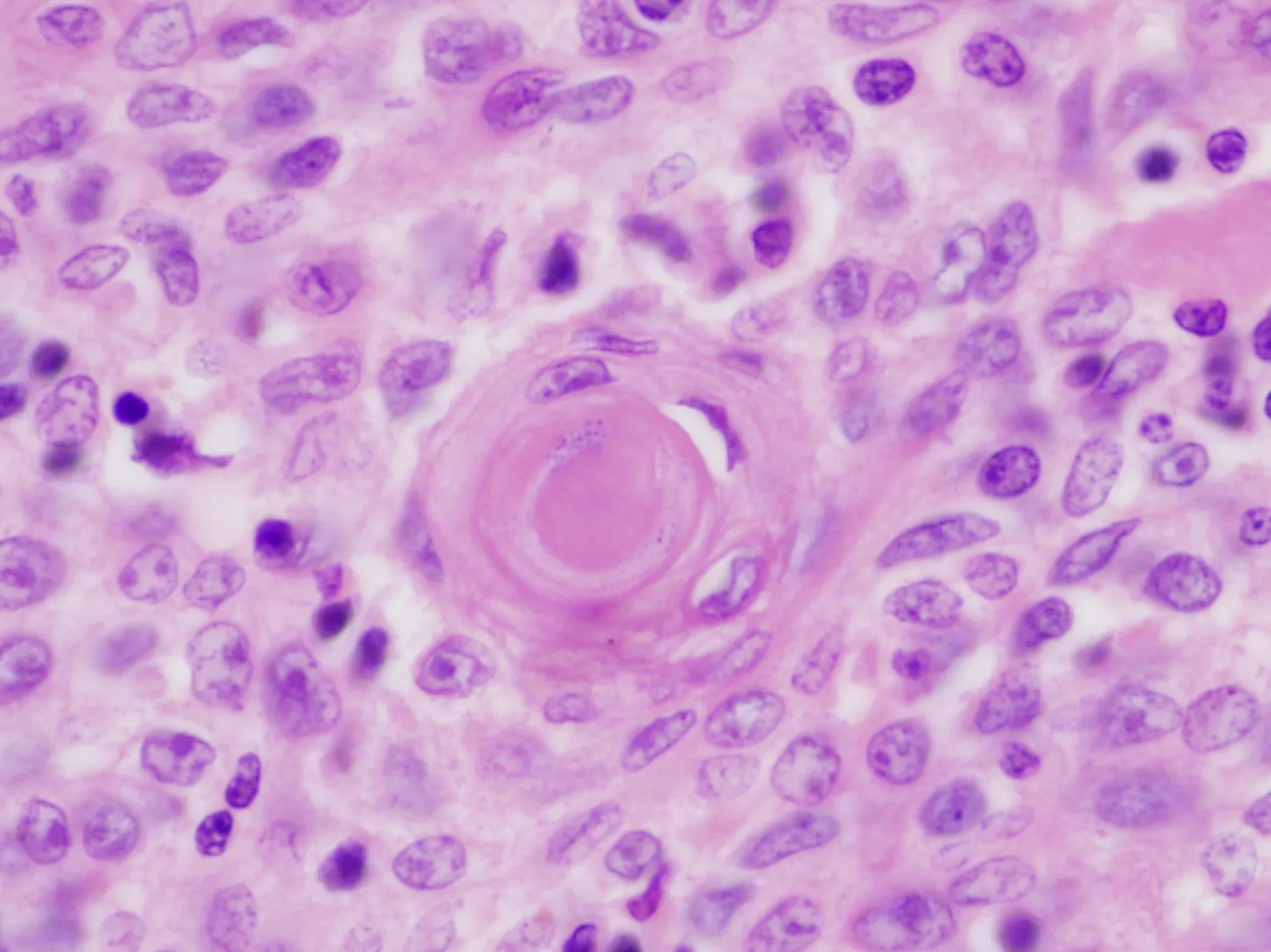
Figure 9.Type B3 thymoma showing abrupt focus of squamous differentiation (center); areas like these can resemble the “squamous eddies” seen in some skin adnexal neoplasms (40×).
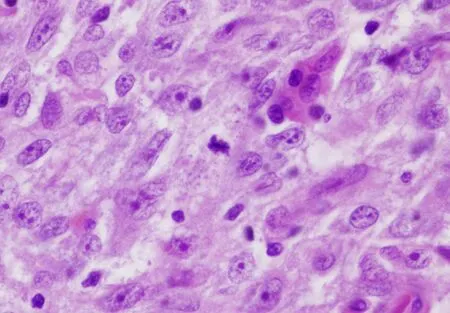
Figure 10.Atypical thymoma predominantly composed of oval to spindle cells (B3, spindle cell type) showing increased size of the cells,increased nuclear chromatin pattern, prominent nucleoli and scattered mitotic figures (60×).Tumors with these features are currently included under “atypical type A thymoma”, but it is also stated that it is not certain whether a pure spindle cell type of B3 thymoma could also exist and that the two would be “very difficult” to separate.
Type B-3 thymoma is probably the most controversial and contentious of the various thymoma categories.Very little is known of its histology and clinical behavior, and very few studies have addressed this particular variant of thymoma in detail.These tumors were recognized in prior classification systems as “epithelialrich” or “polygonal cell type” thymoma[10,12], and were declared to represent a “well-differentiated carcinoma” in the Marino and Müller-Hermelink[18]histogenetic classification.Difficulties for consistently separating them from well-differentiated thymic carcinoma have been continuously cited by several investigators.In the latest WHO book, the diagnostic criteria considered essential for diagnosis include lobular architecture, sheet-like growth pattern, interspersed perivascular spaces, and paucity of lymphocytes.Yet, despite these criteria, difficulties still exist for separating them from well-differentiatedsquamous cell carcinoma, particularly in cases in which prominent squamoid areas or frank islands of squamous differentiation are present.The use of immunohistochemical stains was postulated to be helpful under the assumption that only squamous cell thymic carcinomas were positive for CD5 and CD117;however, a recent study demonstrated that these tumors can also express CD5 in 22.8% of cases and CD117 in up to 13% of cases [Figure 11][27].The use of these stains, therefore, can no longer be accepted as reliable or specific enough for making this distinction.
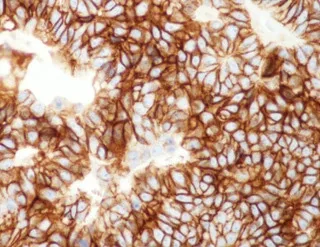
Figure 11.Immunoperoxidase stain for CD117 in B3 thymoma shows strong membrane positivity of the tumor cells (20×).A recent study demonstrated up to 13% positivity for this marker in B3 thymoma, thus limiting its specificity for discriminating these tumors from well-differentiated squamous cell carcinoma of the thymus.
PROBLEM AREAS AND OUTSTANDING ISSUES
Despite all the progress that has been made in recent years regarding our understanding of thymic epithelial neoplasms, a few problem areas remain.The WHO classification represented a great step forward in the field by providing a schema that allowed us to translate the various terminologies in existence prior to the year 2000 into a single unified nomenclature.Having a unified system for translating the results of international studies which had previously utilized different classifications and terminologies permitted leveling the playing field and allowed us to obtain more clarity regarding the biology of these tumors.One of the important effects of this was the increasing realization that, for this particular family of tumors,clinical staging plays the most important role in predicting the prognosis and behavior of the lesions and in planning a rational and practical approach to therapy.Several studies have demonstrated that collapsing some of the various categories in the WHO classification, meaningful prognostic categories emerge without any loss of discriminatory capacity for assessing the behavior of these tumors[15,28-31].Thus, the emergence of the information accrued in recent years, which was only possible due to the availability of the WHO schema(which facilitated the comparison between existing classifications), had allowed us to arrive at the same conclusion that was originally articulated by Dr.Rosai[3]when he first introduced the WHO classification in 1999, mainly, that staging represents the most important prognostic parameter for the assessment of thymic epithelial neoplasms to the extent of reducing histology to a secondary role.Given that the WHO classification was, by its authors’ own admission, introduced simply as a schema to facilitate translation between the various existing classifications and not as a definitivehistologicalclassification, the question arises as to whether now that we have achieved a clearer understanding of the biology of these tumors it may not be time to retire the current WHO schema and replace it for a more definitivehistologicclassification that will more accurately reflect the biology of these tumors.
It is remarkable in this context that the various categories introduced by Dr.Rosai in 1999 and later modified in the subsequent editions of the WHO classification have undergone a slow metamorphosis as new information has helped clarify our views on these tumors.The concept of a “mixed” or type AB thymoma is a prime example of this.The first edition of the WHO on thymic tumors was admittedly a compromise formula devised by Dr.Rosai and members of the WHO panel to accommodate all the conflicting views and terminologies espoused by the different members of the committee entrusted with the new classification[32].It is unfortunate that the panel members were unable to reach a consensus and had to resort to a compromise formula, however helpful that was to resolve the disputes.This compromise formula, however, was never intended as a new histologic classification but merely as a framework for allowing translation of the various terminologies in existence into a common language.At the time, the level of granularity in our understanding of these tumors was still limited as new insights and observations were starting to emerge.The “histogenetic” classification proposed by Marino and Müller-Hermelink[18]in 1985 was greeted initially with much enthusiasm because it promised an appealing biologic basis for classifying these tumors based on their putative histogenesis, which at the time represented a novel approach that had been previously lacking.Unfortunately, as the work of the very proponents of the histogenetic theory and others later demonstrated, the histogenetic basis of their classification was flawed and not supported by numerous immunohistochemical studies that consistently failed to demonstrate any correlation between thephenotype of the cells in their various categories and the normal compartments of the thymus[33-36].For a period of time, it was believed by some that this new histogenetic classification had uncovered histologic types of thymoma that were perceived as being different from the standard categories in the “traditional”classification of Bernatzet al.[10], Lattes and Jonas[11].In particular, the “mixed” type of thymoma and the“predominantly cortical” thymoma did not appear to have any known counterpart in the previous classification schemes.The WHO schema came to clarify some of these issues by providing a framework for assigning the various terms in the different classifications into a common language.But there was still one type of thymoma that remained an “orphan” and was a source of major confusion, the type AB thymoma.This tumor had never been identified or mentioned previously in the literature, and it was conceptually difficult to understand where it fell nosologically as it had no counterpart in any of the previous classifications.Over the past few years, the WHO has experienced a slow transformation in its understanding of this tumor, from the initial premise that it represented a mixture of type A and type B areas within a single tumor[3,19], to the recognition that the type B component was not precisely the same as that in type B thymoma[5], to the admission that the cells in the “type-B-like component” are actually small,spindle cells that are quite different from any of the type B cells[4].All that remains is for the WHO to take the last step in acknowledging that the cells in the “type B-like component” are in fact identical to those in the type A component, and that the only difference between the two components is the number of small lymphocytes contained in the stroma.In other words, that type AB thymoma is nothing other than a lymphocyte-rich type A thymoma (or a lymphocyte-rich spindle cell thymoma, translated into morphologic terms).This realization has now been supported by morphologic studies, immunohistochemistry, electron microscopy, molecular genetics, and by the fact that both categories share virtually identical clinicopathologic features, molecular genetics, and behavior.However, a lymphocyte-rich form of spindle cell thymoma had never been acknowledged previously because the prevailing dogma for many years was that spindle cell thymomas were devoid of lymphocytes or had only a very scant lymphocytic component.We have now come full circle to finally acknowledging that spindle cell thymoma (type A thymoma) can also contain an important and sometimes predominant component of immature T-lymphocytes[22].The identity of type AB thymoma has thus been now clarified as it has become increasingly apparent that it actually represents a variation of type A thymoma and is not a separate and distinct histogenetic category.This has required an adjustment to our bias that spindle cell thymomas are always devoid of lymphocytes; if we dispense with this dogma and acknowledge that there is no reasonable or accepted biological reason why this should be the case, it becomes very easy to recognize type AB thymoma for what it is, a lymphocyte-rich spindle cell thymoma.Another important step forward has been the elimination of the emphasis that was initially placed on the notion that types A and AB thymomas were “benign” tumors without competence for metastases or fatal outcomes.Although most type A and AB thymomas have excellent survival statistics at 5 and 10 years, the WHO now acknowledges that cases with local recurrences or distant metastases are documented, although they provide no specific data regarding malignant behavior for these tumors[4].
Another outstanding issue yet to be addressed by the WHO classification is the status and nosologic position of “atypical type A thymoma”[4].This new entry in the WHO classification has been placed for the time being under the category of type A thymoma and declared to be a subtype of this tumor.Very little exists presently in terms of studies specifically addressing this tumor.In a recent study, we reported a series of cases of spindle cell thymoma that were distinguished by increased cellularity, increase in cytologic atypia with dense chromatin pattern, prominent nucleoli, and obvious mitotic activity[27].We argued that such tumors, on account of their increased cytologic atypia and mitotic activity, should be regarded as part of the spectrum of atypical thymomas and represent a spindle cell variant of type B3 thymoma in the WHO classification.As with all other types of thymoma, their clinical behavior in our study directly correlated with their clinical staging[27].The WHO classification’s statement that there may also be a spindle cell variant of B3 thymoma that can be difficult to separate from atypical type A thymoma is not very helpful and represents an added source of confusion.Additional effort should be expended in further delineating this new entry in the WHO classification, and for further defining the relationship, if any, that atypical type A thymoma has with type B3 thymoma and whether there is a valid rationale for separating it from spindle cell B3 thymoma.As with conventional B3 thymoma of epithelioid or squamoid type, the distinguishing feature separating a B3 type spindle cell thymoma from conventional type A thymoma should be the degree of cytologic atypia.Unfortunately, the WHO is stuck on the concept of density of lymphocytes as one of the primary defining criteria for the various categories, as demonstrated by their persistent use of the term “B-like areas” in the definition of type AB thymoma.Although the density of lymphocytes is certainly a helpful feature for segregating the various types of thymoma, the cytology and degree of atypia of the proliferating neoplastic epithelial cells should also be given equal consideration in the approach to the classification of these tumors.
Another area in which the WHO has revised its thinking is on the issue of the validity of some of the histologic variants outside of the A, AB, B/1,2,3 schema.The latest edition of the WHO has modified its roster of thymoma variants by eliminating two categories from their list; microscopic thymoma and sclerosing thymoma, after reaching the conclusion that neither justifies inclusion as a separate variant of this tumor.The choice of terminology by the WHO for the other two variants that could not be comfortably fit into the standard A, AB, and B categories is also of interest in this regard.The term “metaplastic thymoma”has been adopted by the WHO for an unusual variant of thymoma that was originally described as“thymoma with pseudosarcomatous stroma” on account of the striking spindle cell stromal proliferation with storiform pattern observed in those tumors[37].Shortly after the first paper was published on this entity,a similar publication appeared in the literature describing additional cases of an identical lesion which was designated as “low-grade metaplastic carcinoma of the thymus”[38].When the second edition of the WHO book on thymic tumors appeared[19], the authors recognized that what they had designated as “low-grade metaplastic carcinoma” was actually the same entity previously designated as “thymoma with pseudosarcomatous stroma” and that it did not correspond to a carcinoma but to a thymoma; yet, instead of retaining the original designation offered in the first description, they changed the designation to“metaplastic thymoma”, although the occurrence of a metaplastic process in this tumor was highly speculative and has not yet been conclusively demonstrated.Likewise, the second unusual type of thymoma in the current WHO classification, the so-called “micronodular thymoma with lymphoid stroma”, is a lesion that was initially described as “micronodular thymoma with lymphoid B-cell hyperplasia”[39].This tumor is characterized by a proliferation of spindle to ovoid thymic epithelial cells adopting a micronodular configuration surrounded by a dense polyclonal lymphoid stroma with prominent lymphoid follicular hyperplasia.The images illustrating the chapter in the WHO book on this tumor emphasize the follicular lymphoid hyperplasia displayed by these tumors[4], which is a constant feature in all cases of this thymoma variant, yet they chose to ignore the fact that lymphoid follicular hyperplasia with germinal centers associated with sheets of polyclonal B-lymphocytes is not a feature of the normal thymus and represents an abnormal process of lymphoid hyperplasia, a phenomenon that commonly occurs in patients with myasthenia gravis but can also occur in the setting of conventional thymoma in rare instances.The term“micronodular thymoma with lymphoid stroma” implies that the lymphoid component is similar to that of other types of thymoma, whereas the term “lymphoid B-cell hyperplasia” more accurately described the pathogenic process taking place in these tumors, thereby representing a more accurate name.Yet the original term proposed for these tumors was inexplicably changed for one that is less precise.In any event,and regardless of the choice of terminology, these two unusual variants of thymoma that could not be fit into the ABC schema could be easily incorporated into the overall schema of the WHO rather than separating them as stand-alone tumors.Micronodular thymoma is basically composed of an oval to spindle cell proliferation of thymic epithelial cells that happens to have a prominent B-cell lymphoid hyperplastic stroma and therefore should be regarded as a variant of spindle cell thymoma (type A).Likewise,“metaplastic thymoma” is primarily composed of a neoplastic proliferation of large polygonal epithelioid cells with scant lymphoid elements that happen to be accompanied by a florid spindle cell stromal proliferation; as such, it could be easily incorporated into the type B category.As with all other types of thymoma, behavior for these tumors should be assessed based on staging.Unfortunately, what seems to be standing in the way of such an approach is the strong loyalty of the WHO for the original terminology introduced by Dr.Rosai and the comforting familiarity with the terms that have already been established.While we find this loyalty commendable, it continues to impede the progress that we have gained through new insights and understanding of these tumors in recent years.Another example of this closed approach is the nosologic position of atypical thymomas composed of spindle cells (or B-3 thymoma of spindle cell type).Despite the admission by the WHO that B3 thymoma may also contain spindle cells, and their statement that “whether a pure spindle cell subtype of B3 thymoma exists is unclear”, they still persist in segregating thymomas composed of spindle cells from tumors composed of epithelioid or polygonal cells based on past histogenetic considerations, thus forcing them to create an awkward category of “atypical type A thymoma” instead of conceding that such tumors are the equivalent of the epithelioid cell counterpart of B3 thymoma.Although the “histogenetic” theory proposed in the Marino and Muller-Hermelink classification has become progressively discredited and abandoned, it has essentially morphed and survived in the form of the current WHO schema.Over the past 17 years, since the second edition of the WHO classification of tumors of the thymus was transitioned from Dr.Rosai to a new panel of editors, the WHO has slowly had to adapt to the reality that some of its definitions and criteria required adjustments and modifications and they have since changed several of their postulates.As such, the WHO classification has represented a continuous work in progress, as it should be for any area of scientific endeavor.This metamorphosis, however, has been slow and difficult, and continues to carry with it outdated concepts that have not stood the test of time.As our progress in the molecular genetics of these tumors advances and we gain a better understanding of this process, additional changes are hopefully bound to occur.
CONCLUSION
Thymoma classification continues to be a problem area in pathology and represents a work in progress.It is hoped that a less dogmatic approach can be achieved in the future and that alternate avenues of inquiry can be explored and given their due scrutiny and consideration.It is always difficult for new insights to be met without resistance; however, it is hoped that enlightened individuals will be willing to surrender long-held beliefs that may be contrary to available or emerging evidence and help us move the field forward.
DECLARATIONS
Authors’ contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
The author declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
 Journal of Cancer Metastasis and Treatment2022年3期
Journal of Cancer Metastasis and Treatment2022年3期
- Journal of Cancer Metastasis and Treatment的其它文章
- Moving towards the chemo-free treatment of lymphoma: hype or reality?
- AUTHOR INSTRUCTIONS
- Chronic activation of MUC1-C in wound repair promotes progression to cancer stem cells
- The bone marrow niche landscape: a journey through aging, extrinsic and intrinsic stressors in the haemopoietic milieu
- Diffuse large B-Cell lymphoma: from novel molecular classifications to tailored targeted therapies
- Combined effects of oncolytic vaccinia virus and dendritic cells on the progression of melanoma B16-F10 in mice
