A型肉毒毒素治疗膝骨关节炎疼痛的效果
赵蒙 石奇琳 付奕翎 王琳 李铁山

[摘要]目的探討A型肉毒毒素(BTX-A)治疗膝骨关节炎(KOA)病人疼痛的效果及其可能的机制。方法本研究为单中心、随机、双盲、安慰剂平行对照研究,共纳入79例病人,退出试验9例,揭盲后试验组和安慰剂组各35例,分别给予BTX-A 100 U和生理盐水3 mL单次注射,两组分别于治疗前和注射后2、4、8周评估视觉模拟评分(VAS评分)、西大略湖和麦克马斯特骨关节炎指数(WOMAC指数)、40 m步行时间和膝关节及胫骨前肌压痛阈值,并观察有无不良反应。结果治疗后,两组病人组内不同时间点VAS评分、WOMAC指数、40 m步行时间差异均有统计学意义(F=19.24~265.99,P<0.01);两组间相同时间点比较,BTX-A组病人VAS评分、WOMAC指数、40 m步行时间较安慰剂组有显著改善,差异有统计学意义(F=2.15~105.74,P<0.05)。治疗后,两组病人组内不同时间点膝关节及胫骨前肌压痛阈值均有改善,差异有统计学意义(F=11.24~318.39,P<0.01);两组间相同时间点比较,BTX-A组膝关节及胫骨前肌压痛阈值较安慰剂组显著改善,差异有统计学意义(F=4.39~95.78,P<0.05)。研究过程中两组病人均未出现不良反应。结论BTX-A可能通过改善KOA病人的神经敏化、提高压痛阈,缓解疼痛和改善功能障碍。
[关键词]关节痛;骨关节炎,膝;肉毒毒素类;中枢神经系统致敏
[中图分类号]R684.3[文献标志码]A[文章编号]2096-5532(2022)02-0253-05
doi:10.11712/jms.2096-5532.2022.58.044[开放科学(资源服务)标识码(OSID)]
[网络出版]https://kns.cnki.net/kcms/detail/37.1517.R.20220311.1335.013.html;2022-03-1414:41:47
CLINICAL EFFECT OF BOTULINUM TOXIN A IN TREATMENT OF KNEE OSTEOARTHRITIS ZHAO Meng, SHI Qilin, FU Yiling, WANG Lin, LI Tieshan (Department of Rehabilitation Medicine, The Affiliated Hospital of Qingdao University, Qingdao 266003, China)
[ABSTRACT]ObjectiveTo investigate the clinical effect and possible mechanism of botulinum toxin A (BTX-A) in the treatment of pain in patients with knee osteoarthritis (KOA). MethodsThis study was a single-center randomized, double-blind, placebo-controlled, parallel clinical trial. A total of 79 patients were enrolled, among whom 9 withdrew from the trial, and there were 35 patients in the trial group and 35 in the placebo group after unblinding. The patients in the trial group were given a single injection of BTX-A 100 U, and those in the placebo group were given a single injection of normal saline 3 mL. Before treatment and at 2, 4, and 8 weeks after injection, the two groups were observed in terms of Visual Analog Scale (VAS) score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC),40-meter walking time, and tenderness thresholds of peri-knee and anterior tibial muscles, and adverse reactions were also evaluated. ResultsAfter treatment, both groups had significant changes in VAS score, WOMAC index, and 40-meter walking time at different time points (F=19.24-265.99,P<0.01); compared with the placebo group, the BTX-A group had significant improvements in VAS score, WOMAC index, and 40-meter walking time at each time point (F=2.15-105.74,P<0.05). After treatment, both groups had significant improvements in the tenderness thresholds of knee joint and anterior tibial muscles (F=11.24-318.39,P<0.01); compared with the placebo group, the BTX-A group had significant improvements in the tenderness thresholds of knee joint and anterior tibial muscles at each time point (F=4.39-95.78,P<0.05). No adverse reactions were observed during the study. ConclusionBTX-A can alleviate pain and improve dysfunction in patients with KOA, possibly by improving nerve sensitization and tenderness threshold.
[KEY WORDS]arthralgia; osteoarthritis, knee; botulinum toxins; central nervous system sensitization
膝骨关节炎(KOA)是老年人最常见的关节疾病,临床表现为关节肿痛及活动受限,严重者会造成关节畸形[1-3]。其中疼痛是KOA最突出的临床表现,同时也是病人选择就诊的最主要原因[4-6]。目前KOA尚无有效治愈方法,临床中对于疼痛的控制成为重要的治疗目标之一。临床中常见的有效缓解KOA疼痛的治疗措施包括物理疗法、口服药物、注射治疗及关节置换手术等[7-11],这些方法各有利弊[12-13],临床中需探索更好的治疗KOA病人疼痛的方法。临床中治疗KOA的最优原则应符合治疗次数少、镇痛时间长、经济负担小、镇痛效果强等。关节腔注射A型肉毒毒素(BTX-A)因具有只需要单次注射且镇痛时间长的优点,逐渐成为一种潜在的治疗KOA疼痛的方法。BTX-A是由革兰阳性厌氧芽孢肉毒杆菌在生长繁殖过程中产生的一种细菌外毒素,能通过水解突触前膜相关膜蛋白抑制周围运动神经末梢突触前膜乙酰胆碱释放,引起肌肉的松弛性麻痹,其临床效果可持续3~9个月[14]。本研究通过随机、双盲、安慰剂、平行对照研究,探讨BTX-A治疗KOA疼痛的有效性及其可能机制。
1资料与方法
1.1研究对象
采用单中心、随机、双盲、安慰剂平行对照研究方法,选取2017年9月—2019年12月我院收治的KOA病人79例作为研究对象,退出试验9例,揭盲后按照1∶1的比例将病人随机分配到试验组和安慰剂组中。纳入标准:①符合美国风湿病学会和欧洲抗风湿联盟制定的标准[15],满足下列诊断标准中的A,同时满足B、C、D、E中的任意2条,可诊断为KOA(A:近1个月内反复膝关节疼痛;B:X线平片(站立位或负重位)示影像学改变;C:年龄≥48岁;D:晨僵时间≤30 min;E:活动时有骨摩擦音(感));②经保守治疗效果不佳、注射治疗意愿强烈的病人。排除标准:①病变关节合并感染、关节肿瘤或结核;②凝血功能异常;③存在严重心肺疾病及严重肝、肾功能不全;④合并认知障碍及精神障碍,不能配合治疗及随访;⑤影像学K-L分级4级及膝关节手术治疗史。本研究通过了青岛大学附属医院伦理委员会审查,研究对象自愿接受治疗并签署知情同意书。
1.2超声引导下注射BTX-A治疗方法
采用柯尼卡SONIMAGE HS1彩色超声系统,高频线阵探头(8~12 MHz)引导注射。两组均选择病人一个膝关节作为分析样本,优先纳入优势手一侧的膝关节,病人均取统一体位(平卧位,膝下垫枕使膝关节屈曲30°)。常规消毒皮肤,超声引导下采用平面内进针,穿刺平面为髌上囊平面,膝关节外侧入路进针,超声引导下注射BTX-A 100 U或安慰剂(均用3 mL生理盐水稀释);注射后休息30 min,观察是否出现不良反应。注射方案采用单次注射,观察8周。所有病人均由同一位具备资质、经过培训的康复科医师进行注射。
1.3评价指标
两组分别于治疗前及注射后2、4、8周,进行以下指标评价:①疼痛视觉模拟评分(VAS)[16];②西大略湖和麦克马斯特骨关节炎指数[17](WOMAC指数);③40 m步行时间;④压痛阈值[18](PPT)测定;⑤不良反应发生情况。
1.4统计学处理
应用SPSS 23.0统计软件进行分析。符合正态分布计量资料采用±s表示,数据间比较采用双因素重复测量方差分析;计数资料比較采用χ2检验。以P<0.05为差异有统计学意义。
2结果
2.1两组病人基本资料比较
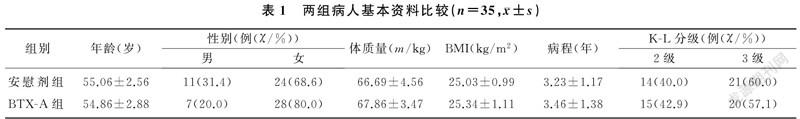
两组年龄、性别、体质量、BMI、病程、K-L分级等比较,差异均无显著性(P>0.05)。见表1。
2.2两组治疗前后VAS评分、WOMAC指数和40 m步行时间比较
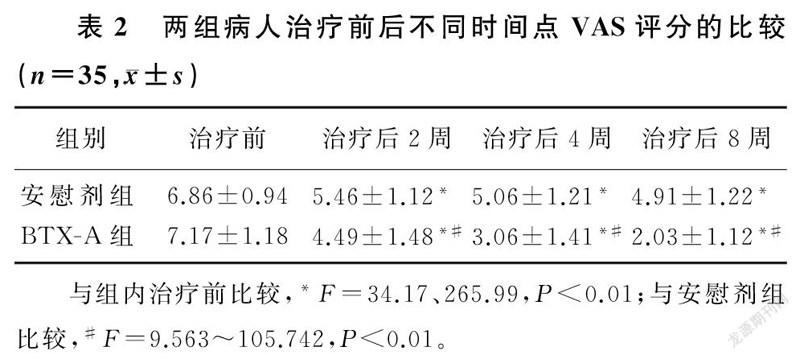
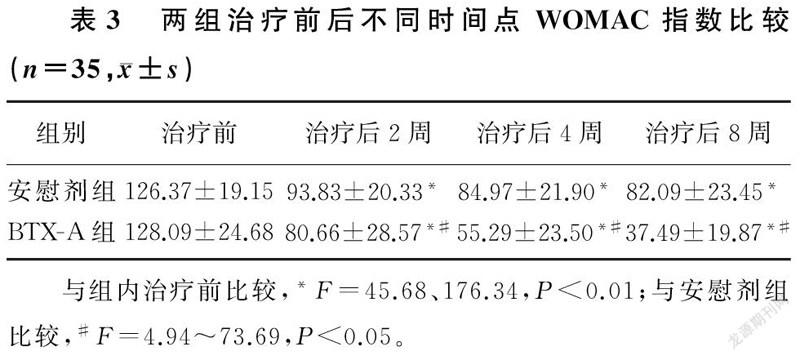
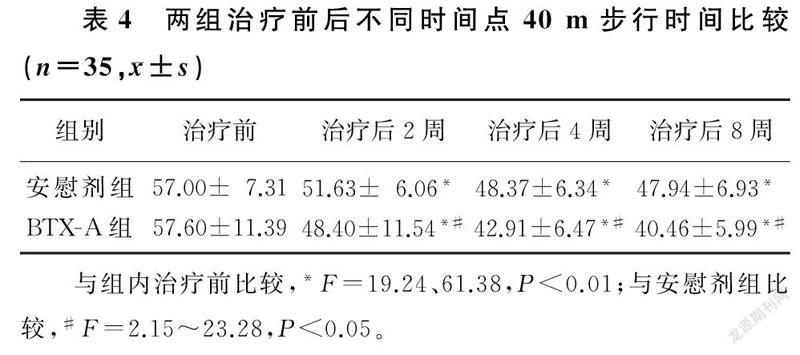
两组不同时间点VAS评分、WOMAC指数、40 m步行时间比较,差异有显著性(F=24.26~333.04,F=5.30~30.27,F=10.61~57.90,P<0.05)。组内比较,两组治疗后2、4、8周VAS评分、WOMAC指数、40 m步行时间较治疗前均有显著改善,差异有统计学意义(F=19.24~265.99,P<0.01);两组治疗后2、4、8周各指标两两比较,差异亦有统计学意义(P<0.01)。两组间比较,治疗前各指标差异无统计学意义(P>0.05);治疗后BTX-A组病人3项指标较安慰剂组均有显著改善,差异有统计学意义(F=2.15~105.74,P<0.05)。提示随访期间病人的疼痛持续缓解,BTX-A组疼痛缓解程度优于安慰剂组。见表2~4。
2.3两组治疗前后不同时间点膝关节及胫骨前肌压痛阈值比较
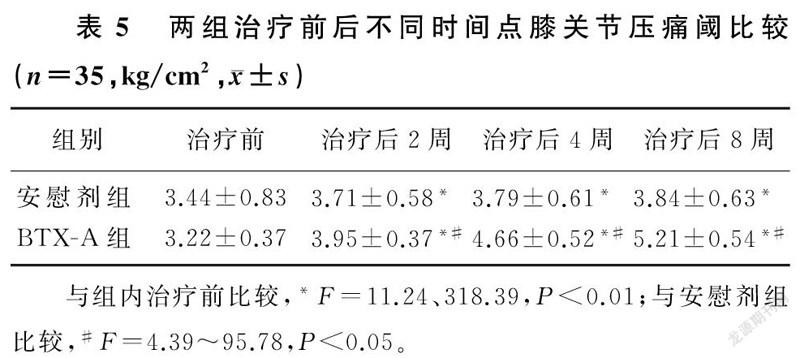
两组不同时间点膝关节及胫骨前肌压痛阈值比较,差异有显著意义(F=419.02、367.88,F=18.96、18.92,F=192.00、176.42,P<0.01)。组内比较,两组治疗后2、4、8周膝关节及胫骨前肌压痛阈较治疗前均有显著改善,差异有统计学意义(F=11.24~318.39,P<0.01);两组治疗后2、4、8周两两比较,差异有统计学意义(P<0.01)。两组间比较,治疗前差异无统计学意义(P>0.05);治疗后BTX-A组病人膝关节及胫骨前肌压痛阈较安慰剂组有显著改善,差异有显著性(F=4.39~95.78,P<0.05)。提示随访期间BTX-A组病人压痛阈持续改善且改善程度优于安慰剂组。见表5、6。
2.5不良事件发生情况
所有KOA病人在注射及随访期间均未有不良事件发生。
3讨论
KOA给中老年人的生活带来了极大的负担,而且随着我国逐步步入老龄化社会,其患病率逐年递增。因此,对KOA的诊断、治疗及综合管理提出了更高的要求[19-20]。
本研究结果显示,治疗后不同时间点BTX-A组病人VAS评分、WOMAC指数、40 m步行时间均较治疗前显著改善,且改善程度优于安慰剂组,差异有统计学意义;提示关节腔注射BTX-A对KOA的疼痛治疗有效,且疗效优于安慰剂组。这与国外的一些研究结果一致。近年来有研究发现,BTX-A对于关节疼痛具有良好的镇痛作用[18-21]。NAJAFI等[22]给予24名女性KOA病人100 U BTX-A单次注射,4周后病人疼痛均有显著改善,说明BTX-A是一种有效的治疗KOA疼痛的方法。最近一项关于BTX-A治疗KOA疼痛的Meta分析中,研究者筛选了8篇随机对照试验进行研究,得出如下结论:BTX-A治疗KOA疼痛具有短期效果[23]。但是关于BTX-A治疗KOA疼痛的研究仍有争议。最近的一项研究中,研究者对121例KOA病人行关节腔BTX-A注射,结果显示,伤害性痛亚组病人给予BTX-A较安慰剂临床疗效差异有显著性,整个人群中疗效差异则无显著性,认为KOA的疼痛具有异质性,BTX-A可能作用于某些特定的KOA疼痛机制,从而缓解病人疼痛[24]。
SIMPSON[25]对压痛阈值测量显示,受累关节的局部疼痛与外周敏感有关,而在起病部位附近或远离起病部位的疼痛与中枢敏感有关。FINGLETON等[26]研究发现,骨关节炎(OA)病人不同解剖部位的压痛阈值的平均值为1.81~5.22 kg/cm,而健康对照组为3.40~11.20 kg/cm2,提示OA病人受累关节及远离关节的部位存在压痛阈值下降的表现,受累关节压痛阈降低提示外周敏化,远离受累关节部位压痛阈下降提示中枢敏化。本研究中膝关节疼痛区域压痛阈值的改变代表外周敏化的改变,胫骨前肌的压痛阈的改变代表中枢敏化的改变。
越来越多的研究发现,外周和中枢神经的敏化是导致KOA疼痛的机制[27-28],在KOA慢性、持续性疼痛中发挥了重要作用。相关研究认为BTX-A可能通过改善KOA的神经敏化进而改善疼痛。BACH-ROJECKY等[21]研究发现,在KOA相关大鼠模型中,BTX-A可以通过降低TRPV1受体的表达,减少P物质和CGRP的释放,降低外周敏化,显著改善大鼠爪子的机械和热敏感性,证实了BTX-A可以改善KOA外周敏化[29-35]。MATAK等[36]研究发现,大鼠外周或神经节内注射肉毒素均可缓解甲醛诱导的面部疼痛,并在三叉神经核发现了被BTX-A剪切的SNAP-25,证实了BTX-A可以通过轴突逆向运输到中枢神经系统发挥镇痛效应。另外,LEE等[37]研究显示,鞘内注射肉毒素可能抑制神经递质和(或)伤害性传入神经中枢终端释放的神经肽,减少中枢敏化,进一步证明BTX-A可以直接抑制中枢敏化。已有随机、双盲、安慰剂对照研究结果显示,BTX-A可以通过改善病人中枢敏化缓解慢性头痛的皮肤异位性疼痛症状,证明BTX-A可以通过改善神经敏化进而改善疼痛[38]。本研究结果与其一致。本研究结果还显示,随着治疗后时间变化,BTX-A组病人疼痛阈值逐渐升高,疼痛持续缓解,且优于安慰剂组。因此推测,BTX-A改善KOA疼痛的机制之一可能是通过抑制外周痛觉感受器参与痛觉的其他递质(如P物质、降钙素基因相关肽、谷氨酸等)的释放,直接阻断外周神经敏化,间接阻断中枢神经敏化,減轻KOA病人疼痛;同时还可能通过轴突逆向运输到神经中枢,减少神经肽的释放直接抑制中枢敏化,进而改善KOA病人的疼痛。
综上所述,BTX-A可以作为治疗KOA病人疼痛的一种方法,BTX-A改善KOA病人疼痛的机制之一可能是通过改善神经敏化,提高压痛阈值,从而缓解疼痛和改善功能障碍。但是本研究样本量较小,只观察了短期疗效和安全性,没有观察长期疗效;下一步研究还需和临床常见的KOA治疗方法进行比较;此外还需对病人进行亚群分析,探讨BTX-A治疗KOA的适宜人群。
[参考文献]
[1]NAJAFI S, SANATI E, KHADEMI M, et al. Intra-articular botulinum toxin type A for treatment of knee osteoarthritis: clinical trial[J]. Toxicon: Official Journal of the International Society on Toxinology, 2019,165:69-77.
[2]ALLEN K D, BONGIORNI D, CAVES K, et al. Stepped exercise program for patients with knee osteoarthritis (STEP-KOA): protocol for a randomized controlled trial[J]. BMC Musculoskeletal Disorders, 2019,20(1):254.
[3]ADIGUN S. Effectiveness of manual therapy for improving pain and function in adults with hip or knee osteoarthritis: a sytematic review[D]. MGH Institute of Health Professions, 2010. https://www.zhangqiaokeyan.com/academic-degree-foreign_mphd_thesis/02061355.html.
[4]SHUKLA D, SREEDHAR S K, RASTOGI V. A comparative study of botulinum toxin: a with triamcinolone compared to triamcinolone alone in the treatment of osteoarthritis of knee[J]. Anesthesia, Essays and Researches, 2018,12(1):47-49.
[5]GEORGIEV T, ANGELOV A K. Modifiable risk factors in knee osteoarthritis: treatment implications[J]. Rheumatology International, 2019,39(7):1145-1157.
[6]VERGES J, VITALONI M, BIBAS M, et al. Global oa ma-nagement begins with quality of life assessment in knee oa patients: a systematic review[J]. Osteoarthritis and Cartilage, 2019,27:S229-S230.
[7]BOON A J, SMITH J, DAHM D L, et al. Efficacy of intra-articular botulinum toxin type A in painful knee osteoarthritis: a pilot study[J]. PM & R: the Journal of Injury, Function, and Rehabilitation, 2010,2(4):268-276.
[8]高嘉翔,陶可,陳坚,等. 运动治疗膝骨关节炎的研究进展[J]. 中华骨与关节外科杂志, 2019,12(12):1014-1019.
[9]HSIEH L F, WU C W, CHOU C C, et al. Effects of botulinum toxin landmark-guided intra-articular injection in subjects with knee osteoarthritis[J]. PM & R: the Journal of Injury, Function, and Rehabilitation, 2016,8(12):1127-1135.
[10]HAN Y H, HUANG H T, PAN J K, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis[J]. Pain Medicine (Malden, Mass), 2019,20(7):1418-1429.
[11]LANDSMEER M L A, RUNHAAR J, VAN MIDDELKOOP M, et al. Predicting knee pain and knee osteoarthritis among overweight women[J]. Journal of ]the American Board of Fa-mily Medicine: JABFM, 2019,32(4):575-584.
[12]DAI W L, ZHOU A G, ZHANG H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials[J]. Arthroscopy: the Journal of Arthroscopic & Related Surgery: Official Publication of the Arthroscopy Association of North America and the International Arthroscopy Association, 2017,33(3):659-670.e1.
[13]COLEN S, VAN DEN BEKEROM M P, MULIER M, et al. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products[J]. BioDrugs: Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy, 2012,26(4):257-268.
[14]LACKOVI Z. New analgesic: Focus on botulinum toxin[J].Toxicon: Official Journal of the International Society on Toxinology, 2020,179:1-7.
[15]ALTMAN R, ASCH E, BLOCH D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association[J]. Arthritis and Rheumatism, 1986,29(8):1039-1049.
[16]SCHWAPPACH J, DRYDEN S M, SALOTTOLO K M. Preliminary trial of intra-articular LMWF-5A for osteoarthritis of the knee[J]. Orthopedics, 2017,40(1):e49-e53.
[17]BAR-OR D, SALOTTOLO K M, LOOSE H, et al. A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee[J]. PLoS One, 2014,9(2):e87910. doi:10.1371/journal.pone.0087910.
[18]WOOLF C J. Central sensitization: implications for the diagnosis and treatment of pain[J]. Pain, 2011,152(3 Suppl):S2-S15.
[19]COURSEAU M, SALLE P V, RANOUX D, et al. Efficacy of intra-articular botulinum toxin in osteoarticular joint pain: a meta-analysis of randomized controlled trials[J]. The Clinical Journal of Pain, 2018,34(4):383-389.
[20]YOSHIMURA N, MURAKI S, NAKAMURA K, et al. Epidemiology of the locomotive syndrome: the research on osteoarthritis/osteoporosis against disability study 2005-2015[J]. Modern Rheumatology, 2017,27(1):1-7.
[21]UEDA K, TAKURA T, FUJIKOSHI S, et al. Longitudinal assessment of pain management among the employed Japanese population with knee osteoarthritis[J]. Clinical Interventions in Aging, 2020,15:1003-1012.
[22]NAJAFI S, SANATI E, KHADEMI M, et al. Intra-articular botulinum toxin type A for treatment of knee osteoarthritis: Clinical trial[J]. Toxicon, 2019,165:69-77.
[23]COURSEAU M, SALLE P V, RANOUX D, et al. Efficacy of intra-articular botulinum toxin in osteoarticular joint pain: a meta-analysis of randomized controlled trials[J]. The Clinical Journal of Pain, 2018,34(4):383-389.
[24]ARENDT-NIELSEN L, JIANG G L, DEGRYSE R, et al. Intra-articular onabotulinumtoxin A in osteoarthritis knee pain: effect on human mechanistic pain biomarkers and clinical pain[J]. Scandinavian Journal of Rheumatology, 2017,46(4):303-316.
[25]SIMPSON L L. The origin, structure, and pharmacological activity of botulinum toxin[J]. Pharmacological Reviews, 1981,33(3):155-188.
[26]FINGLETON C, SMART K, MOLONEY N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis[J]. Osteoarthritis and Cartilage, 2015,23(7):1043-1056.
[27]IYENGAR S, OSSIPOV M H, JOHNSON K W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine[J]. Pain, 2017,158(4):543-559.
[28]趙士杰,任占秀,何秋. A型肉毒素治疗慢性偏头痛机制的研究进展[J]. 中国实用神经疾病杂志, 2019,22(11):1265-1270.
[29]BACH-ROJECKY L, LACKOVI Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain[J]. Croatian Medical Journal, 2005,46(2):201-208.
[30]ZHAO X Y, MENG F G, HU S, et al. The synovium atten-uates cartilage degeneration in KOA through activation of the Smad2/3-Runx1 cascade and chondrogenesis-related miRNAs[J]. Molecular Therapy Nucleic Acids, 2020,22:832-845.
[31]LIAO T Y, DING L, WU P, et al. Chrysin attenuates the NLRP3 inflammasome cascade to reduce synovitis and pain in KOA rats[J]. Drug Design, Development and Therapy, 2020,14:3015-3027.
[32]ZHANG L, XING R L, HUANG Z Q, et al. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis[J]. Mediators of Inflammation, 2019,2019:2165918.
[33]ZHANG L, ZHANG L, HUANG Z Q, et al. Increased HIF-1α in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis[J]. Oxidative Medicine and Cellular Longevity, 2019,2019:6326517.
[34]XIAO Y C, DING L, YIN S J, et al. Relationship between the pyroptosis of fibroblast-like synoviocytes and HMGB1 secretion in knee osteoarthritis[J]. Molecular Medicine Reports, 2021,23(2):97.
[35]KLEIN-WIERINGA I R, DE LANGE-BROKAAR B J, YUSUF E, et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad[J]. The Journal of Rheumatology, 2016,43(4):771-778.
[36]MATAK I, BACH-ROJECKY L, FILIPOVIB, et al. Beha-vioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A[J]. Neuroscience, 2011,186:201-207.
[37]LEE W H, SHIN T J, KIM H J, et al. Intrathecal administration of botulinum neurotoxin type A attenuates formalin-induced nociceptive responses in mice[J]. Anesthesia and Analgesia, 2011,112(1):228-235.
[38]HOLLANDA L, MONTEIRO L, MELO A. Botulinum toxin type a for cephalic cutaneous allodynia in chronic migraine: a randomized, double-blinded, placebo-controlled trial[J]. Neurology International, 2014,6(4):5133.
(本文編辑黄建乡)

